DEVELOPMENT OF POLYETHYLENE-CaCO
3POLYMER
COMPOSITE WITH ADDITION OF SILANE AS COUPLING AGENT
Deswita, Indra Gunawan, Sudirman
Center for Technology of Nuclear Industry Materials Kawasan Puspiptek Serpong, Tangerang 15314
e-mail: [email protected]
ABSTRACT
This research was conducted to determine the effect of adding silane as coupling agent on the physical and mechanical properties of polyethylene-CaCO3 composites. Composite manufacturing processes were carried out with blending method by using Labo Plastomill with various concentrations of CaCO3 and the coupling agent 3-aminopropiltrietoksi silane (3-APE) at speed of 30 rpm for 10 minutes. Analysis of mechanical properties such as tensile strength, elongation at break and hardness as well as the physical nature such as melting point of polyethylene were done before and after the addition of 3-APE. The obtained results showed that increasing 3-APE can lower tensile strength and elongation at break of the composites compared with pure polyethylene. Hardness increased with increasing concentration of 3-APE added to the composite. While the thermal properties showed that with the addition of 3-APE, the melting point of the composite decreased. It means that the tensile strength becomes lower due to the decreasing the crystalline of polyethylene. FT-IR spectral data showed that 3-APE has bonded with polyethylene composites, namely the emergence of a stretching Si-O-Si at the wave number 1123.811 cm-1. Absorption peak at wave numbers around 3400 cm-1 is the absorption of OH and Si-OH, these peaks overlap with each other amine peak of 3-APE. This spectrum shows that the 3-APE reacted with CaCO3.
Keywords: polymer composites, polyethylene, CaCO3, coupling agent, silane, FTIR.
INTRODUCTION
Polyethylene is a type of plastic widely used in consumer goods. This is because polyethylene has many advantages, such as practical, lightweight, can be colored so that it looks attractive, flexible at low temperatures and resistant to chemicals. Polyethylene could be classified as the type of thermoplastic polymer, a material that will become soft when heated and hardens again when cooled. These behaviors can occur repeatedly, without decrease the starting material properties
222
Polyethylene composite is the result of mixing between the polyethylenes as a matrix with another type of material as filler. Filler is added to the matrix to reduce the volume of matrix in composite materials. Cheap filler that will cause lower price of the final product will benefit producers and consumers.
One of the filler materials that can be used in the manufacture of polyethylene composite is CaCO3. This is because CaCO3 does not cause toxicity in humans or animals and do not generate odors. By its nature a composite of polyethylene-CaCO3 can be applied to various products that are safe for health. Relatively small particle size is also the reason for the use of CaCO3 in the manufacture of composites. This is because the small particle size can lead to CaCO3 more easily distributed into polyethylene than other types of materials that have a larger particle size. In addition, the presence of CaCO3 is very abundant in Indonesia so it is easy to get. CaCO3 has also been widely used in various industrial fields because it's cheap.
Although the manufacture of polyethylene composites provide benefits to producers and consumers, the use of CaCO3 can also cause a decline in physical and mechanical properties of polyethylene. This is due to weak interfacial adhesion between the polyethylene and CaCO3, thus it needs to use a coupling agent. By using a coupling agent, the interfacial adhesion between the matrixes with the filler material can be improved. This is because of the coupling agent can assist the formation of bonding between the matrixes with filler. In composite materials, molecules of coupling agent can bind to the matrix at one end and that of filler at the other end. With the bonding at the interface, the composite will have better physical and mechanical properties [2].
