www.elsevier.com / locate / bres
Research report
Visual discrimination learning impairments produced by combined
transections of the anterior temporal stem, amygdala and fornix in
marmoset monkeys
a b a ,
*
aCatherine J. Maclean , David Gaffan , Harry F. Baker
, Rosalind M. Ridley
a
Department of Experimental Psychology, Downing Street, Cambridge CB2 3EB, UK
b
Department of Experimental Psychology, South Parks Road, Oxford OX1 3UD, UK Accepted 19 September 2000
Abstract
Marmoset monkeys(Callithrix jacchus) with bilateral transections of the anterior temporal stem, amygdala and fornix were unable to relearn a 2-choice object discrimination first learnt prior to surgery, and were very severely impaired at relearning a concurrent object discrimination task which they had learnt and relearnt prior to surgery, indicating that they had a dense retrograde amnesia. They also had difficulty learning new visual object discriminations but were only mildly impaired on spatial learning. When tested on new learning of concurrent discriminations 8 to 10 weeks after surgery, three operated monkeys were unable to reach criterion in 400 trials while the remaining two operated monkeys performed within the normal range. The operated monkeys were subsequently shown to be impaired on acquisition of shape discriminations using black objects. These anterograde effects suggest that the impairment runs mainly in the domain
¨
of visual analysis. The monkeys also exhibited many of the features of the Kluver–Bucy syndrome. Histological analysis indicated that in addition to cutting some of the subcortical temporal lobe efferent pathways, the surgical procedures had cut the cholinergic afferents to the temporal neocortex, entorhinal cortex, and hippocampus. In a second experiment we found that treatment with the cholinergic agonist pilocarpine, which is effective in monkeys with specific cholinergic lesions, was unable to remediate the lesion-induced impairments. This suggests that transection of the non-cholinergic afferents, or the temporal lobe subcortical efferents, contributed to the behavioural syndrome and the learning and retention deficits seen in these monkeys. 2001 Elsevier Science B.V. All rights reserved.
Theme: Neural basis of behavior
Topic: Learning and memory systems and functions – animals
Keywords: Temporal stem; Amygdala; Fornix; Discrimination learning; Monkey
1. Introduction nisms originating in other brain areas. Some of the efferent
projections carrying information processed in the ‘ventral In order that behavioural choices can be made in stream’ of the visual association cortex leave the temporal response to visual perceptions it is essential that infor- lobe via the temporal stem [43,59], and the fornix [45]. mation processed in the visual association areas of the Subcortical nuclei send afferent projections through the temporal lobe should escape to other brain areas and that temporal stem, amygdala and fornix into the temporal visual processing in the temporal lobes should be under the lobes [55,61] and these inputs may influence the way in influence of attentional, motivational and hedonic mecha- which visual information is processed.
A wide variety of lesions within the ‘ventral stream’, including transections of the temporal stem, amygdala and fornix, produce impairment on tasks which require the
*Corresponding author. Innes Building, School of Veterinary Medicine,
perception, identification, and recognition of objects.
Madingley Road, Cambridge CB3 0ES, UK. Tel.:144-1223-339-015;
Macaques with transection of the anterior temporal stem
fax:144-1223-339-014.
E-mail address: [email protected] (H.F. Baker). are impaired on retention and new learning of pattern
discriminations [28,63,65] and on performance of ‘recogni- stream from the subcortical afferents which provide the tion’ tasks [11]. Macaques with suction ablations of the modulatory control of visual processing.
amygdala are severely impaired at learning certain types of These subcortical afferent systems include the rising reward-association tasks [17], although macaques with cholinergic projections of the basal forebrain which project axon-sparing, excitotoxic lesions of the amygdala may be most heavily into the temporal and frontal lobes. Choliner-less affected [36]. This suggests that fibres passing through gic cells in the basal nucleus of Meynert (NBM) project, the amygdala may be crucial for certain types of learning. via the temporal stem, to (and through) the amygdala, and Fornix transection causes a severe impairment on tasks to the inferotemporal and perirhinal cortex [55]. Choliner-requiring memory for real scenes [14] or items in fixed gic cells in the vertical limb of the diagonal band (VDB) spatial arrays [15] and a mild impairment on performance project to the main body of the hippocampus and the of ‘recognition’ tasks [13]. None of these lesions, however, entorhinal cortex via the fornix [3] and the supracallosal produce impairments which appear to match the sort of stria of Lancisi (personal observation) while some catastrophic amnesia which can occur in humans with cholinergic projections reach the anterior hippocampus and substantial temporal lobe damage, e.g., [62]. adjacent entorhinal cortex via the temporal stem [32].
Patients with amnesias of various aetiologies have There have been only limited attempts, using excitotox-difficulty in performing ‘recognition’ tasks [1,41,56] and in ins, to produce lesions of these projections in macaques learning visual discriminations presented concurrently [60] although more complete lesions have been produced [1,20,24,42]. Although the precise cognitive requirement by immunotoxic methods in marmoset monkeys. Immuno-for perImmuno-formance of these tasks is open to question, an toxic lesions of the cholinergic cells of the NBM1VDB understanding of the neuroanatomical substrate of per- produce a severe and persistent impairment on object formance of these tasks is pertinent to understanding discrimination learning in marmosets [47,50]. Lesions amnesia. A critical area for ‘recognition’ performance is confined to the NBM produce smaller, transient effects on the perirhinal cortex [9,37] and impairments on slightly visual discrimination learning and retention [12,47,50] modified versions of concurrent discrimination learning while lesions of the VDB produce large, persistent impair-have also been found following ablations confined to ments confined to certain types of conditional learning perirhinal cortex [6–8]. The inferotemporal cortex has also [50]. That these lesions have these cognitive effects been implicated in performance of ‘recognition’ tasks [39] because they deprive the temporal lobe of modulatory and concurrent discrimination learning [29]; see also [10] support is demonstrated by the impairments which follow for a retrospective analysis of work done in one laboratory. crossed unilateral lesions of the NBM / VDB and in-This suggests that, notwithstanding the evidence in favour ferotemporal cortex and hippocampus [5]. Since the trans-of the role trans-of the medial temporal lobe structures in ections of the anterior temporal stem, amygdala and fornix memory, some temporal neocortex (comprising at least performed in macaques by Gaffan et al. [19] destroy the inferotemporal and perirhinal cortex) also makes a substan- cholinergic afferents to the temporal lobe from the NBM
tial contribution to memory functions. and the VDB, respectively, it seemed appropriate to
Patients with large medial temporal lobe lesions (e.g., explore the effects of this lesion in marmosets as well as [31,54]) exhibit a much denser amnesia than do patients macaques. Learning impairments which were the conse-with fornix transection [16] or damage restricted largely to quence solely of the loss of these afferents might be the hippocampus [46]. Monkeys with large temporal lobe ameliorated by cholinergic agonists since this treatment lesions (e.g., [38–40,64]) also exhibit denser impairments has been found to be effective in monkeys with fornix than do those with smaller medial temporal lobe lesions transections [52] or NBM and / or VDB lesions [49–51]. [66] or fornix transection [13]. Horel [25] appreciated the A further behavioural feature worthy of comparison importance of the temporal neocortex in memory forma- between macaques and marmosets is the occurrence of the
¨
tion and argued that much experimental and clinical data Kluver–Bucy syndrome. Bilateral temporal lobectomy in ¨
apparently implicating the medial temporal lobe structures, macaques produces the Kluver–Bucy syndrome which especially the hippocampus, in memory formation could be comprises ‘psychic blindness’, and inappropriate emotional interpreted as demonstrating an involvement of the tempo- and appetitive responses to visual stimuli [33]. Many of
¨
ral stem. Gaffan et al. [19] have recently demonstrated a the signs of the Kluver–Bucy syndrome have been seen in very dense amnesic syndrome in macaques with combined macaques with transections of the temporal stem [28], or transection of the anterior temporal stem, amygdala and following amygdalectomy [22,27,30] or inferotemporal fornix. Macaques with this combination of lesions were cortical ablation [2,27,30]. Horel showed that signs of the
¨
more severely impaired on the object-in-place task and the Kluver–Bucy syndrome could also be produced by pre-delayed matching-to-sample task than were macaques with venting visual input into the temporal lobe by making only some of these lesions. Macaques with all three lesions crossed unilateral lesions of the striate cortex and in-were also severely impaired on concurrent discrimination ferotemporal cortex together with a transection of the learning. Gaffan et al. [19] argue that this severe impair- posterior corpus callosum [26]. We have recently observed
¨
impair-ments in marmosets with ablations confined to the in- matched for learning ability on the basis of performance on ferotemporal cortex [53]. Marmosets with lesions of the these tasks. Monkeys from the two sources named above NBM1VDB or fornix transection alone show learning were distributed between the two new groups. Monkeys in
¨
impairments but not the Kluver–Bucy syndrome Group C2 (n54) were unoperated and monkeys in Group ¨
[47,48,50,52]. The occurrence of the Kluver–Bucy L2 (n54) had the same surgical procedure as monkeys in syndrome in marmosets with transection of the anterior Group L1.
