www.elsevier.com / locate / bres
Research report
Sodium-ascorbate cotransport controls intracellular ascorbate
concentration in primary astrocyte cultures expressing the SVCT2
transporter
*
Jasminka Korcok, Raphael Yan, Ramin Siushansian, S. Jeffrey Dixon, John X. Wilson
Department of Physiology, Faculty of Medicine and Dentistry, The University of Western Ontario, London, Ontario, Canada Accepted 8 August 2000
Abstract
1
Expression of the Na -ascorbate cotransporter, SVCT2, was detected in rat brain and in primary cultures of cerebral astrocytes by Northern blot analysis. SVCT2 expression in cultured astrocytes increased in response to the cyclic AMP analog, dibutyryl cyclic AMP. A
1
mathematical model of ascorbic acid transport was developed to evaluate the hypothesis that Na -ascorbate cotransport across the plasma membrane regulates the steady state intracellular concentration of ascorbic acid in these cells. The outcomes predicted by this model were compared to experimental observations obtained with primary cultures of rat cerebral astrocytes exposed to normal and pathologic conditions. Both cotransport activity and intracellular ascorbic acid concentration increased in astrocytes activated by dibutyryl cyclic
1
AMP. Conversely transport activity and ascorbic acid concentration were decreased by hyposmotic cell swelling, low extracellular Na
1
concentration, and depolarizing levels of extracellular K . In cells incubated for up to 3 h in medium having an ascorbic acid concentration typical of brain extracellular fluid, the changes in intracellular ascorbic acid concentration actually measured were not
1
significantly different from those predicted by modeling changes in Na -ascorbate cotransport activity. Thus, it was not necessary to specify alterations in vitamin C metabolism or efflux pathways in order to predict the steady state intracellular ascorbic acid concentration. These results establish that SVCT2 regulates intracellular ascorbic acid concentration in primary astrocyte cultures. They further indicate
1
that the intracellular-to-extracellular ratio of ascorbic acid concentration at steady state depends on the electrochemical gradients of Na and ascorbate across the plasma membrane. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters modulators transporters and receptors
Topic: Uptake and transporters
Keywords: Ascorbic acid; Transport; Mathematical model; Brain cell
1. Introduction vitamin through plasma membrane transporters. Intracellu-lar AA functions as an enzyme cofactor and may contrib-Vitamin C occurs almost entirely in its reduced form, ute to antioxidant defense in brain cells. The concentration ascorbic acid (AA), in normal brain [4,10,16,18,19]. While and redox state of vitamin C are crucial for these functions. the steady state concentration of AA is 200–400 mM in On the one hand, high concentrations of AA protect brain brain extracellular fluid, it is approximately 10-fold higher cells from ischemic, excitotoxic and oxidative injury in the cellular compartment. Brain cells cannot synthesize [9,15,16,23,35]. On the other hand, high concentrations of vitamin C from glucose de novo. Instead, they obtain the oxidized vitamin C (dehydroascorbic acid, DHAA) are
cytotoxic [20] and especially neurotoxic [12].
Vitamin C transport systems have been characterized in
Abbreviations: AA, ascorbic acid; DHAA, dehydroascorbic acid; an experimental model of brain cells, namely, primary
dBcAMP, dibutyryl cyclic AMP; HPLC, high-performance liquid chroma- cultures of rat cerebral astrocytes. Cultured astrocytes tography
achieve intracellular AA concentrations ([AA] ) as high asi
*Corresponding author. Tel.: 11-519-661-3475; fax: 1
1-519-661-8 mM when incubated with physiologic levels of extracel-3827.