One of the coupling agents that can be used to improve the interfacial adhesion between the matrix and filler is silane. Silane as a coupling agent in the manufacture of composite provides a number of advantages. Mechanical properties especially tensile strength, flexibility and compressive strength increased significantly with the use of silane [2]. The use of silane as a coupling agent can also increase the modulus and hardness and lower melting point, but did not change the tensile strength of HDPE-talc composites [3]. Other studies describe that the addition of three aminopropiltrietoksi silane (3-APE) provides various influences on perlite-filled HDPE composites. Influence happens depends on the nature of HDPE used, such as melt index flow, density, and branching. For HDPE with a higher density, the addition of 3-APE could improve the tensile strength, but it was not significant. While for HDPE with a lower density and short branches, the addition of 3-APE lowered the tensile strength. It is due to the branches which are contained in HDPE [4]. The addition of 3-APE on HDPE composites rubber wood (a powder) increased the tensile strength and the elongation at break of composite. However, increasing the concentration of 3-APE lowered the tensile strength [5].
Based on the above reasons, we conducted research to determine the effect of addition of 3-aminopropiltrietoksi silane as coupling agent on physical and mechanical properties of polyethylene-CaCO3 composites.
EXPERIMENTAL METHOD
The materials used are Linear Low Density Polyethylene (LLDPE) produced by PT Titan Petrochemicals Indonesia which acts as a matrix, CaCO3 as filler, 3-aminopropiltrietoksi silane as a coupling agent, liquid nitrogen, and mica plastic from Yasicha.
223 In this research, the manufacture of polymer composites in which polyethylene as a matrix, and CaCO3 as filler, the concentration of CaCO3 was varied from 30%w/w up to 70%w/w (5% interval, total weight 40 grams), to create a composite of LLDPE-CaCO3 , process was begun by entering the fointo Labo Plastomil. Firstly LLDPE is allowed to warm up and milled at a temperature of 130o C to melt completely. CaCO3 are then added slowly while continuing to mill. Having been added CaCO3, Labo Plastomil closed and left for 10 minutes with 30 rpm rotational speed.
The results obtained by using Labo Plastomil were in form of composite. Then do the downsizing measure into small sheet with scissors, was done 10 grams small sheets of composite were placed inside the size of the printing press 15x15 cm film, and then inserted into the hot pressing machine with a pressure of 150 kg/cm2 at a temperature of 130o C for 5 minutes. After five minutes the sheet of hot press removed then inserted into the cold press with a pressure of 5 tons for 2 minutes. Plastic film is then formed in the dumb bell shape using the mold tool Die-Cut based on the ASTM D-1822-1, a total of five pieces which can be used for testing physical and mechanical properties.
The addition of 3-APE is done in the methods for making polyethylene-CaCO3 composites without the addition of 3-APE. The CaCO3 is first mixed with a solution of 3-APE 20% respectively as much as 5 ml, 10 ml, and 15 ml. Mixing process was conducted by stirring and then dried in the open air for 10 minutes.
RESULTS AND DISCUSSION
a. Mechanical properties of composites
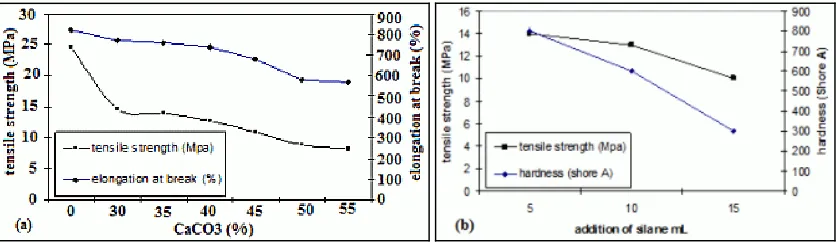
Composite tensile test results of LLDPE-CaCO3 before the addition of 3 - APE is shown in Figure 1(a). Based on the data obtained, the addition of CaCO3 concentrations as much as 30 % caused the tensile strength LLDPE composites decreased by 40.3 % and continued to decline drastically to 74.9 % in the addition of as many as 70 % CaCO3. Decreasing tensile strength of composites of LLDPE-CaCO3 because of LLDPE is a material which is non polar providing compatibility of the less well between both materials in the mixing. In addition, the bond formed between them is weak. Interactions that occur between LLDPE with CaCO3 is only physical interaction without chemical bonding so that only the interfacial adhesion LLDPE-CaCO3 formed, resulting in the transfer of inter-phase tension of the mixture becomes obstructed and there is concentrated tension in the interfacial region of LLDPE-CaCO3. This caused the formation of craze areas that generated from the interfacial region. Craze areas that is formed will be increased in line with the increasing concentration of CaCO3.