temporal stem, amygdala and fornix would therefore be
unlikely to be just a consequence of impaired visual 2.2. Surgery discrimination learning ability.
Some authors have considered the symptoms of the For surgery, each monkey was premedicated with 0.05 ¨
Kluver–Bucy syndrome in macaques to be the behavioural ml ketamine (100 mg / ml, i.m., Vetalar, Pharmacia, Lux-manifestation of visual agnosia, i.e., a gross impairment of embourg) and anaesthetised with 18 mg / kg alphaxolone– visual analysis [21,33]. Although macaques with small alphadolone (i.m., Saffan, Schering-Plough, Welwyn
Gar-¨
inferotemporal lesions do not exhibit florid Kluver–Bucy den City, UK). Dexamethasone (2 mg / kg, i.m., Merck, signs, it has been argued that their learning impairment Sharpe and Dohme, Harlow, UK) was given just before also arises from some sort of difficulty with visual analysis surgery, to limit cerebral oedema. Each monkey was rather than from a memory impairment per se [18]. It is placed in a stereotaxic frame and, following incision of the therefore pertinent to ask whether the behavioural dis- skin and retraction of the temporal muscles, a large cranial turbance and / or the learning impairment seen after trans- flap was removed to allow a clear view of the dorsal and ection of the anterior temporal stem, amygdala, and fornix lateral surfaces of the brain. A large stellate incision was could also be attributed to difficulties in visual analysis. made in the dura mater over the surface of one hemisphere and the dural flaps retracted. A small hole was made by suction through the corpus callosum between the
hemi-2. Materials and methods spheres at co-ordinates AP 17.0 to 18.0 mm [57]. The
bifurcation of the fornix was identified and each arm of the
2.1. Animals fornix was elevated and transected, either by using a
hooked, 19-gauge hypodermic needle (by RMR), or by
2.1.1. Experiment 1 direct suction ablation (by DG).
Ten laboratory-born common marmosets (Callithrix Immediately after the fornix transection had been made,
jacchus), six male and four female, were used in Experi- the temporal stem and amygdala was transected. A suction ment 1. They were each aged about 12 months and incision was made in the left temporal lobe about 2 mm weighed 300–350 g at the beginning of the experiment. below the lateral sulcus and angled down at about 458. Before surgery, the monkeys were trained on a series of This steep approach was taken so that auditory cortex, discrimination learning tasks (see below) and were as- rather then visual cortex, was compromised by the surgery. signed to one of two groups matched for learning ability. The incision was continued until the floor of the cranium Group C1 (n55) were unoperated controls and Group L1 was encountered and the incision was extended rostrally to (n55) received bilateral fornix transection and a bilateral within 2 mm of the temporal pole and caudally to the temporal lobe incision which transected the temporal stem beginning of the lateral ventricle adjacent to the anterior and the amygdala. At the end of Experiment 1, the extremity of the hippocampus. This incision was intended monkeys in Group L1 were perfused for histological brain to traverse the white matter of the superior temporal gyrus,
analysis. leaving the insula and temporal neocortex intact, and to
transect the temporal stem and amygdala. The lateral
2.1.2. Experiment 2 sulcus was left intact because this contains the middle
In Experiment 2, five of the unoperated monkeys from cerebral artery, damage to which could have produced a Group C1 of Experiment 1 together with five other large cerebral infarction. The dura mater were closed using marmosets which were unoperated control monkeys from 6 / 0 vicryl sutures and the procedure was repeated in the another study [50] and which had a comparable degree of right hemisphere. The cranial flap was replaced, the cognitive testing, were used to assess the effect of a temporal muscles reattached to the skull with cholinergic receptor agonist (pilocarpine) on the lesion- cyanoacrylate glue (Vetbond, Animal Care Products, Ger-induced cognitive impairment. One monkey was sub- many), and the skin sewn with 3 / 0 vicryl sutures. sequently removed from the study because histological
analysis revealed that its lesion failed to comply with the 2.3. Cognitive testing intended removal and another, unoperated monkey could
maintain motivation, the monkeys were fed their normal i.p.), the monkeys were deeply anaesthetised with 1.0 ml daily diet of bread, fruit and monkey pellets after testing sodium pentobarbitone (200 mg / ml, i.p.) and perfused each day. Three types of task were used: coloured object transcardially with 250 ml ice-cold phosphate-buffered (Object) discrimination (either as single discriminations or saline (PBS), pH 7.4, followed by 300 ml ice-cold 4% as 10 concurrent discriminations), black object (Shape) paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The discrimination, and spatial discrimination. brains were removed and postfixed for 2 h in ice-cold 4% For Object discrimination, two small plastic coloured paraformaldehyde in phosphate buffer, after which they junk objects were presented over two food-wells in the were inspected visually. Diagrammatic drawings were WGTA and the monkey had to learn to displace the made of the cortical damage in monkeys in Groups L1 and appropriate object to retrieve a small food reward (usually L2 and the brains of monkeys in Group L2 were also a piece of marshmallow). The left–right position of the photographed. The brains were then cut coronally into two objects varied pseudorandomly but the reward was blocks that were transferred to 30% sucrose–PBS at 48C always found under the same object. For Shape discrimina- for 2 to 3 days. The blocks were transferred to fresh 30% tion, two small junk objects painted black all over were sucrose–PBS after 24 h. Using a freezing stage microtome, used as stimuli. These two tasks differ in the type of sections were cut throughout the brain. Sections (40mm) perceptual analysis needed for task solution. While were stained for acetylcholinesterase (AChE) activity coloured objects can be distinguished by overall appear- [34,51] or with Cresyl Violet.
ance or by identifying differences in individual features (e.g., differently coloured parts), the black objects can only
be distinguished by perceiving differences in the shape of 3. Results
the whole object.