E-mail address: [email protected] (J.X. Wilson). lular AA for 3 h [24]. Longer incubation periods to do not 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
raise [AA] further [24]. Because the hydroxyl groups ati were ubiquitous but had relatively high expression in positions 2 and 3 ionize with pK values of 4.17 and 11.57, brain. In the present study, we report that astrocytes in most molecules of vitamin C exist as the monovalent anion primary culture express SVCT2 and that the expression ascorbate at physiologic pH. Simple diffusion of AA, level is increased by cyclic AMP. Subsequently, we ascorbate and DHAA through cell membranes is a slow develop a mathematical model to evaluate the hypothesis
1 1
process [19]. However, a much faster, Na -dependent that Na -ascorbate cotransport activity regulates [AA] .i
mechanism of ascorbate uptake has been observed in the Actual values for transport rates and intracellular con-plasma membrane of rat and mouse astrocytes. This centrations were obtained using primary cultures of cere-mechanism is an example of electrogenic, secondary active bral astrocytes exposed to normal and pathologic
con-1
transport and it is driven by the membrane potential and ditions. When measured rates for Na -dependent AA
1 1
transmembrane Na gradient. The rate of Na -ascorbate uptake were entered as parameters in the model, the cotransport is stimulated in astrocytes that have been predicted values for [AA] were found to conform closelyi
depleted of intracellular AA [33] as well as in astrocytes to experimental observations. Furthermore, changes in 1
that have been activated by dibutyryl cyclic AMP Na -ascorbate cotransport activity were sufficient to ex-(dBcAMP) or forskolin [26,31,32]. Conversely, this co- plain the sustained influences of several pathologic con-transport activity is inhibited by incubation in media ditions on [AA] .i
1
having low Na concentration [31], low osmolality [25] or 1
depolarizing K levels [34]. Furthermore, the rate of 1
Na -dependent ascorbate influx slows as [AA] rises afteri 2. Materials and methods reintroduction of a physiologic AA concentration to the
medium, with most of this downregulation completed 2.1. Cell cultures 1
within 3 h [33]. In addition to Na -ascorbate cotransport,
astrocytes also take up DHAA through facilitative hexose The experimental protocols were approved by the transporters and reduce it to AA intracellularly [26]. This University of Western Ontario Council on Animal Care. recycling of DHAA to AA depends on the rate of Primary cultures of astrocytes were prepared from the formation of DHAA in the extracellular fluid, is inhibited neopallium of 1-day-old Wistar rats according to our by physiologic concentrations of glucose and is not published procedure [26]. All subsequent treatments were
1
influenced by Na [26]. The only pathway by which AA carried out at 378C unless otherwise noted. The astrocytes has been demonstrated to exit astrocytes is through vol- were grown to confluence in horse serum-supplemented, ume-sensitive organic anion channels [25]. These channels minimum essential medium that did not contain detectable become permeant to ascorbate in response to hyposmotic AA (,1mM) [25]. Like astrocytes in situ, these cells are cell swelling. However, this response is only transitory and coupled by connexin43-positive gap junctions and contain the plasma membrane permeability to ascorbate returns to glial fibrillary acidic protein [11]. They were used for normal within 3 min even though regulatory volume transport experiments after 3–4 weeks in culture.
decrease is incomplete [25].
It is possible that [AA]i is influenced by several 2.2. Experimental procedures mechanisms, namely, ascorbate uptake, DHAA uptake and
recycling to AA, and ascorbate efflux. However, the effects Northern blot analysis was performed according to a of some of these mechanisms may be transient and not modification of a published procedure [22]. Total RNA determinant for [AA] at steady state. Although severali was isolated from whole brain of neonatal rat and primary transport systems and metabolic pathways have been astrocyte cultures using TRIzol (GIBCO). RNA was shown to influence AA levels in cells, their relative separated on 0.6% formaldehyde-agarose gel, transferred importance has remained controversial [19]. Indeed, their to nylon membrane and cross-linked by UV irradiation. respective roles may vary between tissues. For instance, The amount of RNA loaded in each lane was 15mg. The the brain differs from many other organs by having an blot was hybridized overnight with a 1 kb rat SVCT2 extracellular AA concentration that is several-fold higher cDNA 32P-labelled probe, in ULTRAhyb (Ambion) at than plasma levels. As a result, the extracellular AA 428C. Following hybridization, membrane was washed concentration in brain (200–400 mM) is sufficient to 2315 min in 0.13SSC, 0.1% S.D.S. and 2330 min in
1
saturate high affinity Na -ascorbate cotransport systems 0.13SSC, 0.1% S.D.S. at 428C. The signal was detected
[31]. by exposing the membrane to X-ray film. The blots were
Recently, the transporters SVCT1 and SVCT2 were then stripped and reprobed for rat 18S ribosomal RNA cloned from both rat and human and shown to induce under the same conditions to normalize RNA loading. The
1
Na -dependent uptake of L-ascorbate when expressed intensities of SVCT2 and 18S rRNA autoradiographic
heterologously [2,14,27–29]. Northern blot analysis of images were quantified by scanning densitometry and were mammalian tissues detected transcripts encoding SVCT1 expressed as the ratio, SVCT2 mRNA / 18S rRNA.
