Brain Research 886 (2000) 108–112
www.elsevier.com / locate / bres
Interactive report
Cellular bases of functional brain imaging: insights from neuron-glia
1
metabolic coupling
*
Pierre J. Magistretti
´ ´ ´
Institut de Physiologie, Faculte de Medecine, Universite de Lausanne 7, Rue du Bugnon, CH-1005 Lausanne, Switzerland
Accepted 18 September 2000
1. Basic principles and questions spectroscopy (MRS) [5]. Paradoxically, despite the high degree of technological sophistication of these brain In a seminal article published over a hundred years ago imaging methods, the precise nature of the mechanisms in the Journal of Physiology [25], Charles Sherrington and of the cell types involved in coupling and in generat-postulated that ‘[the brain possesses] intrinsic mechanisms ing metabolic signals, is still debated [12]. In order to by which its vascular supply can be varied locally in elucidate the physiological bases of this coupling it is correspondence with local variations of functional activi- crucial to better understand the cellular and molecular ty’; he postulated also that ‘chemical products of cerebral bases of brain energy metabolism and in particular the metabolism . . . cause variations of caliber of the cerebral nature of the signals produced by neuronal activity which vessels’. Evidence for a direct coupling between neuronal lead to the localized increases in glucose utilization. activity and one of the parameters of energy metabolism, Over the last few years it has become clear that highly that is blood flow, was clearly formulated for the first time. regulated metabolic interactions occur between neurons This fundamental principle in brain physiology has been and astrocytes, providing a conceptual framework for the extended to brain energy metabolism as evidence indicates interpretation of new data on the molecular aspects of that neuronal activity is tightly coupled to glucose utiliza- brain energy metabolism [29]. On the basis of in vitro tion [27]. Since in normal conditions, glucose is the observations obtained in primary astrocyte cultures, our exclusive energy substrate for the brain, from the metabol- laboratory has proposed in 1994 an operational model that ic point of view, this coupling implies that spatially could account for the coupling of neuronal activity with localized and temporally restricted increases over basal energy metabolism [18]. Central to this model is the role of glucose utilisation occur in register with neuronal activity glutamate released during synaptic activity and its reuptake in brain areas subserving specific modalities. The ramifica- via specific transporters into astrocytic processes that tions of this physiological concept are multiple; specifical- ensheath synaptic contacts.
ly, they are at the core of all the techniques currently used for functional brain imaging. Indeed, the signals detected
with functional brain imaging techniques are based on the 2. The central role of astrocytes in coupling synaptic
coupling between neuronal activity and energy metabo- activity with energy metabolism
lism. Thus, positron emission tomography (PET) can
monitor the increase in cerebral blood flow, in glucose Structural and functional arguments suggest that, in utilization and oxygen consumption [22,4]; the degree of view of their cytological characteristics, astrocytes play a blood oxygenation yields the signals that are detected with central role in the distribution of energy substrates from functional magnetic resonance imaging (fMRI) [15]. The the circulation to neurons [29,7]. Thus, while the ratio changes in the local concentration of certain metabolic between neurons and non-neuronal cells depends on intermediates, in particular lactate and glucose, associated species, brain areas or developmental ages, it is a well with neuronal activity, is revealed by magnetic resonance established fact that neurons contribute at most 50% of cerebral cortical volume [9,14]. Astrocytes are stellate cells
1 (hence their name) with multiple fine processes some of
Published on the World Wide Web on 12 October 2000.
which are in close apposition to capillary walls. In fact the
*Tel.:141-21-692-5542; fax:141-21-692-5595.
E-mail address: [email protected] (P.J. Magistretti). entire surface of intraparenchymal capillaries is covered by
.J
.
