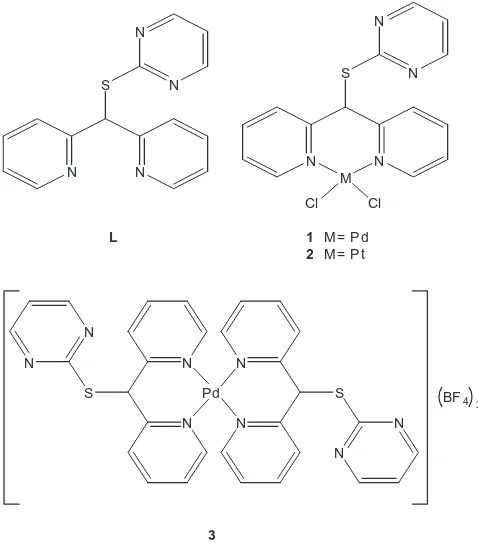
Six-membered ring chelate complexes of Pd(II) and Pt(II) derived from
di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane
Gabriela Rodríguez-García
a, Noemí Andrade-López
a,⇑, José G. Alvarado-Rodríguez
a,
Diego Martínez-Otero
a, Rafael Moreno-Esparza
b, Marcos Flores-Álamo
baCentro de Investigaciones Químicas, Universidad Autónoma del Estado de Hidalgo, Ciudad Universitaria, Carretera Pachuca-Tulancingo km 4.5, Colonia Carboneras, Mineral de la Reforma, Hgo. C.P. 42076, Mexico
bFacultad de Química (UNAM), Edificio B. Ave. Universidad 3000, C.P. 04510, Coyoacán, Mexico D.F., Mexico
a r t i c l e
i n f o
Article history:
Received 21 January 2011 Accepted 15 February 2011 Available online 26 February 2011
Dedicated to Professor Raymundo Cea Olivares on the occasion of his 60th birthday
Keywords:
NMR
X-ray diffraction Pd(II) and Pt(II) complexes Di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane
a b s t r a c t
Cis-[MLCl2] complexes of di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane ligand (L), where M = Pd (1), and
M = Pt (2) have been synthesized. Reaction of1withLin presence of Na[BF4] and hot acetonitrile
pro-duced the complex [PdL2](BF4)2(3). Complexes1–3and ligandLhave been characterized by elemental
analyses, IR and NMR spectroscopy. Crystal structures of1,3andLwere determined by single crystal X-ray diffraction analyses, showing nonplanar structures with the pyridinic rings twisted around the bridging carbon and theipsocarbon bonds.1and3displayed a bidentate coordination ofLto the palla-dium atom with the formation of six-membered chelate rings, where the local geometry at pallapalla-dium atom was distorted square planar. In3the palladium atom was coordinated to two dipyridyl ligands through two of the pyridinic nitrogen atoms to form a cationic complex stabilized by two tetrafluorobo-rate counter-ions.
Ó2011 Elsevier Ltd. All rights reserved.
1. Introduction
A great variety of complexes of Pd(II) and Pt(II) containing dipyridyl ligands with a bridging group have been utilized as cata-lysts in modern organic synthesis[1–5]. In these complexes has been described the formation of five- and six-membered chelate rings where the dipyridyl ligands are mainly coordinated in a bidentate mode. In the dipyridyl complexes of Re(I)[6,7], Fe(III)
[8], Zn(II)[9,10], Pd(II)[11,12], Pt(II)[13]and Rh(I)[14], the size of the chelate ring is influenced by the structural features of the li-gands; thus in di-2-pyridyl ligands with a rigid bridging group the formation of five-membered chelate rings is favored [5,6,11,14]
while with more flexible groups six-membered chelate rings are formed[7–14]. In some Pt(II) analog complexes, the chelate ring size has been related with the reactivity of these complexes to-wards oxidative addition reactions[15,16]. On the other hand, sev-eral structural aspects as intermolecular interactions by hydrogen bonding[17,18], as well as the absence of the steric hindrance around the nitrogen atoms of the pyridinic rings in complexes of Pd(II) and Pt(II) of dipyridyl ligands[13,19]have been associated with anticarcinogenic activity.