1
(in mM): 134 NaCl, 5.4 KCl, 1.8 CaCl , 0.8 MgSO , 102 4 ANOVA and Dunnett’s test were used to evaluate the 1
glucose and 20 Hepes (pH 7.3). The final Na con- effect of dBcAMP on SVCT2 expression levels. A ran-centration of this medium was 138 mM and its osmolality domised block factorial design was employed to evaluate was 300 mOsm. Other transport media were made by the effects of treatments on observed AA concentrations by changing the NaCl or KCl concentrations. The specific two-way ANOVA. Additionally, the paired t-test was used
1
changes were as follows: (i) Isoosmotic low-Na transport to compare the observed AA concentrations with the 1
media containing either 35 or 80 mM Na were prepared theoretical values. Finally, the effects of treatments on
14
by partial replacement of NaCl with the chloride salt of [ C]AA efflux rates were analysed using one-way 1
N-methyl-D-glucamine . (ii) Hyposmotic transport ANOVA. P,0.05 was considered to be significant for all
medium was prepared by omission of 58 mM NaCl, so that statistical tests. 1
the final Na concentration was 80 mM and the osmolality 1
was 206 mOsm. (iii) Isoosmotic high-K transport
medium was prepared by substitution of 50 mM KCl for 3. Results 1
an equal concentration of NaCl, thus raising the K 1
concentration to 55.4 mM. (iv) Hyperosmotic normal-K SVCT2 was detected by Northern blot analysis in whole transport medium was prepared by addition of sufficient rat brain and primary astrocyte cultures (Fig. 1). DBcAMP
N-methyl-D-glucamine chloride to increase the osmolality increased astrocytic expression of SVCT2 after a latent
1
to 400 mOsm. (iv) Hyperosmotic high-K transport medium was prepared by addition of 50 mM KCl to
1
increase both the K concentration and the osmolality.
14
Stock solutions of AA andL-[1- C]AA (8 mCi / mmol,
Dupont Canada) were prepared at 48C and contained homocysteine to prevent oxidation of the vitamin [24]. These stock solutions were diluted in prewarmed transport media immediately before addition to cell cultures.
Initial rate of AA uptake was measured by incubating 1
astrocytes in isoosmotic normal-Na transport medium
14
containing [ C]AA (5mM, 378C). Uptake was linear with time for at least 90 s. To study intracellular accumulation of AA in the presence of a physiologic concentration of extracellular AA, the astrocytes were incubated for the indicated periods in transport media containing 200 mM AA (with 160mM homocysteine to prevent AA oxidation). Washing the cultures with ice-cold Tris-sucrose solution (pH 7.3) terminated uptake. [AA] was determined byi
acidic extraction and HPLC-based electrochemical assay (assay sensitivity was 2 pmol AA) [25]. Total cell protein was determined by the Lowry assay.
For experiments investigating AA efflux, astrocytes were first loaded with the radiolabeled vitamin by
incuba-1
tion for 60 min in isoosmotic normal-Na transport
14
medium containing 200 mM [ C]AA (with 160 mM homocysteine). Next, the cells were washed and incubated in the indicated transport media containing 200 mM unlabeled AA. Aliquots of media were collected at timed intervals and then the cells were harvested. The radioactive contents of the media and cells were measured by liquid scintillation counting.
2.3. Statistics
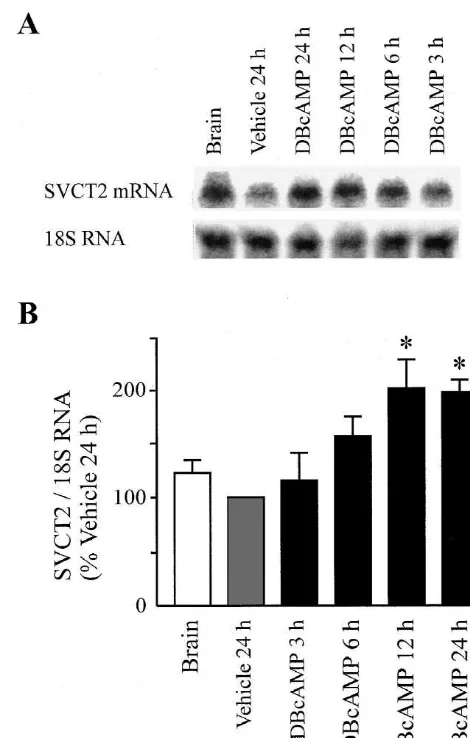
Fig. 1. Northern blot analysis of SVCT2 expression in total RNA from rat brain and primary cultures of cerebral astrocytes. Confluent primary Data are presented as mean6S.E.M. values from n
cultures of rat astrocytes were incubated with dBcAMP (0.25 mM) or number of independent experiments, with triplicate
period of 12 h (Fig. 1). The initial rates of AA uptake after 24 h of treatment were 6962 nmol / g protein / min in vehicle control and 10464 nmol / g protein / min in dBcAMP-treated astrocytes (n57 experiments, P,0.05).