Magistretti
/
Brain
Research
886
(2000
)
108
–
112
109
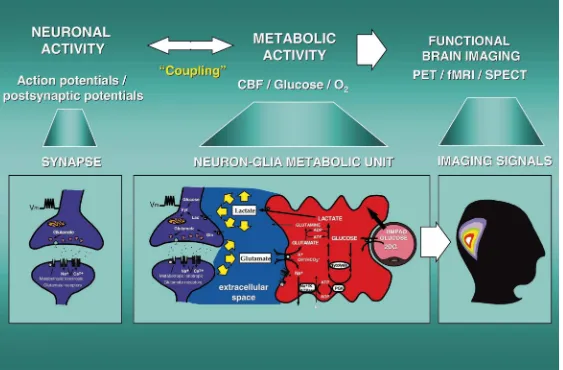
Fig. 1. Glutamatergic synapse (left panel). Presynaptically released glutamate depolarizes postsynaptic neurons by acting at specific receptor subtypes. The action of glutamate is terminated by an
1 1
efficient glutamate uptake system located primarily in astrocytes (middle panel). Glutamate is cotransported with Na , resulting in an increase in the intra-astrocytic concentration of Na , leading to an
1 1 1 1
activation of the astrocyte Na / K -ATPase. Activation of the Na / K -ATPase stimulates glycolysis, i.e. glucose utilization and lactate production. Lactate, once released by astrocytes, can be taken up by neurons and serve them as an adequate energy substrate. This model, which summarizes in vitro experimental evidences indicating glutamate-induced glycolysis, is taken to reflect cellular and molecular events occurring during activation of a given cortical area. Neuron-glia metabolic unit(middle panel). Following neuronal activation and synaptic glutamate release, glutamate reuptake into
1 1
astrocytes triggers increased glucose uptake from capillaries via activation of an isoform of the Na / K -ATPase which is highly sensitive to ouabain, possibly thea2isoform [18,19]. Glucose is then processed glycolytically to lactate by astrocytes which are enriched in the muscle form of LDH (LDH ). The exchange of lactate between astrocytes and neurons is operated by monocarboxylate5
transporters. Lactate is then converted to pyruvate since neurons contain the heart form of LDH (LDH ). Pyruvate, via the formation of acetylCoA by pyruvate dehydrogenase (PDH), enters the TCA1
cycle thus generating 18 ATP/ lactate. These molecular mechanisms operate an astrocyte-neuron lactate shuttle [12,1]. Relevance of metabolic trafficking between astrocytes and neurons for
8 18
FDG-PET(right panel). The proposed model of glutamate-induced glycolysis in astrocytes implies that the activity-linked uptake of FDG monitored with PET, reflects primarily an astrocyte-based
18
110 P.J. Magistretti / Brain Research 886 (2000) 108 –112
these specialized processes called astrocytic end-feet [21]. pling are now beginning to be elucidated; a critical
1 1
involvement of the Na / K -ATPase is likely since oua-This cytoarchitectural arrangement implies that astrocytes
bain completely inhibits the glutamate-evoked 2-DG up-form the first cellular barrier that glucose entering the brain
take by astrocytes [18,19]. Indeed, recent evidence ob-parenchyma encounters, and it makes them a likely site of
86
tained in our laboratory using Rubidium uptake to prevalent glucose uptake (Fig. 1). In addition, glucose
directly monitor the activity of the pump, shows that transporters of the GLUT1 type are expressed on astrocytic
1 1
glutamate activates the Na / K -ATPase [19]. This effect end-feet [13]. Other astrocyte processes are wrapped
of glutamate is likely due to the mobilization of a subunit around synaptic contacts which possess receptors for a
of the pump which is highly sensitive to ouabain, probably variety of neurotransmitters as well as reuptake sites, in
the a2 subunit [19]. particular for glutamate. In fact there is evidence obtained
There is ample evidence from studies in a variety of from quantitative morphometric studies supporting the
cellular systems including the brain, kidney, vascular notion that astrocytes are polarized cells, with one process
smooth muscle and erythrocytes, that increases in the contacting a cell of mesodermal origin (most frequently an
1 1
activity of the Na / K -ATPase stimulate glucose uptake endothelial cell of the capillary) and a multitude of process
and glycolysis [17,19,11]. Consistent with this view, that are intertwined within the neuropil ensheathing
synap-glutamate stimulates the glycolytic processing of glucose tic contacts. These synaptically-targeted lamellar processes
in astrocytes, as indicated by the increase in lactate release represent approximately 80% of an astrocyte membrane
[18]. The proposed stoichoimetry of the molecular steps surface and are devoid of organelles [24]. Overall, these
involved in the coupling between glutamate uptake and features imply that astrocytes are ideally positioned to
glucose utilization is the following: the uptake of one sense increases in synaptic activity and to couple them
glutamate with 3 sodium ions triggers the entry of one with energy metabolism.