For spatial discrimination tasks, a four-hole test board 3.1. Postoperative behaviour was used in which there were two food-wells 4.5 cm apart
(centre to centre) on the left side of the test tray and two 3.1.1. Experiment 1
food-wells 4.5 cm apart on the right side of the test tray. Following surgery, the monkeys were transferred to The distance between the inner food-wells was 10 cm. The large incubators maintained at |288C. All monkeys were left side pair of food-wells, or the right side pair of given 0.2 ml paracetamol syrup if judged necessary. There food-wells, was covered with two identical stimuli accord- was some fitting in three monkeys, which was controlled ing to a pseudorandom schedule (and the other two food- by one or two doses of benzodiazepine (0.1 ml of 2 wells were open and empty). For spatial learning, reward mg / ml, i.m.). The monkeys were hand-fed and -watered as was to be found under the left stimulus of the pair on every required and their physiological recovery over the next 2 to trial, i.e., irrespective of the left / right position of the pair 3 days was uneventful. Their capacity for self-care was, of stimuli. This task could not be solved by the monkey however, compromised for a further 3 to 4 days by a maintaining a particular bodily position within the WGTA. disinclination to drink and, to some extent, eat without For spatial reversal, the reward was now to be found under assistance. Although they had good appetites, they ignored the right stimulus of the pair irrespective of the left / right the waterspout and most of the food, only eating food position of the pair of stimuli on each trial. which they encountered accidentally. Some monkeys had For each task, trials were presented until the monkey to be taught to use the waterspout by smearing the spout had reached a predetermined criterion (e.g., 27 correct with sugar syrup. Sometimes, when offered a piece of responses in 30 consecutive trials, 27 / 30). If the criterion banana, they would not distinguish between the banana was not reached within a predetermined number of trials and the hand of the person holding it, tending to nibble (e.g., 300) the monkey was deemed to have failed the task. both. They spent much time rooting about in the fleece that In all these tasks the monkey learns to associate one of the lined the bottom of the incubator or cage. The monkeys two stimuli (or positions in the case of spatial discrimina- were uncharacteristically tame, even after making allow-tion) with reward (i.e., one object or position acquires ance for their physiological recovery from surgery (see secondary reinforcing properties). In concurrent coloured Fig. 1). When left alone they lacked initiative and showed object discrimination 10 pairs of coloured objects were little interest in their surroundings. On occasions this presented to the monkey, one pair at a time over the amounted to ‘time out’, i.e., blank staring (with or without food-wells and in the same order (pair 1, pair 2 . . . pair 10; a drifting head movement). Sometimes they orientated to a pair 1, pair 2 . . . etc) for each monkey until the monkey non-event. This gave the appearance of hallucinatory reached criterion or performed a set number of trials. behaviour but obviously this cannot be proved. Thus, the monkeys exhibited the ‘psychic blindness’, ‘paradoxical
2.4. Histology tameness’, and ‘paresis of attention’ which form part of the
¨
surgery) the four lesioned monkeys were assessed with a checklist twice each day (see Table 1).
3.2. Cognitive testing
3.2.1. Experiment 1
For clarity, the design and results of each stage of Experiment 1 are described together. The mean learning scores (number of trials to criterion6S.E.M.) for each group on each task are shown in Table 2. Data were analysed for within- and between-group effects by analysis of variance (ANOVA). Groups of tasks of the same type or related tasks (e.g., postoperative retention) were analysed in a repeated measures general linear model design, followed by post hoc comparisons between groups using
t-tests. Where appropriate, the degrees of freedom were
reduced to take account of non-homogeneity of variances.
3.2.1.1. Preoperative testing. Before surgery, monkeys were tested on a number of tasks. The first was a simple coloured object discrimination (Object 1) presented until the monkeys had reached a criterion of 90 correct re-sponses in 100 consecutive trials (90 / 100). This task provided the necessary training in the basic requirements of task performance in the WGTA. This was followed by
¨
Fig. 1. Photograph of marmoset with Kluver–Bucy syndrome taken 6
two further simple coloured object discriminations, Object
weeks after bilateral transections of the temporal stem, amygdala and
2 to 27 / 30 and Object 3 to 9 / 10. This provided further
fornix. The marmoset is gently nibbling a gloved finger at an open cage
practice and prepared the monkeys for acquisition of
door. (The aggressive bite of a normal marmoset is extremely painful,
and most marmosets would attempt to escape through an open cage door.) different discriminations to a low criterion during
concur-rent testing. The monkeys were then tested on a concurconcur-rent By about 6 days after surgery most of the monkeys were coloured object discrimination task (Conc 1) in which 10 capable of self-care and were returned to their home cages. pairs of coloured objects were presented, one pair on each Testing was resumed 7 to 10 days after surgery. Per- trial in the same order, until an overall criterion of 90 / 100 formance in the WGTA was not compromised by the consecutive trials was reached (see Fig. 2). This task was
behaviour described above. intended to place a burden on memory for objects. The
monkeys were then rested for about 30 days after which they were tested on retention of this concurrent
discrimina-3.1.2. Experiment 2 tion (Ret Conc 1) to 90 / 100 (see Fig. 2). This allowed us
Following surgery for Experiment 2, monkeys showed to assess whether this species was capable of retaining a the same behavioural syndrome as described above. A concurrent discrimination over a period of time and
¨
more formal assessment of the Kluver–Bucy syndrome ensured that these discriminations were well learnt. was made in these monkeys, in addition to the postopera- Repeated-measures ANOVA on the preoperative tasks tive welfare charts kept for all monkeys. During the time showed no group effect and no group3task interaction. that they were in incubators (at least one week after The two groups were, therefore, well matched for learning
Table 1 ¨
Signs of Kluver–Bucy Syndrome in monkeys in group L2 (n54)
Needing to be hand-fed and -watered beyond normal post-op care 4 / 4 monkeys
Then only eating food that was accidentally encountered 4 / 4 monkeys
Then rooting for food by smell, and plunging the face into the fleece 3 / 4 monkeys
Unaggressively nibbling observers’ fingers 4 / 4 monkeys
Nibbling faeces 3 / 4 monkeys
Being extra tame relative to postoperative condition 4 / 4 monkeys
Lacking behavioural initiative 4 / 4 monkeys
Tolerating being touched on the nose, ears and body 4 / 4 monkeys
Table 2
Mean learning scores (6S.E.M.) for tasks used in Experiment 1
Tasks Control group Lesion group Post hoc tests ANOVA
Preoperative:
ability before surgery. Following a 30 day rest period, Object 1 to 27 / 30. They were deemed to have failed the there was no difference between the groups on retention of task if criterion was not reached within 200 trials. They the concurrent discrimination. All monkeys showed a were then tested on retention of the concurrent discrimina-substantial reduction in learning score on retention testing tion (2nd Ret Conc 1) to 90 / 100 (see Fig. 2). In this case indicating that these discriminations were held within long- they were deemed to have failed the task if they did not
term memory reach criterion in 500 trials. Repeated-measures ANOVA
on these tasks showed a highly significant group effect 3.2.1.2. Postoperative testing. Retention of tasks learned [F1,85492.18, P,0.001], task effect [F1,8577.95, P,
preoperatively: Behavioural testing resumed 7–10 days 0.001], and group3task interaction [F1,8593.87, P, after surgery. The monkeys were tested first on retention of 0.001].
Post hoc comparisons showed that the lesioned group was impaired relative to the control group on both re-tentions. All lesioned monkeys failed to reach criterion in 200 trials of retention of Object 1. Four of five lesioned monkeys failed to reach criterion on the concurrent re-tention task in 500 trials (see Fig. 2). Since the original learning scores for concurrent discrimination task had been below 500 trials for four of these five monkeys, this failure represents an impairment in relearning as well as in retention.