We next developed a mathematical model to evaluate 1
the hypothesis that Na -ascorbate cotransport activity regulates [AA] . The mechanisms addressed by the mathe-i
matical model are illustrated in Fig. 2 and the details are presented in the DISCUSSION below. Uptake rate through
1
the Na -ascorbate co-transport system is designated as J .1
Volume-insensitive AA efflux from astrocytes is J ; this2
efflux may occur by reversal of the co-transport system or by other volume-insensitive pathways. The AA efflux pathway that is activated transiently by cell swelling is J .3
DHAA formation, uptake and intracellular reduction to AA are shown as discrete steps in Fig. 2 but are designated altogether as J in the model. The differential equation4
describing the rate of change of [AA] as a function ofi
time was solved numerically and solutions are presented as the theoretical AA concentrations plotted in Figs. 3 and 4. The results of the HPLC-based electrochemical assay showed that the astrocytes cultured in vitamin C-free medium did not contain intracellular AA initially. How-ever, the cells gradually accumulated the vitamin when incubated with physiologic concentrations of AA (200
1
mM) and Na (138 mM) (Fig. 3). Activation of astrocytes by dBcAMP increased the AA uptake rate and steady state
[AA] . The [AA]i i observed at each time point were Fig. 3. Accumulation of AA in control and dBcAMP-activated as-compared to values predicted by the mathematical model. trocytes. Primary cultures of rat astrocytes were grown to confluence in For both the vehicle control and dBcAMP-activated as- vitamin C-free medium. Subsequently, dBcAMP-activated (0.25 mM, top trocytes, observed [AA] was not significantly differenti panel) and control (bottom panel) astrocytes were incubated with 200mM AA for the indicated times in isoosmotic medium containing 138 mM from predicted values (Fig. 3). This finding demonstrates
1
Na before being harvested and assayed for intracellular AA con-that the model is capable of predicting [AA] using valuesi centration. Plotted as open circles are mean6S.E.M. values for cell AA
for J calculated from previously published V1 max and Km concentration from 4 experiments. Curves represent the theoretical values
1
values for Na -ascorbate cotransport by control and predicted by the mathematical model that is described in the text. dBcAMP-activated astrocytes [31]. Furthermore, the effect
of dBcAMP on steady state [AA]i was modeled by
changing J , without altering J , J or J (J1 2 3 4 250.012[AA] ,i J350, and J450).
1 Incubation of astrocytes in media that decreased Na -dependent ascorbate uptake rates led to decreased steady state [AA] (Fig. 4). The mathematical model was used toi
calculate theoretical values for steady state [AA] usingi
values for J calculated from transport parameters obtained1
in earlier studies (as presented in the Legend of Fig. 4). When the other fluxes were kept constant (J250.012[AA] ,i J350, and J450), the [AA] values predicted were noti
significantly different from the concentrations actually measured (Fig. 4).
The equations modeled the effects of treatments on [AA] by varying the AA uptake rate and leaving the effluxi
Fig. 2. Model of vitamin C recycling by cultured astrocytes. AA uptake rate unchanged. To further test the validity of the
assump-1
through the Na -AA cotransporter is designated as J , efflux through1 tion that efflux rates do not change, we measured these volume-insensitive pathways as J , and efflux through volume-activated2
rates under various experimental conditions. Table 1 shows pathways as J . DHAA formation, uptake and intracellular reduction to3
the efflux rates obtained when astrocytes were incubated in AA are shown as discrete steps in this illustration but are designated
1 1
Fig. 4. Accumulation of AA in astrocytes incubated under conditions of changed ionic gradients. Primary astrocyte cultures were incubated with
1 1
200mM AA for 3 h in normal, low Na , hyposmotic or high K medium before being harvested and assayed for intracellular AA concentration. Bars show mean6S.E.M. values from 4 experiments. The theoretical values were generated from the equation described in the Discussion using the following parameters: J o1 50.39mmol / g protein / min for the
1
isoosmotic normal-Na condition (based on transport data in [31]);
1
J o1 50.22mmol / g protein / min for the isoosmotic low-Na condition (35
1
mM Na ) [31]; J o1 50.27 mmol / g protein / min for the hyposmotic condition [25]; and J o1 50.26mmol / g protein / min for the hyperosmotic
1
high-K condition [34].