glucose which, through glycolysis produces 2 ATPs one of From the foregoing, we postulated that astrocytes may
which is consumed by the pump resulting in the extrusion play a prominent role in coupling neuronal activity to local
of 3 sodium ions, while the other fuels the enzymatic increases in glucose utilization during activation as
ob-conversion of glutamate to glutamine, an ATP-requiring, served in animals and humans. To explore this hypothesis
astrocyte-specific reaction catalyzed by glutamine synthase we have studied glucose utilization by mouse cerebral
3
[12]; the glycolytic processing of glucose results in cortex astrocytes in culture using the H-2-deoxyglucose
approximately 2 lactate molecules produced per 1 glucose (2-DG) as a marker of glucose utilization. We have shown
molecule, i.e. a stoichoimetrical relationship between that glutamate stimulates 2-DG uptake and
phosphoryla-glucose and lactate, as expected. The increase in lactate tion in astrocytes in a concentration-dependent manner
formation from extracellular glucose evoked by glutamate with an EC50 of approximately 80mM [18]. This effect is
is expressed with an EC approximately 80 mM, a value not receptor-mediated since it can not be prevented nor 50
which is very similar to the transport rate of glutamate into mimicked by glutamate receptor antagonists and agonists,
1
astrocytes, and to the stimulation of glucose utilization and respectively [18]. Rather, the involvement of a Na
-depen-of the pump activity by glutamate [18,19]. dent glutamate transporter is strongly supported by a set of
These data indicate that glutamate stimulates aerobic pharmacological evidences. Indeed the glutamate-mediated
glycolysis (i.e. the transformation of glucose into lactate in increase in glucose utilization is mimicked by D-aspartate
the presence of sufficient oxygen) in astrocytes by a but not D-glutamate, a pharmacological signature for an
1 1
mechanism involving an activation of the Na / K -AT-effect mediated by glutamate transporters; as expected
Pase. from a phenomenon mediated by a Na-dependent process
such as the glutamate transport, removal of extracellular
1
Na inhibits the metabolic effect of glutamate [18,7].
4. Glutamate-stimulated lactate production by
Finally, inhibition of glutamate uptake by known
transpor-astrocytes provides an energy source for neurons
ter inhibitors such as threo-hydroxyaspartate results in the inhibition of the glutamate-stimulated glucose utilization
This view raises the question of the usefulness of lactate by astrocytes [18]. Our results clearly indicated a tight
1
as an energy substrate for neurons. A vast array of coupling between Na -dependent glutamate uptake and
experimental data has been accumulated over the years, glucose utilization by astrocytes (Fig. 1), a finding later
indicating that in vitro, lactate can adequately maintain confirmed by Sokoloff and associates [28].
synaptic activity in the absence of glucose [29,26]. Al-though it is generally Al-thought that in vivo, lactate is not an adequate substrate for the brain since it crosses only
3. Astrocytes couple the activity of glutamatergic
1 marginally the blood brain barrier [16], recent magnetic
synapses with glucose utilization: role of the Na /
1 resonance evidence indicates a significant transfer of
K -ATPase
cou-nism described above (Fig. 1), or if applied to in vitro data in vivo would support the notion of a transient preparations, lactate may in fact be consumed preferen- glycolytic processing of glucose during activation. tially to glucose, particularly during periods of intense The model proposed for the coupling between neuronal activity [29,26]. activity and glucose utilization, on the basis of studies at Since lactate can be used as an energy fuel by neurons, the cellular level (Fig. 1) would be consistent with an we considered the possibility that a selective distribution of initial glycolytic processing of glucose occurring in as-lactate dehydrogenase (LDH) isoenzymes could exist trocytes during activation, resulting in a transient lactate among lactate-producing and lactate-consuming cells. In- overproduction, followed by a recoupling phase during deed LDH is the enzyme that catalyzes the interconversion which lactate would be oxidized by neurons. The spatio-of lactate and pyruvate. We have raised polyclonal anti- temporal ‘window’ during which a lactate peak could be bodies against the LDH1 (heart-type) and the LDH5 detected by MRS would depend on the rapidity and degree (muscle-type) subunits and used them for immunohisto- of recoupling existing between astrocytic glycolysis and chemistry of human hippocampus and visual cortex. The neuronal oxydative phosphorylation.