Figure 1. Tensile strength and elongation at break. (a) LLDPE-CaCO3 composite before addition of 3-APE.
224
LLDPE-CaCO3 surface contact as the inception of the craze areas when the loading applied can be reduced by the formation of interfacial adhesion strength. Interfacial adhesion formation by using the coupling agent should be able to increase the tensile strength of composites. However, in this study the tensile strength of composites of LLDPE-CaCO3 decreases with the addition of coupling agent. It can be seen from the Figure 1(b). Addition of 3-APE are decreasing the composite tensile strength. Decrease in tensile strength occurs in composites due to the weak chemical bond formed between the composite with 3-APE. Hydrolysis which occurred on 3-APE to form a silanol group with ethanol was not accompanied by the addition of acid, so that the silanol group formed less stable. Hydrolysis of silane to form stable silanol group can occur at around pH 3.
Another factor that affected the decreasing tensile strength of composites is the agglomeration of CaCO3 in a matrix of LLDPE. The formation of agglomerates causing the mechanical properties of composites becomes lower. The agglomeration of CaCO3 distributed regularly in the composite filler materials was caused by the small particle of CaCO3. Filler with a large surface area will provide more contact between the filler and matrix, so that the mechanical properties of the composite increases. Small particles have large surface areas compared with large ones. But the filler with fine particles tend to agglomerate which can be detrimental to mechanical properties, especially the impact of the strength of these composites.
The addition of 3-APE as much as 5 ml caused the tensile strength decreased by 5.3 % in the composite of LLDPE. Composite tensile strength of LLDPE with the addition of 3-APE at the same volume, both when the concentration of CaCO3 30 % and 70 % decreasing compared to before the addition of 3-APE. However, the decline was not significant.
The 3-APE hydrolysis reaction to form silanol group are shown below:
In LLDPE composites with CaCO3 concentration of 30 %, the addition of 3-APE were 5 ml causing tensile strength decreased by 5.3 % while the addition of 3-APE as much as 15 ml, tensile strength decreased by 34.6 %. This means that increasing 3-APE added to the composite, the tensile strength would decrease. This decrease was due to the amount of coupling agent has exceeded the number of existing polyethylene matrix that resulted in coupling reactions agent follow with the warming effect.
Elongation at break
Elongation at break of LLDPE-CaCO3 composite decreases with increasing concentration of CaCO3, as shown in Figure 1. Elongation at break of LLDPE-CaCO3 composite decreased drastically at a concentration of 30 % CaCO3. This means that increasing the concentration of CaCO3 in the composite will decrease the elongation at break of LLDPE-CaCO3 composites. Decrease in elongation at break which occurs because when loading the pull, the greater the concentration of CaCO3 greater the occurrence of potential snag early on CaCO3 surface contact with LLDPE because a very large density differences between CaCO3 and LLDPE. In addition, the contact interface of CaCO3 with the adhesion of LLDPE occurs so that the knot is relatively less strong.
225 amount added is relatively small, the plasticizing agent is acting as an anti plasticizer that may reduce the elongation at break.
At the concentration of CaCO3 30 %, the addition of 3-APE at the same volume caused an increased elongation at break of LLDPE composites. Elongation at break of LLDPE composite at 30 % concentration of CaCO3 by the addition of 5 ml 3-APE were increased compared to before the addition of 3-APE. However, an increase was not significant. While at the concentration of CaCO3 70 % elongation at break decreased significantly. This difference occurs because the occurrence of agglomeration CaCO3 in LLDPE matrix. CaCO3 agglomerates distributed regularly in the composite. Small particle size of filler has caused a subtle way that particles tend to agglomerate (agglomeration). Increasing the concentration of CaCO3 will increase the occurrence of potential snag early on CaCO3 surface contact with LLDPE due to a very large density differences between CaCO3 and LLDPE.