14
Fig. 5. Efflux of [ C]AA from astrocytes incubated under conditions of changed ionic gradients. Primary astrocyte cultures were loaded with
14
of hyperosmolality or depolarization were observed. We [ C]AA, and then were washed and incubated for 10 min in the indicated 1
transport media containing 200 mM unlabeled AA. In the case of then investigated whether depolarizing levels of K affect
1
isoosmotic high-K medium, this efflux incubation period is long enough AA efflux under isoosmotic conditions, because this better 1
to reverse Na -glutamate cotransport but too brief to swell astrocytes and approximates the change in extracellular fluid that occurs
activate volume-sensitive organic anion channels [21]. Shown are the 14
in ischemic brain [1,3]. No significant effect of isoosmotic- levels of [ C]AA in the transport medium (top panel) or remaining in 1
high K medium on AA efflux was observed (Fig. 5). This astrocytes (bottom panel) at the end of the efflux period. * P,0.05 1
compared to control (isoosmotic medium containing normal concen-finding indicates that depolarization by extracellular K , at
1 1
trations of Na and K ). a concentration typical of ischemic brain [1,3], does not
1
reverse the Na -ascorbate cotransporter. The lack of effect 1
of hyperosmolality and high-K contrasts with the stimu-lation of AA efflux by hyposmotic medium observed in the
4. Discussion same experimental protocol (Fig. 5).
The present study showed that cultured cerebral as-trocytes express SVCT2. Expression of this protein in Xenopus oocytes [2,14,27] conferred vitamin C transport
Table 1 activity that resembles that of astrocytes [31,34], with
1 14 1
Lack of effect of a depolarizing concentration of K on [ C]AA efflux respect to Na -dependence, electrogenicity, specificity and a
from astrocytes incubated in hyperosmotic medium
affinity for L-ascorbate. Furthermore, dBcAMP increased
14 14
Efflux medium Cell [ C]AA Medium [ C]AA astrocytic expression of SVCT2 and rate of ascorbate (mmol / g protein) (dpm / culture) uptake comparably. These similarities indicate that SVCT2
1
Isoosmotic 5.4 mM K 2064 651687 mediates ascorbate uptake in these cells.
1
Hyperosmotic 5.4 mM K 2363 710696 We developed a mathematical model that predicts the
1
Hyperosmotic 55.4 mM K 2265 6326139
rise in [AA] that occurs when AA-depleted astrocytes arei
aAstrocytes were loaded with [ C]AA (20014 mM in isoosmotic normal- 1
incubated with physiologic concentrations of AA and Na
1
1
Na medium), and then washed and incubated for 10 min in the indicated (Fig. 2). Uptake rate through the Na -ascorbate cotrans-transport media containing 200 mM unlabeled AA. Shown are the
14 port system is designated as J . Volume-insensitive AA
1
mean6S.E.M. values for the levels of [ C]AA in cells and media at the
efflux from astrocytes is J ; this efflux may occur by end of 10 min efflux incubations (n53 experiments). No significant 2
volume-insensitive pathways. The AA efflux pathway that is astrocytes [26]. This recycling can be expected to mini-activated transiently by cell swelling is J . DHAA forma-3 mize the toxicity of the oxidized form of vitamin C. Since tion, uptake and intracellular reduction to AA are shown as DHAA uptake is mediated by facilitative hexose
transpor-1
discrete steps in Fig. 2, but are designated altogether as J4 ters and not by the Na -ascorbate cotransport system [26], in the mathematical model. The rate of change of [AA] isi it is possible to model changes in the transport of each the sum of the rates of AA accumulation, less the rates of form of vitamin C independently. Incubation with high AA disappearance. Therefore, concentrations of DHAA (200mM) increases the [AA] ini
cultured astrocytes transiently when glucose is absent from d[AA] / dti 5J12J22J31J .4 the incubation medium [26]. In the present experiments
however, because homocysteine was included in the It has been shown previously that when AA-depleted
incubation medium to prevent oxidation of AA to DHAA astrocytes are incubated with 200 mM AA, J decreases1
and glucose was included to block facilitative hexose exponentially to a lower bound of 67% after 18 h [33].