results show that the immunoreactivity against LDH (the5 Finally, the model proposed in Fig. 1 is consistent with form enriched in lactate-producing tissues) is restricted to a the notion that the signals detected during physiological
18
population of astrocytes while neurons are stained only by activation in humans with F-2 DG PET and autoradiog-an autoradiog-antibody directed against LDH (the form enriched in1 raphy in laboratory animals may reflect predominantly lactate-consuming tissues) [1,10]. These data thus support uptake of the tracer into astrocytes [12,18]. This conclu-the idea that some astrocytes would preferentially process sion does not question the validity of the 2 DG-based glucose glycolytically into lactate which, once released, techniques, rather it provides a cellular and molecular basis could be transformed by neurons into pyruvate and enter for these functional brain imaging techniques (Fig. 1). the TCA cycle to serve as an energy fuel. It should be The mechanisms of glutamate-mediated neuron-glia stressed that one molecule of lactate entering the TCA metabolic coupling that we have demonstrated over the last cycle through the LDH-catalyzed reaction, can yield, in five years by using combined functional in vitro and normoxic conditions, 17 ATPs. structural and functional ex vivo localization approaches In summary, since glutamate release occurs following have provided an initial basis to better understand the the modality-specific activation of a brain region, these cellular and molecular steps involved in neuro-metabolic data and the proposed model are consistent with the view coupling. However a number of questions still need to be that during activation, glutamate uptake into astrocytes addressed; in particular the different time constants for leads to increased glucose utilization and lactate pro- synaptic activity and the metabolic response at the basis of duction, which can be subsequently used by neurons to the signals detected with PET, need to be considered. In meet their energy needs. Further support for this notion of this context it is likely that the glutamate-mediated cou-an ‘astrocyte-neuron lactate shuttle’ in the brain (Fig. 1), pling at neuron-astrocyte metabolic units in an activated has been provided by the recent identification of two cortical area will be compounded through mechanisms in lactate transporters, MCT-1 and MCT-2, selectively ex- which the extracellular space acts as a spatial and temporal pressed in astrocytes or neurons. Thus, MCT-1 is enriched metabolic buffer.
in astrocytes in culture while MCT-2 is predominantly expressed in neurons [2,20].
References
[1] P.G. Bittar, Y. Charnay, L. Pellerin, C. Bouras, P.J. Magistretti,
5. Relevance to functional brain imaging
Selective distribution of lactate dehydrogenase isoenzymes in neu-rons and astrocytes of human brain, J. Cereb. Blood Flow. Metab.
Results obtained in a variety of in vivo paradigms both
16 (1996) 1079–1089.
in laboratory animals and in humans, support the existence [2] S. Broer, B. Rahman, G. Pellegri, L. Pellerin, J.-L. Martin, S.¨ of such a transient lactate production during activation. Verleysdonk, B. Hamprecht, P.J. Magistretti, Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1
Thus, marked increases in the concentration of
extracellu-(MCT1) expressing Xenopus laevis oocytes, J. Biol. Chem. 272
lar lactate have been monitored by microdialysis studies in
(1997) 30096–30102.
rat striatum and hippocampus during physiological sensory
[3] L.K. Fellows, M.G. Boutelle, M. Fillenz, Physiological stimulation
stimulation [3]. This activity-linked increase in lactate is increases nonoxidative glucose metabolism in the brain of the freely completely inhibited by the glutamate uptake inhibitor moving rat, J. Neurochem. 60 (1993) 1258–1263.
THA, thus providing further support to the existence of [4] R.S.J. Frackowiak, G.L. Lenzi, T. Jones, J.D. Heather, Quantitative measurement of regional cerebral blood flow and oxygen
metabo-glutamate stimulated glycolysis during activation [6]. 15
lism in man using O and positron emission tomography: theory,
Magnetic resonance spectroscopy (MRS) in humans has
procedure and normal values, J. Comput. Assist. Tomogr. 4 (1980)
also revealed that during physiological activation of the 727–736.
visual system, a transient lactate peak is observed in [5] J. Frahm, G. Kruger, K.-D. Merboldt, A. Kleinschmidt, Dynamic¨
112 P.J. Magistretti / Brain Research 886 (2000) 108 –112
during focal brain activation in man, Magn. Reson. Med. 35 (1996) activity to glucose utilization, Proc. Natl. Acad. Sci. USA 91 (1994)
143–148. 10625–10629.
1 1
[6] A.E. Fray, R.J. Forsyth, M.G. Boutelle, M. Fillenz, The mechanisms [19] L. Pellerin, P.J. Magistretti, Glutamate uptake stimulates Na / K -controlling physiologically stimulated changes in rat brain glucose ATPase activity in astrocytes via activation of a distinct subunit and lactate — a microdialysis study, J. Physiol. 496 (1996) 49–57. highly sensitive to ouabain, J. Neurochem. 69 (1997) 2132–2137. [7] C. Golgi, Sulla fine anatomia degli organi centrali del sisterna [20] L. Pellerin, G. Pellegri, J.-L. Martin, P.J. Magistretti, Expression of
nervoso, Milano, Hoepli, 1886. monocarboxylate transporter mRNA in mouse brain: Support for a [8] B. Hassel, A. Brathe, Cerebral metabolism of lactate in vivo: distinct role of lactate as an energy substrate for the neonatal vs. evidence for neuronal pyruvate carboxylation, J. Cereb. Blood Flow adult brain, Proc. Natl. Acad. Sci. (USA) 95 (1998) 3990–3995. Metab. 20 (2000) 327–736. [21] A. Peters, S.L. Palay, H. de F. Webster, The Fine Structure of the [9] H.K. Kimelberg, M.D. Norenberg, Astrocytes. Sci. Am. 260 (1989) Nervous System: Neurons and Their Supporting Cells, W.B.