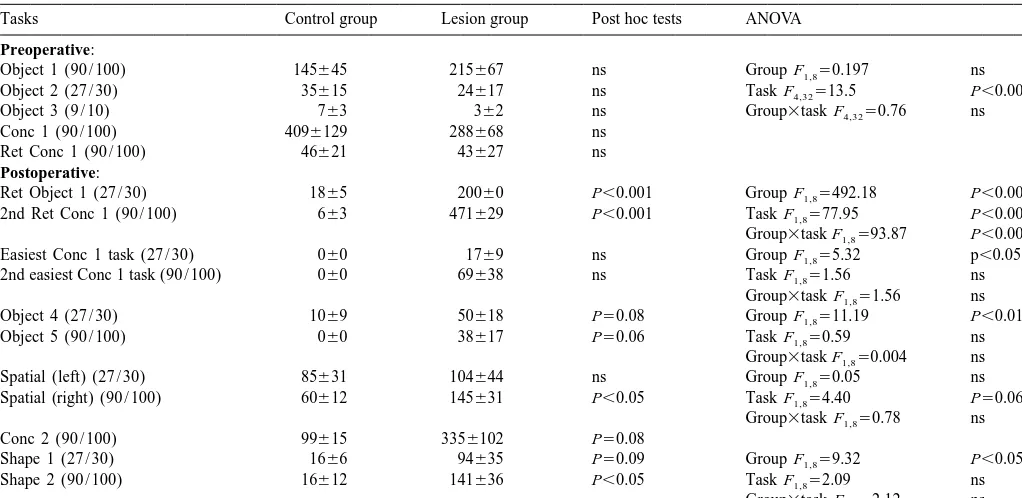
The learning score to 9 / 10 for retention of each component discrimination of the concurrent discrimination task was also calculated (Fig. 3A). Where a monkey did not reach a criterion of 9 / 10 on a component discrimina-tion, the total number of trials on that task for that monkey was used. Repeated measures ANOVA across the
com-Fig. 2. The effect of the lesion on learning and retention of concurrent ponent discriminations revealed that there was a highly
discriminations before and after surgery (Expt. 1). Histogram shows mean significant group effect [F 523.04, P,0.001], a signifi-1,8
trials to criterion (1S.E.M.). Conc 1 is first set of 10 concurrent cant component effect [F 52.48, P,0.05] and a 9,72
discriminations learnt preoperatively, retained preoperatively and retained
group3component interaction [F9,7252.01, P,0.05]. Post
again postoperatively. Conc 2 is a new set of 10 concurrent
discrimina-hoc examination revealed that the lesioned group was
tions learnt postoperatively. (See also Table 2). ***P,0.001, comparing
0.001]. Post hoc analysis revealed a significant group difference at all levels of difficulty except the easiest.
3.2.1.3. Further testing of tasks relearnt postoper
-atively. Since the lesioned monkeys had failed to reach
criterion on both postoperative retention tasks and on all but one of the components of the retention of the concur-rent discrimination task, it was important to consider the extent to which these monkeys were capable of maintain-ing sustained performance in the WGTA. All monkeys were therefore retested to 27 / 30 on retention of the component they had each found easiest to relearn in the concurrent discrimination task. All monkeys were then retested to 90 / 100 on the component which they had each found second easiest to relearn in the concurrent discrimi-nation task. Repeated-measures ANOVA on the relearning scores for these two tasks showed a significant group difference over both tasks [F1,855.32, P,0.05]. Post hoc comparisons did not reach significance on either task; all control monkeys scored zero on both these tasks whereas, for the lesioned group, scores ranged from 0–50 on the first task and 0–200(fail) on the second task. Thus some lesioned monkeys who had been able to master a task to a criterion of 9 / 10 had further difficulty carrying perform-ance through to a criterion of 27 / 30 or 90 / 100. This could be interpreted as a mnemonic impairment which is evident after the visual analysis of objects has been sufficient to produce discrimination performance to a low criterion.
Acquisition of visual discriminations: After retention
testing, the marmosets were tested on acquisition of a new simple coloured object discrimination (Object 4) to a criterion of 27 / 30 and a second new simple coloured
Fig. 3. (A) Postoperative retention of a concurrent discrimination set first
object discrimination (Object 5) to a criterion of 90 / 100.
learnt prior to surgery (2nd Ret Conc 1 in Table 2). (B) Postoperative
learning of a new set of 10 concurrent discriminations (Conc 2 in Table Repeated measures ANOVA revealed a significant group
2). Histogram shows mean trials to criterion (1S.E.M.) for each effect [F 511.19, P,0.01], but no task effect and no 1,8
component discrimination which are ordered according to increased task
group3task interaction. Post hoc comparisons approached
difficulty for the control group.
significance for both tasks. Scores for these two tasks were zero or near zero for control monkeys. Thus lesioned monkeys were impaired on acquisition of visual discrimi-nations even where the tasks were extremely easy for discriminations of this concurrent task. Retention scores control monkeys.
for the control group were near zero for all tasks so it was Spatial discrimination tasks: In a further attempt to
not possible to correlate impairment in lesioned monkeys dissociate visual discrimination impairments from other with difficulty for unoperated monkeys across component difficulties of sustained performance, the monkeys were
discriminations. tested on simple spatial learning (to 27 / 30) followed by
The scores for each component discrimination of the spatial reversal (to 90 / 100).
retention of the concurrent task were also ordered accord- Repeated measures ANOVA revealed no group effect, a ing to difficulty for each monkey and then averaged at small task effect [F1,854.40, P50.069] and no group3task each level of difficulty. This allowed, for example, com- interaction. Post hoc comparisons, however, revealed a parison of performance between the groups on the com- small difference between groups on the spatial reversal ponent discrimination which each monkey found easiest, task (P,0.05). Thus overall there was only minimal or hardest, etc. Repeated measures ANOVA on these impairment on these spatial tasks, which contrasts with the scores revealed a significant group effect [F1,8523.04, major impairment seen on visual discrimination tasks.
P,0.001], a significant difficulty effect (necessarily) and a Further acquisition of visual discriminations: Monkeys
further 10 component discriminations, using wholly novel and in some cases may only be discriminable on the basis objects, to 90 / 100. Three lesioned monkeys failed this task of overall shape. Repeated ANOVA across these two tasks in 500 trials but the other two monkeys learnt the task with revealed a significant group effect [F91,8059.32, P,0.05], scores within the range of the control monkeys (see Fig. but no task effect and no group3task interaction. Post hoc 2). The difference between groups approached significance analysis indicated that the difference between the two
(P50.08). groups approached significance on the first task, which was
Repeated measures ANOVA of the scores to 9 / 10 for tested to a lower criterion (27 / 30) and attained signifi-each component discrimination of this concurrent task cance on the second task, which was tested to a higher revealed a significant group effect [F1,856.05, P,0.05] criterion (90 / 100). The impairment on black object dis-and a significant component effect [F9,7253.85, P,0.001)] crimination especially when tested to a higher criterion but no group3component interaction. Post hoc compari- suggests that defects in visual analysis and in mnemonic sons for each individual component revealed no com- processing are involved in producing the learning impair-ponents for which the two groups differed significantly ment.
although the score for the lesioned group was above that of
the control group for all components except one, where the 3.2.2. Experiment 2 scores were nearly equal (see Fig. 3B). There was a
significant correlation between component difficulties ac- 3.2.2.1. Preoperative testing. Monkeys in Experiment 2 ross the two groups (r50.7962, P,0.01). In other words, were tested on retention of the first object discrimination both the lesioned and control monkeys found the same that they had ever learnt (2nd Ret Object 1 for monkeys component discriminations harder than some other com- from both sources), followed by acquisition of a new ponent discriminations. Since learning difficulty for control object discrimination (Object 6) and two new shape monkeys probably reflects differences in discriminability discriminations (Shape 3 and Shape 4). Repeated measures between the two objects used in each component, this ANOVA showed no group difference over these tasks correlation indicates that the learning impairment shown indicating that the groups were well matched for learning by lesioned monkeys was also influenced by the same ability prior to surgery. (Table 3)
differences in discriminability between objects.