transporters, the mathematical model assigned a value of Therefore,
zero to the rate of DHAA recycling to AA. The model also
J15J o(0.671 10.33 exp(2st)) assumed that the AA efflux rate was a linear function of
[AA] . Thus, the success of our model is consistent with a where J o is the Michaelian initial rate of AA uptake at1 i
predominant role for AA uptake, compared to DHAA time zero and s is a parameter to be determined. When the
recycling or AA efflux, in determining steady state [AA]
extracellular AA concentration is 200mM, i
in control and activated astrocytes under physiologic
J o1 5200Vmax/ [Km1200].
conditions.
1
When cells are incubated with AA in isoosmotic normal- The role of the Na -ascorbate cotransport system was 1
Na medium, the relevant parameters are Vmax50.46 evaluated further by incubating astrocytes under conditions
mmol / g protein / min and Km532 mM for control as- of altered ionic gradients. The effect of lowered extracellu-1
trocytes and Vmax50.69mmol / g protein / min and Km534 lar Na on the observed [AA] was the same as thati
mM for dBcAMP-activated astrocytes [31]. Therefore, predicted mathematically on the basis of changes in the Km
1
J o1 50.39mmol / g protein / min for control astrocytes, and and Vmax of Na -ascorbate cotransport. This indicates that
J o1 50.59 mmol / g protein / min for activated astrocytes. the intracellular-to-extracellular ratio of AA at steady state 1 Because J decreases to 75% of its original (time zero)1 depends on the electrochemical gradients of Na and value after 3 h and to 67% after 18 h incubation with 200 ascorbate across the plasma membrane. Most of the AA
21
mM AA [33], s50.0079 min . The volume-insensitive inside astrocytes is localized to the cytosol [24]. It is component of AA efflux (J ) was assumed to be propor-2 possible that higher levels of total intracellular AA can be tional to [AA] . The best fit between predicted andi achieved in neurons as a result of subcellular compart-observed values was found when J250.012[AA] . Thei mentation, for example, into secretory granules that are volume-sensitive component of AA efflux was assumed to relatively more abundant in neurons.
1
be negligible (J350) during incubations in isoosmotic Extracellular K concentration rises to depolarizing media. The rate of recycling of DHAA was also assumed levels after cerebral ischemia [1,3] or general anesthesia 1 to be negligible (J450). The reasons for the latter assump- [17]. We incubated astrocytes with hyperosmotic high-K tion were that homocysteine was present in the transport media to depolarize the plasma membrane during AA
1 medium to minimize oxidation of AA to DHAA, and uptake incubations. The effect of elevated extracellular K glucose was present to compete with DHAA for uptake on astroglial AA uptake was a diminished [AA] consistenti
1
through facilitative hexose transporters. The differential with the decrease in the Vmaxof Na -ascorbate cotransport. equation was solved using the fourth order Runge-Kutta We also incubated astrocytes with isoosmotic (Fig. 5) and
1
scheme [13]. hyperosmotic (Table 1) high-K media to depolarize the
We found that the mathematical model accurately plasma membrane during AA efflux incubations. Astroglial 1
predicted the higher [AA]i in dBcAMP-activated as- AA efflux was not increased by K under either iso-1
trocytes solely on the basis of their accelerated Na - osmotic or hyperosmotic conditions. In the case of iso-1
ascorbate cotransport rate. Moreover, the model predicted osmotic high-K medium, the efflux incubation period was 1
the steady state [AA] values for astrocytes exposed toi long enough to reverse Na -glutamate cotransport (10
1 1
pathologic changes of medium osmolality, Na and K min) but too brief to swell astrocytes and activate volume-1
based on the observed inhibition of Na -ascorbate co- sensitive organic anion channels [21]. This finding sug-1
transport by these conditions. gests that K , even at the high level found in the DHAA has three adjacent carbonyl groups in a ring extracellular fluid of ischemic brain [1,3], cannot reverse
1
structure, which is not seen elsewhere in biologic systems. the electrogenic Na -ascorbate cotransporter.