Due to the importance of the structural studies of the Pd(II) and Pt(II) complexes of dipyridyl ligands and continuing with our stud-ies in coordination chemistry of 10 group elements[11,12,20], we report herein the structural study in solution and solid state of complexes 1–3 derived from the di-(2-pyridyl)pyrimidin-2-yls-ulfanylmethane ligand (L), in order to evaluate the formation of six-membered chelate rings,Fig. 1.
2. Experimental
2.1. Reagents and general procedures
All manipulations of air and moisture sensitive materials were carried out under dinitrogen using Schlenk techniques un-less otherwise stated. Solvents were dried by standard methods with appropriate reagents and distilled prior their use. Di-(2-pyr-idyl)ketone, 2-mercaptopyrimidine, PdCl2, PtCl2 and Na[BF4]
were commercially obtained (Aldrich) and used as received. Melting points were recorded on a Mel-Temp II apparatus and reported without correction. Elemental analyses were performed on a Perkin–Elmer Series II CHNS/O Analyzer 2400. IR spectra were recorded on a FT-IR 200 Perkin–Elmer spectrophotometer in the 4,000–400 cm 1 range using KBr pellets. Raman spectra
in solid state were recorded in the 4,000–100 cm 1 range on a
0277-5387/$ - see front matterÓ2011 Elsevier Ltd. All rights reserved.
doi:10.1016/j.poly.2011.02.028
⇑Corresponding author. Tel./fax: +52 771 71 72000. E-mail address:[email protected](N. Andrade-López).
Contents lists available atScienceDirect
Polyhedron
Perkin–Elmer Spectrum GX NIR FT-RAMAN spectrophotometer with 10–280 mW laser power and 4 cm 1resolution. NMR
spec-tra were recorded on a Jeol GSX 400 and Varian VNMRS 400 spectrometers;1H (399.78 MHz) and13C{1H} (100.53 MHz)
spec-tra were obtained in DMSO-d6 solution. Chemical shifts (ppm) are relative to the frequency of TMS, seeTable 1. NMR unequiv-ocal assignments of the1H and13C{1H} chemical shifts in
Land the complexes 1and 2 were made by two-dimensional hetero-nuclear and homohetero-nuclear correlation experiments (hetcor, cosy and noesy), while for3were made by two-dimensional homonu-clear correlations experiments (cosy and noesy). The single-crys-tal X-ray structure determination of the L and the complex 1 were performed at room temperature on a CCD SMART 6000 dif-fractometer using Mo K
a
radiation (k= 0.71073 Å, graphite monocromator). Data were integrated, scaled, sorted, and aver-aged using theSMARTsoftware package. X-ray diffraction data of3 were collected on an Oxford Diffraction GEMINI CCD diffrac-tometer at 140 K with graphite-monochromated Mo Ka radiation (k= 0.71073 Å). Data were integrated, scaled, sorted, and aver-aged using the CRYSALISsoftware package [21].
The structures were solved by direct methods, usingSHELXTLNT
Version 5.1 and refined by full-matrix least squares against F2
[22]. All non-hydrogen atoms were refined anisotropically. The po-sition of the hydrogen atoms were kept fixed with a common iso-tropic displacement parameter. Crystallographic data are given in
Table 3and selected bond and angles are given inTable 4.
2.2. Synthesis of di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane (L)
A mixture of di-(2-pyridyl)chloromethane (0.871 g, 4.26 mmol) and 2-mercaptopyrimidine (0.477 g, 4.26 mmol) in 20 mL of dry CH3OH was refluxed for 24 h. Removal of the solvent under vacuum
was followed by addition of 20 mL of water and acidified with HCl 2 M and stirred for 1 h. The aqueous layer was neutralized with K2CO3and extracted with 20 mL of chloroform; this solution was
fil-tered and dried by means of a column filled with anhydrous Na2SO4.
The slow evaporation of the solvent yielded a yellow solid. From a saturated solution of acetonitrile were obtained colorless crystals by slow evaporation. Yield: 80%, (0.954 g). M.p.: 79–80°C. IR:
m
(cm 1) = 1,639 (C@N); 1,585, 1,462 (C@C). Anal. Calc. for C15H12N4S: C, 64.26; H, 4.31. Found: C, 64.39, H, 4.37. EI:m/z281
[M++1].