The scores for each component discrimination were also 3.2.2.2. Postoperative testing. Monkeys were retested on ordered according to difficulty for each monkey and then retention of the first object discrimination which they had averaged for each level of difficulty. Repeated measures ever learnt (3rd Ret Object 1), followed by acquisition of a ANOVA on these scores revealed a significant group effect new object discrimination (Object 7), retention of the [F1,856.05, P,0.05], a significant difficulty effect (neces- shape discrimination first learnt prior to surgery (Ret sarily) and a significant group3difficulty interaction Shape 3), and acquisition of a new shape discrimination [F9,7255.06, P,0.001]. Post hoc analysis revealed a (Shape 5). Repeated measures ANOVA across these tasks significant group difference only on the three tasks that revealed a significant group difference (F1,6530.25, P, each monkey found most difficult. This suggests that the 0.01) and a significant group3task interaction (F3,185 impairment, which had ameliorated considerably from the 10.42, P,0.001). Post hoc t-tests showed a significant time of the postoperative retention of the other concurrent group difference on the first (object) retention task (t5 discrimination task, was now confined to learning about 5.336, P,0.01) and on acquisition of the shape discrimi-objects which the monkeys found most difficult to dis- nation (t55.189, P,0.01).
mea-Table 3
Mean learning scores (6S.E.M.) for tasks used in Experiment 2
Tasks Control group Lesion group Post hoc tests ANOVA
Preoperative:
sures ANOVA showed a significant group effect (F1,65 cortex. In the other three monkeys in Group L1, the 31.713, P,0.001) but no drug effect and no group3drug perirhinal cortex, like the inferotemporal cortex was lateral interaction. Thus although these monkeys showed a learn- to the incision. In two monkeys in Group L1, the amygdala ing impairment as a result of surgery, this impairment was was cut neatly so that half was medial to the cut while the not ameliorated by treatment with a cholinergic agonist. other half was lateral to the cut. In the other three monkeys in Group L1, the amygdala lesion was larger. In one
3.3. Histology monkey in Group L1, there was a very small amount of
bilateral damage in the hippocampus and in another
3.3.1. Experiments 1 and 2 monkey in Group L1 there was substantial unilateral
On gross inspection of the brains of monkeys in Group damage to the hippocampus. The tail of the caudate L1 and Group L2, very little damage was visible on the nucleus was damaged unilaterally in two monkeys but was cortical surface (see Figs. 4 and 5). The incision on the intact bilaterally in the other three monkeys in Group L1. lateral surface was confined to cortex above the superior The tail of caudate nucleus was damaged bilaterally in all temporal dimple and was, therefore, in auditory rather than monkeys in Group L2. In Group L1, the fornix was visual association cortex. In one monkey in Group L1 transected bilaterally in three monkeys; a thread of tissue, (shown on the far right in Fig. 5A), there was some which appeared to consist only of ependymal lining, damage to the superior temporal cortex in the left hemi- bridged the lesion site in one monkey and in another sphere only. One monkey in Group L2 (also shown on the monkey, a very small amount of fornical tissue remained far right in Fig. 5B) sustained a large infarct in the left intact on one side. In Group L2, the fornix was transected temporal lobe although the incision did not reach the in all monkeys. The lateral geniculate nuclei were intact medial ventral surface of the cortex, such that the temporal bilaterally in all monkeys.
stem was not completely sectioned. This monkey also had
a large medial temporal lobe lesion in the right hemisphere 3.3.3. Acetylcholinesterase histochemistry (Figs. 8 and
and was excluded from experimental analysis. 9)
In all monkeys in Group L1 and Group L2, there was
3.3.2. Cresyl Violet staining substantial loss of AChE staining throughout the medial
In the temporal lobes in all monkeys, an incision could and inferior temporal lobe. AChE staining was severely be seen parallel to, but starting about 2 mm below, the reduced throughout the hippocampus and entorhinal cortex lateral sulcus in the anterior half of the temporal lobe. bilaterally except in the one monkey in Group L1 in which (Examples of this incision are illustrated in AChE stained a small amount of fornical tissue remained, where AChE sections in Figs. 6 and 7). This incision descended staining was not so greatly reduced in the hippocampus in diagonally from the lateral cortical surface to the medial that hemisphere. Some AChE staining remained in these ventral cortical surface. The incision extended from at least areas probably as a consequence of cholinergic inputs to 3 mm behind the temporal pole (i.e., at the level of the the posterior areas via the supracallosal stria, which were amygdala) posteriorly for|5 mm (i.e., to the anterior part only partially transected by the incision in the corpus of the hippocampus) and traversed the temporal stem in all callosum.
Fig. 4. Photographs of lateral, frontal, and ventral views of the brain from a lesioned monkey from Expt 2. There was no visible damage on the dorsal surface. On the lateral surfaces the arrows indicate a slit in the dorsal temporal cortex between the lateral sulcus and the superior temporal dimple. Viewed from the front and below, arrows indicate the extension of the slit. The monkey illustrated is the second from the left in Fig. 5B.
cortex. There was a loss of AChE staining in that part of and had presumably cut the temporal lobe efferents to the the amygdala which was lateral to the surgical incision. frontal lobe and dorsomedial thalamus via the temporal There was also a loss of AChE staining in the most stem / amygdala (see [23]) and to the mammillary bodies anterior part of the hippocampus. This part of the hip- and anterior thalamic nuclei via the fornix. This lesion pocampus receives its cholinergic input from the NBM via would, however, have left intact the visual inputs from the a ventral pathway and staining in this area is usually occipital lobe to the temporal lobe and their reciprocal spared by fornix transection. The loss of this staining is connections. The lesions in the two experiments had been therefore probably a consequence of the temporal stem intended to be the same although, in practice, the temporal
lesion. lobe incision in the second experiment had extended very
slightly further back than in the first experiment such that 3.3.4. Summary of histological findings the tail of the caudate nucleus was damaged bilaterally in Transection of the temporal stem and amygdala had cut the monkeys in Experiment 2 but not in those in Experi-the cholinergic input to that part of Experi-the amygdala and ment 1.