kainate-induced neurotoxicity in the rat hippocampus, Brain Res. extracellular fluid [5,6,8]. This pattern is consistent with
727 (1996) 133–144. rapid release of AA from swollen astrocytes in ischemic
[10] M. Miele, M. Fillenz, In vivo determination of extracellular brain brain regions. AA released during this astroglial swelling ascorbate, J. Neurosci. Methods 70 (1996) 15–19.
may contribute to scavenging of reactive oxygen species in [11] C.C.G. Naus, J.F. Bechberger, S. Caveney, J.X. Wilson, Expression of gap junction genes in astrocytes and C6 glioma cells, Neurosci. the extracellular fluid. Hyposmotic medium stimulates AA
Lett. 126 (1991) 33–36. efflux because it swells astrocytes and thereby activates
[12] J.W. Patterson, D.W. Mastin, Some effects of dehydroascorbic acid volume-sensitive organic anion channels that are permeant on the central nervous system, Am. J. Physiol. 167 (1951) 119–126. to ascorbate [25]. However, the increase in the plasma [13] W.H. Press, S.A. Teukolsky, W.T. Vetterling, B.P. Flannery, Numeri-cal Recipes in C: The Art of Scientific Computing, 2nd Edition, membrane permeability is transient and terminates within
Cambridge University Press, New York, 1992. 1–3 min [25]. This explains why modeling the decrease in
1 [14] D.P. Rajan, W. Huang, B. Dutta, L.D. Devoe, F.H. Leibach, V. the Vmax of Na -ascorbate cotransport was sufficient to Ganapathy, P.D. Prasad, Human placental sodium-dependent vitamin predict the steady state [AA]i of cells incubated in C transporter (SVCT2): molecular cloning and transport function, hyposmotic medium. Thus, it is likely that transient Biochem. Biophys. Res. Commun. 262 (1999) 762–768.
[15] A. Ranjan, D. Theodore, R.P. Haran, M.J. Chandy, Ascorbic acid changes in efflux do not explain the effects of these
and focal cerebral ischaemia in a primate model, Acta Neurochir. pathologic conditions on steady state [AA] .i 123 (1993) 87–91.
[16] G.V. Rebec, R.C. Pierce, A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopa-minergic and glutamatergic transmission, Prog. Neurobiol. 43 Acknowledgements (1994) 537–565.
[17] C.R. Ries, E. Puil, Ionic mechanism of isoflurane’s actions on thalamocortical neurons, J. Neurophysiol. 81 (1999) 1802–1809. We thank Dr. M.A. Hediger (Department of Medicine,
[18] R.C. Rose, Cerebral metabolism of oxidized ascorbate, Brain Res. Brigham and Women’s Hospital) for generously providing
628 (1993) 49–55.
the rat cDNA for SVCT2 and Dr. T.M. Underhill (School [19] R.C. Rose, J.X. Wilson, Ascorbate membrane transport properties, of Dentistry, University of Western Ontario) for advice on in: L. Packer, J. Fuchs (Eds.), Vitamin C in Health and Disease, Northern blot analysis. Supported by grant OGP0002200 Marcel Dekker, Inc, New York, 1997, pp. 143–162.
[20] R.C. Rose, J.L. Choi, A.M. Bode, Short term effects of oxidized from the Natural Sciences and Engineering Research
ascorbic acid on bovine corneal endothelium and human placenta, Council of Canada.
Life Sci. 50 (1992) 1543–1549.
[21] E.M. Rutledge, M. Aschner, H.K. Kimelberg, Pharmacological 3
characterization of swelling-induced D-[ H]aspartate release from primary astrocyte cultures, Am. J. Physiol. 274 (1998) C1511–
References C1520.
[22] J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular Cloning: A [1] J. Astrup, S. Rehncrona, B.K. Siesjo, The increase in extracellular Laboratory Manual, Cold Spring Harbour Laboratory, Cold Spring
potassium concentration in the ischemic brain in relation to the Harbour, New York, 1989.
preischemic functional activity and cerebral metabolic rate, Brain [23] M.A. Sciamanna, C.P. Lee, Ischemia / reperfusion injury of forebrain Res. 199 (1980) 161–174. mitochondria and protection by ascorbate, Arch. Biochem. Biophys. [2] R. Daruwala, J. Song, W.S. Koh, S.C. Rumsey, M. Levine, Cloning 305 (1993) 215–224.
and functional characterization of the human sodium-dependent [24] R. Siushansian, J.X. Wilson, Ascorbate transport and intracellular vitamin C transporters hSVCT1 and hSVCT2, FEBS Lett. 460 concentration in cerebral astrocytes, J. Neurochem. 65 (1995) 41–
(1999) 480–484. 49.