Saun-44–52. ders, Philadelphia, 1991.
[10] J.D. Laughton, Y. Charnay, B. Belloir, L. Pellerin, P.J. Magistretti, [22] M.E. Phelps, S.C. Huang, E.J. Hoffman, C. Selin, L. Sokoloff, D.E. C. Bouras, Differential messenger RNA distribution of lactate Kuhl, Tomographic measurement of local cerebral glucose metabol-dehydrogenase LDH-1 and LDH-5 isoforms in the rat brain, ic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation Neuroscience 96 (2000) 619–625. of method, Ann. Neurol. 6 (1979) 371–388.
1
[11] P. Lipton, K. Robacker, Glycolysis and brain function: [K ]0 [23] J. Prichard, D. Rothman, E. Novotny, E. Petroff, T. Kuwabara, M.
1
stimulation of protein synthesis and K uptake require glycolysis, Avison, A. Howseman, C. Hanstock, R. Shulman, Lactate rise
1
FASEB J. 42 (1983) 2875–2880. detected by H NMR in human visual cortex during physiologic [12] P.J. Magistretti, L. Pellerin, D.L. Rothman, R.G. Shulman, Energy stimulation, Med. Sci. 88 (1991) 5829–5831.
on demand, Science 283 (1999) 496–497. [24] A. Rohlmann, J.R. Wolff, Subcellular topography and plasticity of [13] S. Morgello, R.R. Uson, E.J. Schwartz, R.S. Haber, The human gap junction distribution on astrocytes, in: D.C. Spray, R. Der-blood–brain barrier glucose transporter (GLUT1) is a glucose mietzel (Eds.), Gap Junctions in the Nervous System, R.G. Landes, transporter of gray matter astrocytes, Glia 14 (1995) 43–54. Austin, 1996, pp. 175–192.
[14] J. O’Kusky, M. Colonnier, A laminar analysis of the number of [25] C.S. Roy, C.S. Sherrington, On the regulation of the blood supply of neurons, glia and synapses in the visual cortex (area 17) of the adult the brain, J. Physiol. (Lond.) 11 (1890) 85–108.
macaque monkey, J. Comp. Neurol. 210 (1982) 278–290. [26] A. Schurr, C.A. West, B.M. Rigor, Lactate-supported synaptic [15] S. Ogawa, D.W. Tank, R. Menon, J.M. Ellermann, S.G. Kim, H. function in the rat hippocampal slice preparation, Science 240
Merkle, K. Ugurbil, Intrinsic signal changes accompanying sensory (1988) 1326–1328.
14
stimulation: Functional brain mapping with magnetic resonance [27] L. Sokoloff, M. Reivich, C. Kennedy et al., The [ C]deoxyglucose imaging, Proc. Natl. Acad. Sci. USA 89 (1992) 5951–5955. method for the measurement of local cerebral glucose utilization: [16] W.M. Pardridge, W.H. Oldendorf, Transport of metabolic substrates theory, procedure, and normal values in the conscious and
anes-through the blood–brain barrier, J. Neurochem. 28 (1977) 5–12. thetized albino rat, J. Neurochem. 28 (1977) 897–916.
[17] J.C. Parker, J.F. Hoffman, The role of membrane phosphoglycerate [28] S. Takahashi, B.F. Driscoll, M.J. Law, L. Sokoloff, Role of sodium in the control of glycolytic rate by active cation transport in human and potassium ions in regulation of glucose metabolism in cultured red blood cells, J. Gen. Physiol. 60 (1967) 893–916. astroglia, Proc. Natl. Acad. Sci. USA 92 (1995) 4616–4620. [18] L. Pellerin, P.J. Magistretti, Glutamate uptake into astrocytes [29] M. Tsacopoulos, P.J. Magistretti, Metabolic coupling between glia