temporal cortex which was lateral to the incision and to a substantial proportion of the temporal cortex caudal to the
incision. The fornix transection had cut the greater part of 4. Discussion
the cholinergic input to the hippocampus and entorhinal
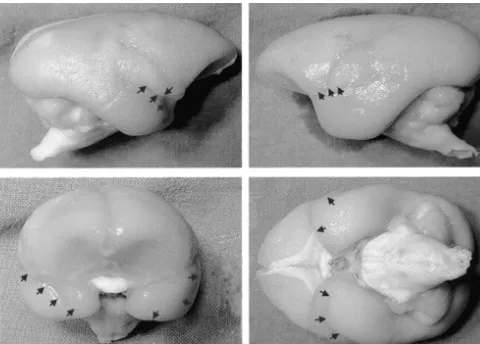
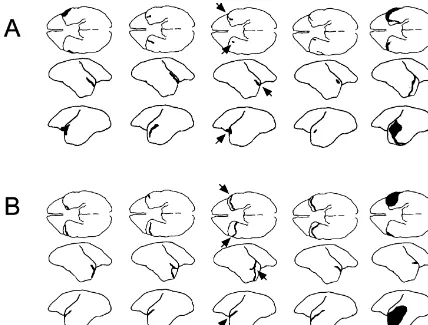
Fig. 5. Diagrams illustrating brain surface in monkeys with bilateral transections of the temporal stem, amygdala and fornix. (A) Animals from Expt 1. (B) Animals from Expt 2. For one monkey in each experiment the arrows indicate where the damage may be seen. Only one monkey in Expt 1 (shown on the far right) had any substantial surface damage and this was only in the left hemisphere. One monkey intended for Expt 2, and shown on the far right, sustained a large infarct in the left hemisphere resulting in substantial cortical damage. This monkey also sustained other subcortical damage in the right hemisphere and was excluded from Expt 2.
lesioned monkeys was able to relearn the first object performance in the unoperated monkeys. The impairment discrimination and four of the five lesioned monkeys were in the lesioned monkeys could, however, arise from either also unable to relearn the concurrent discriminations a failure in discrimination ability (apperceptive agnosia) or whereas the unoperated monkeys showed almost perfect a failure to associate objects with reward in long-term retention of these tasks. The lesioned monkeys were memory (a form of amnesia or associative agnosia). The significantly impaired on relearning nine of the 10 dis- lesioned monkeys’ eventual success on these tasks may criminations of the concurrent set indicating that the reflect the use of a slow habit-forming mechanism in the overall concurrent impairment was not a consequence of absence of a faster memory-forming mechanism.
failure to relearn only a subset of the discriminations. The spatial learning tasks assessed the possibility that Lesioned monkeys were then assessed on their ability to the lesioned monkeys had a general learning or perform-reach a more stringent criterion on two tasks for which ance deficit. A very mild impairment on spatial reversal they had successfully attained the same low criterion as the was found. This was comparable to the effect of fornix unoperated monkeys. This was done in an attempt to assess transection in this species [52], and suggests that transec-discrimination and learning ability in circumstances where tion of the anterior temporal stem and amygdala does not the unoperated monkeys showed perfect performance. add to this fornix-induced impairment and does not Lesioned monkeys were impaired in these two tasks and produce a general cognitive decrement.
Fig. 6. Sections through one hemisphere of a marmoset brain at stereotaxic levels AP 10.5 (anterior) to AP 7.5 (posterior). Top: normal marmoset brain immunostained with ME20.4IgG, an antibody which recognises the low-affinity p75 neurotrophin receptor protein which, in marmosets, is located on the cholinergic cells of the basal forebrain [35]. Thin arrows indicate the output pathways of the NBM. Thick arrows indicate that the output pathway of the VDB travels through the fornix and terminates in the hippocampus and entorhinal cortex. A5amygdala; H5hippocampus. Bottom: sections through lesioned marmoset brain, stained for AChE activity (a marker for cholinergic activity in target areas) showing transection of the temporal stem and amygdala, which interrupts the cholinergic projection to the temporal neocortex, and fornix transection, which interrupts the cholinergic projection into the hippocampus and entorhinal cortex.
pattern discriminations learnt preoperatively but were not them to cope with easy object discriminations, even though subsequently impaired at new concurrent learning of object these and the other lesioned monkeys were subsequently or pattern discriminations presented in the same way. shown to be impaired on more difficult shape discrimina-These results suggest that the rhinal cortex may play a tions. In comparable test situations, marmosets with lesions specific role in retention of preoperatively acquired knowl- of the cholinergic projections from the NBM to the edge. Two of the marmosets in the current experiment temporal (and frontal) cortex also show substantial re-conform to this pattern of impairment whereas the other covery of function over time [12,47,50].
three marmosets were as impaired on acquisition as they The lesioned marmosets in this study exhibited a ¨
were on retention of concurrent discriminations. This latter Kluver–Bucy syndrome comparable to that seen in mar-pattern of impairments resembles that seen after inferotem- mosets with bilateral inferotemporal cortex ablations [53]. poral ablations in marmosets [53] or macaques [18]. The syndrome ameliorated with time after either lesion so Comparison of the size and location of the transections and that all affected monkeys (except one with a temporal of the distribution of the loss of cortical AChE staining in cortical ablation) became capable of self-care after about a individual monkeys failed to suggest any anatomical basis week. Nonetheless, the behaviour of monkeys with either for the differences between the performance of these lesion remained socially inappropriate for the duration of monkeys. One of the monkeys which learnt the new the experiments. Marmosets with bilateral NBM1VDB concurrent set with comparative ease had shown the lowest lesions or crossed NBM1VDB and inferotemporal1
¨
Fig. 7. A further series of sections stained for AChE activity illustrating the lesion and the extent of the cholinergic depletion. There is no damage or loss of AChE activity in the frontal section (AP14.5). The lesion extends from AP 11.5 to AP 7.5. The fornix transection is evident between AP 9.5 and AP 5.5. Loss of AChE staining, indicted by the line drawn around the cortex, is evident in the temporal cortex lateral to the lesion and extends behind the lesion as far as AP20.5.
¨
Kluver–Bucy signs. Equally, it is unlikely that damage to information to forward areas. The destruction of the some specific medial temporal lobe structure (e.g., the ‘forward’ temporal lobe efferents in the current study may amygdala) is responsible for this behaviour since the produce a disconnection equivalent to that produced by inferotemporal ablations in our previous study did not inferotemporal or amygdala ablations and this
disconnec-¨
encroach on the amygdala. tion may underlie the Kluver–Bucy syndrome exhibited by
¨
One interpretation of these data is that the Kluver–Bucy these three lesions.
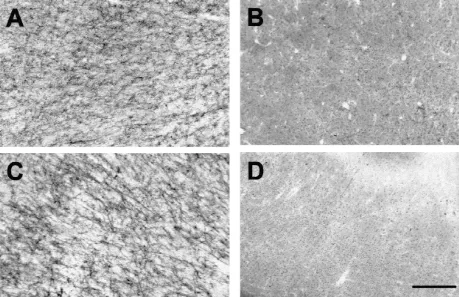
Fig. 8. Photomicrographs of sections stained for AChE, in a monkey with transections of the temporal stem, amygdala and the fornix. (A) Dorsolateral frontal cortex and (B) anterior temporal cortex, in the same section through the brain (at AP 11.5). (C) Parietal cortex and (D) posterior temporal cortex, in the same section through the brain (at AP 2.5). The transection of the temporal stem and amygdala has deprived most of the temporal cortex of its cholinergic innervation while leaving that in the frontal and parietal cortices intact. Scale bar50.25 mm.
entirely preclude a role for the cholinergic loss in the conjunction with anterior temporal stem and amygdala learning impairments seen in this study. lesions in macaques implies that the densest amnesia The marmosets studied in these experiments differ results from a combination of lesions to temporal neocorti-somewhat from the macaques with a comparable lesion cal and medial structures. In marmosets it is less easy to studied by Gaffan et al. [19]. The macaques did not exhibit determine whether a learning deficit results from an
¨
a ‘florid’ Kluver–Bucy syndrome, i.e., symptoms which amnesia or from an associative agnosia but, whichever were visually obvious and which temporarily interferred interpretation is preferred, the effect of this lesion on with self-care. This suggests that the lesion in the mar- acquisition and retention is severe. In marmosets, addition mosets effectively disabled the temporal lobes whereas the of damage to the medial temporal structures to temporal effect of the lesion in the macaques, though severe, was neocortical damage also results in additional cognitive more specific. The tasks used by Gaffan et al. [19] were dysfunction [5,53].