In LLDPE composites with CaCO3 concentration of 30 %, the addition of 3-APE were 5 ml causing elongation increased by 1.3 % while the addition of 3-APE as much as 15 ml, elongation at break decreased by 61 %. This means increasing 3-APE added to the composite, the lower the elongation at break. This decrease was due to the amount of coupling agent has exceeded the number of existing polyethylene matrix that resulted in coupling reactions agent follow with the warming effect. Different things happen on LLDPE composites with a concentration of 70 % CaCO3. In this composite elongation increased again on addition of 3-APE as much as 15 ml. This is because the distribution of CaCO3 in the composite is not uniform.
Hardness
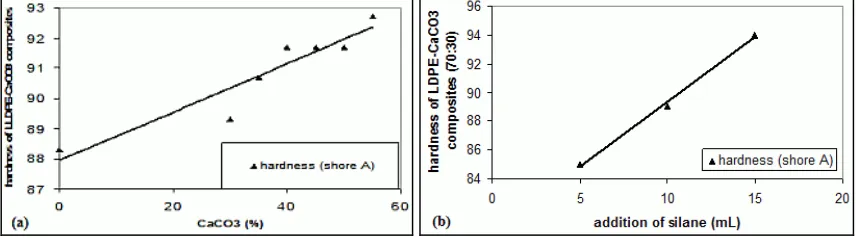
Hardness Test carried out by using the hardness Shore A. Shore A values indicate the distance / depth of penetration of the indenter on the surface of the test material. Results with a Shore A hardness measurement shown on the Figure 2(a). The figure show that hardness on composites increased with increasing concentration of CaCO3. This is because the CaCO3 has a greater density than LLDPE, thus CaCO3 as filler could increase the hardness of composite. This means that CaCO3 gave the same effect in increasing hardness on composites.
Figure 2: Hardness measurement (a). LLDPE-CaCO3 composite before addition of 3-APE. (b) LLDPE-CaCO3
composites (70:30) as the addition of 3-APE.
226
hardness of the composite will increase. In LLDPE composites with CaCO3 concentration of 30 %, the addition of 3-APE as much as 5 ml causes the hardness increased by 5.6 % while the addition of 3-APE as much as 15 ml, hardness increased by 6.7 %. Increasing hardness is because the more volume of 3-APE were added then the greater the effect of 3-APE in acting as an anti-plasticizer.
b. Thermal properties analysis with Differential Scanning Calorimetry (DSC)
Thermal properties analysis was conducted to determine the effect of adding 3-APE on physical properties, namely the melting point of LLDPE-CaCO3 composite. The melting point of pure LLDPE is at 121 ° C.
Addition of CaCO3 caused melting point of LLDPE composites decreased, because of the addition of CaCO3 can reduce the bonding energy between the polymer chains so that the crystalline regions of LLDPE decreased. Increasing CaCO3 added to the composite, the crystalline regions of the composite decreased, result in decreasing the melting point. Addition of CaCO3 was also decrease the energy requirement to separate the polymer chains in the composite. The energy required by composite of LLDPE with 30 % CaCO3 is around 33.76 J/g.
Addition of 3-APE also decreases the melting point of the composite. In LLDPE melting point decreased to 115.48 from 122.84 ° C. This decreasing melting point was due to 3-APE prevent the mobility of LLDPE composites so that the degree of crystallinity of LLDPE was increased. However, the effect of 3-APE in preventing the mobility of the composites is relatively low and therefore decreasing the melting point was also not significant. Addition of 3-APE can also reduce the energy required to separate the polymer chains in the composite. Decreasing energy that occurs is proportional to the decrease in melting point. In LLDPE composites with 30 % CaCO3 concentration, the addition of 3-APE as much as 5 ml caused the energy required by composite of LLDPE decreased from33.76 J / g to 32.32 J / g.