[3] A. Eckholm, K. Katsura, T. Kristian, M. Liu, J. Folbergrova, B.K. [25] R. Siushansian, S.J. Dixon, J.X. Wilson, Osmotic swelling stimulates Siejso, Coupling of cellular energy state and ion homeostasis during ascorbate efflux from cerebral astrocytes, J. Neurochem. 66 (1996) recovery following brain ischemia, Brain Res. 604 (1993) 185–191. 1227–1233.
¨
[4] R.A. Grunewald, Ascorbic acid in the brain, Brain Res. Rev. 18 [26] R. Siushansian, L. Tao, S.J. Dixon, J.X. Wilson, Cerebral astrocytes (1993) 123–133. transport ascorbic acid and dehydroascorbic acid through distinct [5] L. Hillered, L. Persson, H.G. Bolander, A. Hallstrom, U. Ungerstedt, mechanisms regulated by cyclic AMP, J. Neurochem. 68 (1997)
Increased levels of ascorbate in the striatum after middle cerebral 2378–2385.
artery occlusion in the rat monitored by intracerebral microdialysis, [27] H. Tsukaguchi, T. Tokui, B. Mackenzie, U.V. Berger, X.Z. Chen, Y. Neurosci. Lett. 95 (1988) 286–290. Wang, R.F. Brubaker, M.A. Hediger, A family of mammalian
1
[6] L. Hillered, P. Nilsson, U. Ungerstedt, U. Ponten, Trauma-induced Na -dependent L-ascorbic acid transporters, Nature 399 (1999) increase of extracellular ascorbate in rat cerebral cortex, Neurosci. 70–75.
Lett. 113 (1990) 328–332. [28] H. Wang, B. Dutta, W. Huang, L.D. Devoe, F.H. Leibach, V.
1
[7] Y. Kinuta, H. Kikuchi, M. Ishikawa, M. Kimura, Y. Itokawa, Lipid Ganapathy, P.D. Prasad, Human Na -dependent vitamin C transpor-peroxidation in focal cerebral ischemia, J. Neurosurg. 71 (1989) ter 1 (hSVCT1): primary structure, functional characteristics and 421–429. evidence for a non-functional splice variant, Biochim. Biophys. Acta [8] Y. Matsuo, T. Kihara, M. Ikeda, M. Ninomiya, H. Onodera, K. 1461 (1999) 1–9.
Kogure, Role of neutrophils in radical production during ischemia [29] Y. Wang, B. Mackenzie, H. Tsukaguchi, S. Weremowicz, C.C. and reperfusion of the rat brain: effect of neutrophil depletion on Morton, M.A. Hediger, Human vitamin C (L-ascorbic acid) transpor-extracellular ascorbyl radical formation, J. Cereb. Blood Flow ter SVCT1, Biochem. Biophys. Res. Commun. 267 (2000) 488–494. Metab. 15 (1995) 941–947. [30] Y.H. Wang, K.R. Dhariwal, M. Levine, Ascorbic acid bioavailability [9] D.G. MacGregor, M.J. Higgins, P.A. Jones, W.O. Maxwell, M.W. in humans: ascorbic acid in plasma, serum and urine, Ann. NY
[31] J.X. Wilson, Ascorbic acid uptake by a high-affinity sodium-depen- regulation of ascorbate transport activity in astrocytes, Neurochem. dent mechanism in cultured rat astrocytes, J. Neurochem. 53 (1989) Res. 15 (1990) 1037–1043.
1064–1071. [34] J.X. Wilson, E.M. Jaworski, S.J. Dixon, Evidence for electrogenic [32] J.X. Wilson, S.J. Dixon, Ascorbic acid transport in mouse and rat sodium-dependent ascorbate transport in rat astroglia, Neurochem.
astrocytes is reversibly inhibited by furosemide, SITS and DIDS, Res. 16 (1991) 73–78.

![Fig. 5. Efflux of [ C]AA from astrocytes incubated under conditions of14changed ionic gradients](https://thumb-ap.123doks.com/thumbv2/123dok/3139645.1382801/5.612.311.552.62.359/fig-efux-astrocytes-incubated-conditions-changed-ionic-gradients.webp)