those used to assess ‘amnesia’ in monkeys, namely the In summary this study has shown that combined transec-object-in-place task, delayed matching-to-sample, and con- tions of the temporal stem, amygdala and fornix produce current object–reward association. The largest impairments substantial impairments on object and shape discrimination on these tasks have been seen, respectively, after lesions of learning. Data from other experiments indicate that these the perirhinal cortex / fornix [44], perirhinal cortex [9,37] effects are not seen after fornix transection alone and may and inferotemporal / perirhinal cortex [10] although milder not be seen after transection of the anterior temporal stem impairments on these tasks may be seen following lesions alone. These learning impairments are accompanied by
¨
Fig. 9. Photomicrographs of sections stained for AChE in a monkey with transections of the temporal stem, amygdala and fornix and in an unoperated monkey. CA1 region of the hippocampus in (A) an unoperated monkey, and (B) a lesioned monkey. Entorhinal cortex in (C) an unoperated monkey and (D) a lesioned monkey. The fornix transection has deprived these areas of their cholinergic input. Scale bar50.25 mm.
basal forebrain and contralateral ablations of the inferotemporal
may contribute to the visual discrimination learning
im-cortex and hippocampus in monkeys, Neuroscience 98 (2000) 243–
pairments that these monkeys exhibit. These results
com-251.
plement those of Gaffan et al. [19] who have already [6] M.J. Buckley, D. Gaffan, Impairment of visual object-discrimination shown substantial amnesic effects of this combination of learning after perirhinal cortex ablation, Behav. Neurosci. 111
(1997) 467–475.
lesions in macaques.
[7] M.J. Buckley, D. Gaffan, Learning and transfer of object-reward associations and the role of the perirhinal cortex, Behav. Neurosci. 112 (1998) 15–23.
[8] M.J. Buckley, D. Gaffan, Perirhinal cortex ablation impairs visual
References object identification, J. Neurosci. 18 (1998) 2268–2275.
[9] M.J. Buckley, D. Gaffan, E.A. Murray, Functional double dissocia-[1] J.P. Aggleton, R.M. Nicol, A.E. Huston, A.F. Fairbairn, The tion between two inferior temporal cortical areas: perirhinal cortex performance of amnesic subjects on tests of experimental amnesia in versus middle temporal gyrus, J. Neurophysiol. 77 (1997) 587–598. animals: delayed matching-to-sample and concurrent learning, [10] E.A. Buffalo, L. Stefanacci, L.R. Squire, S.M. Zola, Re-examination Neuropsychologia 26 (1988) 265–272. of the concurrent discrimination learning task: the importance of the
¨
[2] K. Akert, R.A. Gruesen, C.N. Woolsey, D.R. Meyer, Kluver–Bucy anterior inferotemporal cortex, area TE, Behav. Neurosci. 112 syndrome in monkeys with neocortical ablations of temporal lobe, (1998) 3–14.
Brain 83 (1961) 480–498. [11] R.A. Cirillo, J.A. Horel, P.J. George, Lesions of the anterior [3] J.R. Alonso, H.S. U, D.G. Amaral, Cholinergic innervation of the temporal stem and the performance of delayed match-to-sample and primate hippocampal formation: II. Effects of fimbria / fornix trans- visual discriminations in monkeys, Behav. Brain Res. 34 (1989) ection, J. Comp. Neurol. 375 (1996) 527–551. 55–69.
[4] H.F. Ridley, R.M. Baker, Use of the common marmoset (Callithrix [12] A. Fine, C. Hoyle, C.J. Maclean, T.L. LeVatte, H.F. Baker, R.M.
jacchus) in psychopharmacological research, in: M.H. Joseph, L.J. Ridley, Learning impairments following injection of a selective Waddington (Eds.), Working Methods in Neuropsychopharmacologi- cholinergic immunotoxin, ME20.4 IgG-saporin, into the basal cal Research, Manchester University Press, Manchester, 1986, pp. nucleus of Meynert in monkeys, Neuroscience 81 (1997) 331–343. 41–73. [13] D. Gaffan, Recognition impaired and association intact in the [5] H.C. Barefoot, H.F. Baker, R.M. Ridley, Synergistic effects of memory of monkeys after transection of the fornix, J. Comp.
[14] D. Gaffan, Amnesia for complex naturalistic scenes and for objects [37] M. Meunier, J. Bachevalier, M. Mishkin, E.A. Murray, Effects on following fornix transection in the rhesus monkey, Eur. J. Neurosci. visual recognition of combined and separate ablations of the 4 (1992) 381–388. entorhinal and perirhinal cortex in rhesus monkeys, J. Neurosci. 13 [15] D. Gaffan, Scene-specific memory for objects: a model of episodic (1993) 5418–5432.
impairment in monkeys with fornix transection, J. Cog. Neurosci. 6 [38] M. Mishkin, Memory severely impaired by combined but not by (1994) 305–320. separate removal of amygdala and hippocampus, Nature 273 (1978) [16] D. Gaffan, E.A. Gaffan, Amnesia in man following transection of 297–298.
the fornix. A review, Brain 114 (1991) 2611–2618. [39] M. Mishkin, A memory system in the monkey, Philos. Trans. R. [17] D. Gaffan, S. Harrison, Amygdalectomy and disconnection in visual Soc. Lond. B. Biol. Sci. 298 (1982) 85–95.
learning for auditory secondary reinforcement by monkeys, J. [40] E.A. Murray, M. Mishkin, Visual recognition in monkeys following Neurosci. 7 (1987) 2285–2292. rhinal cortical ablations combined with either amygdalectomy or [18] D. Gaffan, S. Harrison, E.A. Gaffan, Visual identification following hippocampectomy, J. Neurosci. 6 (1986) 1991–2003.
inferotemporal ablation in the monkey, Q. J. Exp. Psychol. 38B [41] M. Oscar-Berman, R.T. Bonner, Matching- and delayed matching-(1986) 5–30. to-sample performance as measures of visual processing, selective [19] D. Gaffan, A. Parker, A. Easton, Dense amnesia in the monkey after attention, and memory in aging and alcoholic individuals,
transection of fornix, amygdala and anterior temporal stem,
Neuro-psychologia 23 (1985) 639–651. psychologia (in press).
[42] M. Oscar-Berman, S.M. Zola-Morgan, Comparative neuropsycholo-[20] E.A. Gaffan, J.P. Aggleton, D. Gaffan, C. Shaw, Concurrent and
gy and Korsakoff’s syndrome. II. Two-choice visual discrimination sequential pattern learning by patients with Korsakoff amnesia,
learning, Neuropsychologia 18 (1980) 513–525. Cortex 26 (1990) 381–397.
[43] D.N. Pandya, H.G.J.M. Kuypers, Cortico-cortical connections in the [21] N. Geschwind, Disconnexion syndromes in animals and man, Brain
rhesus monkey, Brain Res. 13 (1969) 13–36. 88 (1965) 237–294.
[44] A. Parker, D. Gaffan, The effect of anterior thalamic and cingulate [22] G.V. Goddard, Functions of the amygdala, Psychol. Bull. 62 (1964)
cortex lesions on object-in-place memory in monkeys, Neuro-89–109.
psychologia 35 (1997) 1093–1102. [23] S. Goulet, F.Y. Dore, E.A. Murray, Aspiration lesions of the
[45] C.E. Poletti, G. Cresswell, Fornix system efferent projection in the amygdala disrupt the rhinal corticothalamic projection system in
squirrel monkey: an experimental degeneration study, J. Comp. rhesus monkeys, Exp. Brain Res. 119 (1998) 131–140.