In LLDPE composites with the same concentration of CaCO3 is 70 %, the increasing 3-APE added then the melting point and the energy required to separate the polymer chains will also be lower. The addition of 5 ml 3-APE the melting point is 117.74 - 122.55 ° C and the amount of heat needed is 13.99 J / g. While the addition of 15 ml 3-APE the melting point is 117.63 - 122.06 ° C and the amount of energy needed is 12.66 J / g. This decrease was due to 3-APE prevent mobility so that the degree of crystallinity of LLDPE become lower and the tensile strength was also lower.
c. Functional Group Identification by Fourier Transform Infra Red (FT-IR)
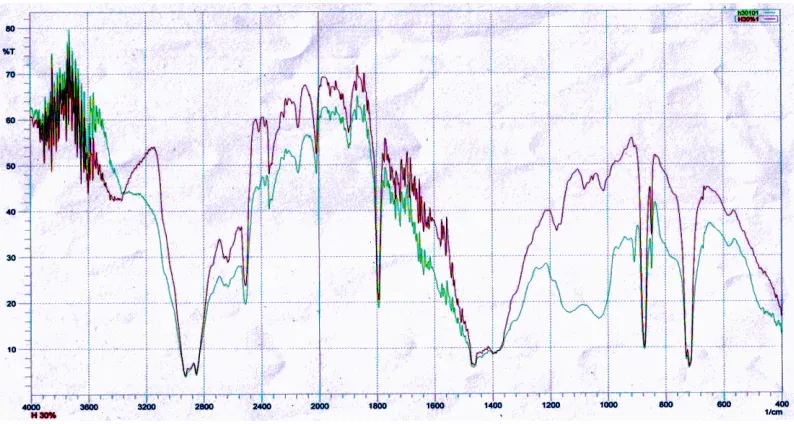
Polyethylene, CaCO3, 3-APE and polyethylene-CaCO3 composite before and after the addition of 3-APE each identified its functional group by using FT-IR. Based on data from FT-IR spectrum of LLDPE are very aesthetically in the appendix, we see the CH stretching absorption band for the CH3, CH2, and CH at wave numbers around 3200-2600 cm-1, CH bending absorption band found at wave numbers around 1500 to 1420 cm- 1, CH2 bending absorption band at wave number 1400-1340 cm-1 and bending absorption band of C-CH3 at wave numbers 1500-1275 cm-1.
227 After 3-APE added to polyethylene-CaCO3 composites the presence of wave number of 1123.11 which is associated as the stretching vibrations of Si-O-Si appears. Peaks at wave numbers around 3400 cm-1 can be associated as OH absorption. These peaks overlap with each other amine peak of 3-APE.
Figure 3: The FTIR spectrum of LLDPE-CaCO3 composite before and after addition of 3-APE.
CONCLUSION
1. Addition of 3-APE caused tensile strength, elongation at break and the melting point of LLDPE-CaCO3 composite decreased. However, the hardness of the composite increased. 2. The increasing concentration of 3-APE added to the composite, the tensile strength,
elongation at break and a melting point of the composite would decrease, but the hardness will increase.
REFERENCES
[1]. COWD, M.A, “Kimia Polimer”. Terjemahan : Harry Firma. Bandung : ITB Press, (.1991).
[2]. PLUEDDELMANN, E. P.. “Coupling and Interfacial Agents and Their Effect on Mechanical Properties”. Elsivier Applied Science Publisher, New York, (1986).
[3]. AKIN-ÖKTEM, GΫLSU AND TINCER. Preparation and Characterization of Perlite-Filled High-Density Polyethylene. I. Mechanical Properties. Journal of Apllied Polymer Science 54, (1994),1103-1114.
[4]. TIEQI LI, GUANGCONG LIU AND KUN QI. “Reconsideration of the Effect of a Coupling Agent in Talcum-Filled Plastics”. Journal of Apllied Polymer Science, 67, (1998), 1227-1236.