Neurol. 175 (1977) 101–128. [24] K.L. Hood, B.R. Postle, S. Corkin, An evaluation of the concurrent
[46] N.L. Rempel-Clower, S.M. Zola, L.R. Squire, D.G. Amaral, Three discrimination task as a measure of habit learning: performance of
cases of enduring memory impairment after bilateral damage limited amnesic subjects, Neuropsychologia 37 (1999) 1375–1386.
to the hippocampal formation, J. Neurosci. 16 (1996) 5233–5255. [25] J.A. Horel, The neuroanatomy of amnesia; a critique of the
[47] R.M. Ridley, H.C. Barefoot, C.J. Maclean, P. Pugh, H.F. Baker, hippocampal memory hypothesis, Brain 101 (1978) 403–445.
¨
[26] J.A. Horel, E.G. Keating, Partial Kluver–Bucy syndrome produced Different effects on learning ability following injection of the by cortical disconnection, Brain Res. 16 (1969) 281–284. cholinergic immunotoxin ME20.4IgG-saporin into the diagonal band
¨
[27] J.A. Horel, E.G. Keating, L.J. Misantone, Partial Kluver–Bucy of Broca, basal nucleus of Meynert, or both in monkeys, Behav. syndrome produced by destroying temporal neocortex or amygdala, Neurosci. 113 (1999) 303–315.
Brain Res. 94 (1975) 347–359. [48] R.M. Ridley, S. Gribble, B. Clark, H.F. Baker, A. Fine, Restoration [28] J.A. Horel, L.J. Misantone, Visual discrimination impaired by of learning ability in fornix-transected monkeys after fetal basal cutting temporal lobe connections, Science 193 (1976) 336–338. forebrain but not fetal hippocampal tissue transplantation, Neuro-[29] E. Iwai, M. Mishkin, Further evidence on the locus of the visual science 48 (1992) 779–792.
area in the temporal lobe of the monkey, Exp. Neurol. 25 (1969) [49] R.M. Ridley, T.K. Murray, J.A. Johnson, H.F. Baker, Learning 585–594. impairment following lesion of the basal nucleus of Meynert in the [30] E. Iwai, M. Yukai, J. Watanabe, K. Hikosaka, H. Suyama, S. marmoset: modification by cholinergic drugs, Brain Res. 376 (1986)
Ichikawa, A role of amygdala in visual perception and cognition in 108–116.
macaque monkeys (Macaca fuscata and Macaca mulatta), Tohoku [50] R.M. Ridley, P. Pugh, C.J. Maclean, H.F. Baker, Severe learning J. Exp. Med. 161 (Suppl.) (1990) 95–120. impairment caused by combined immunotoxic lesion of the [31] N. Kapur, S. Barker, E.H. Burrows, D. Ellison, J. Brice, L.S. Illis, cholinergic projections to the cortex and hippocampus in monkeys,
K. Scholey, C. Colbourn, B. Wilson, M. Loates, Herpes simplex Brain Res. 836 (1999) 120–138.
encephalitis: long term magnetic resonance imaging and neuro- [51] R.M. Ridley, N.A. Samson, H.F. Baker, J.A. Johnson, Visuospatial psychological profile, J. Neurol. Neurosurg. Psychiatry 57 (1994) learning impairment following lesion of the cholinergic projection to
1334–1342. the hippocampus, Brain Res. 456 (1988) 71–87.
[32] C.A. Kitt, S.J. Mitchell, M.R. DeLong, B.H. Wainer, D.L. Price, [52] R.M. Ridley, H.D. Thornley, H.F. Baker, A. Fine, Cholinergic Fibre pathways of basal forebrain cholinergic neurons in monkeys, neural transplants into hippocampus restore learning ability in Brain Res. 406 (1987) 192–206. monkeys with fornix transections, Exp. Brain Res. 83 (1991) 533–
¨
[33] H. Kluver, P.C. Bucy, Preliminary analysis of functions of the 538.
temporal lobes in monkeys, Arch. Neurol. Psychiat. 42 (1939) [53] R.M. Ridley, K.A. Warner, C.J. Maclean, D. Gaffan, H.F. Baker, ¨
979–1000. Visual agnosia and Kluver–Bucy syndrome in marmosets (Callithrix [34] G.B. Koelle, The histochemical identification of acetylcholinesterase jacchus) following ablation of the inferotemporal cortex, with
in cholinergic, adrenergic and sensory neurons, J. Pharmacol. Exp. additional mnemonic effects of immunotoxic lesions of cholinergic Ther. 114 (1955) 167–184. projections to medial temporal areas, Submitted to Brain Research [35] C.J. Maclean, H.F. Baker, A. Fine, R.M. Ridley, The distribution of (2000).
p75 neurotrophin receptor-immunoreactive cells in the forebrain of [54] W.E.B. Scoville, B. Milner, Loss of recent memory after bilateral the common marmoset (Callithrix jacchus), Brain Res. Bull. 43 hippocampal lesions, J. Neurol. Neurosurg. Psychiatry 20 (1957)
(1997) 197–208. 11–21.
animal model of amnesia: performance of amnesic patients on tests [62] B.A. Wilson, D. Wearing, Prisoner of consciousness: a state of just designed for the monkey, Behav. Neurosci. 102 (1988) 210–221. awakening following herpes simplex encephalitis, in: R. Campbell, [57] H. Stephan, G. Baron, W.K. Schwerdtfeger, The Brain of the M.A. Conway (Eds.), Broken Memories, Blackwell, Oxford, 1995,
Common Marmoset (Callithrix jacchus): A Stereotaxic Atlas, pp. 14–30.
Springer, Berlin, 1980. [63] S.M. Zola-Morgan, L.R. Squire, Preserved learning in monkeys with [58] J.A. Thornton, L.A. Rothblat, E.A. Murray, Rhinal cortex removal medial temporal lesions: sparing of motor and cognitive skills, J.
produces amnesia for preoperatively learned discrimination prob- Neurosci. 4 (1984) 1072–1085.
lems but fails to disrupt postoperative acquisition and retention in [64] S.M. Zola-Morgan, L.R. Squire, Medial temporal lesions in mon-rhesus monkeys, J. Neurosci. 17 (1997) 8536–8549. keys impair memory in a variety of tasks sensitive to human [59] G.W. Van Hoesen, E.H. Yeterian, R. Lavizzo-Mourey, Widespread amnesia, Behav. Neurosci. 99 (1985) 22–34.
corticostriate projections from temporal cortex of the rhesus mon- [65] S.M. Zola-Morgan, L.R. Squire, M. Mishkin, The neuroanatomy of key, J. Comp. Neurol. 199 (1981) 205–219. amnesia: amygdala–hippocampus versus temporal stem, Science [60] M.L. Voytko, D.S. Olton, R.T. Richardson, I.K. Gorman, J.T. Tobin, 218 (1982) 1337–1339.
D.L. Price, Basal forebrain lesions in monkeys disrupt attention but [66] S.M. Zola, L.R. Squire, E. Teng, L. Stefanacci, E.A. Buffalo, R.E. not learning and memory, J. Neurosci. 14 (1994) 167–186. Clark, Impaired recognition memory in monkeys after damage [61] M.J. Webster, J. Bachevalier, L.G. Ungerleider, Subcortical con- limited to the hippocampal region, J. Neurosci. Meth. 20 (2000)