2.3. Synthesis of complexes1–3
2.3.1. Cis-dichloro{di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane}palladium(II) (1)
To a solution of PdCl2(0.063 g, 0.357 mmol) in 20 mL of hot
ace-tonitrile were added 0.100 g (0.357 mmol) ofL. The mixture was refluxed for 24 h and cooled to room temperature to give a yellow solution. The slow evaporation of the solvent yielded a yellow solid that was washed with 20 mL of chloroform and 20 mL of diethyl-ether. Complex2 was obtained as red crystals from a saturated solution of dimethylsulfoxide. Yield: 98% (0.159 g). M.p.: 235– 236°C (dec.). IR:
m
(cm 1) = 1,627 (C@N); 1,559 1,438 (C@C); 455 (Pd–N); 339 (Pd–Cl). Anal. Calc. for C15H12N4SPdCl20.5H2O: C,38.60; H, 2.81. Found: C, 38.58, H, 2.45. FAB+:m/z423 [M+–Cl]. Compound2was prepared following a similar procedure for the formation of the complex1.
2.3.2. Cis-dichloro{di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane}platinum (II) (2)
PtCl2 (0.075 g, 0.285 mmol) in hot acetonitrile (20 mL) and
(0.080 g, 0.285 mmol) of L. The mixture was stirred; after 24 h was observed the formation of a brown solution. The slow evapo-ration of the solvent yielded a brown solid that was washed with 20 mL of chloroform and 20 mL of diethylether. Yield: 80% (0.124 g). M.p.: 202–204°C (dec.). IR:
m
(cm 1) = 1,607 (C@N); 1,546, 1,477 (C@C); 446 (Pt–N); 334 (Pt–Cl). Anal. Calc. for C15H12N4SPtCl21/3CHCl3; C, 31.42; H, 2.12. Found: C, 30.97; H,2.08. FAB+:m/z546 [M+].
2.3.3. Bis-{di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane}palladium(II) tetrafuoroborate (3)
To a solution of1(0.100 g, 0.218 mmol) in 30 mL of hot acetoni-trile were added 0.480 (0.437 mmol) of Na[BF4]. The mixture was
stirred and refluxed for 1 h. To the mixture were added 0.061 mL (0.218 mmol) ofLand refluxed for 24 h. The mixture was filtered and a yellow solution was obtained. The evaporation of the solvent yielded a yellow solid that was washed with 20 mL of chloroform and 20 mL of diethylether. The complex3was obtained as yellow crystals from a saturated mixture of acetonitrile–acetone–water in a 1:1:1 ratio using a total volume of 25 mL. Yield: 90% (0.165 g). M.p.: 181°C (dec.). IR:
m
(cm 1) = 1,605 (C@N); 1,555, 1,483 (C@C); 1,057 (B–F); 462 (Pd–N). Anal. Calc. for C30H24N4S2PdB2F8CHCl3: C38.78; H 2.62. Found: C, 38.85; H 2.62. FAB+:m/z755 [M+–BF
4].
3. Results and discussion
3.1. Synthesis of di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane (L)
Lwas prepared from the reaction of di-(2-pyridyl)chlorometh-ane with 2-mercaptopyrimidine in an equimolar ratio, see
Scheme 1.
3.2. Synthesis of the mono-chelate complexes1and2and the bis-chelate complex3
The complexes1 and2 were prepared from the reaction ofL with PdCl2and PtCl2respectively in an equimolar ratio in hot
ace-tonitrile,Scheme 2. Complex3was synthesized from the reaction of1withLin presence of Na[BF4],Scheme 3. Complexes1–3were
Fig. 1.Structures of di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane (L) and the complexes1–3.
obtained as air-stable solids, soluble in dimethylsulfoxide and hot acetonitrile, and insoluble in methanol and chloroform.
3.3. Vibrational spectroscopy
IR spectrum of the free ligand L displayed a weak band at 1,639 cm 1assigned to the
m
(C@N) absorption. For complexes1,2 and3the
m
(C@N) absorption is shifted to lower frequencies with re-spect toLat 1,627, 1,607 and 1,605 cm 1, respectively [11]. The IR spectrum of3showed a band in 1,057 cm-1that was assigned bycomparison to the
m
(B–F) absorption[23]. Raman spectra of the com-plexes 1–3 displayed bands attributable tom
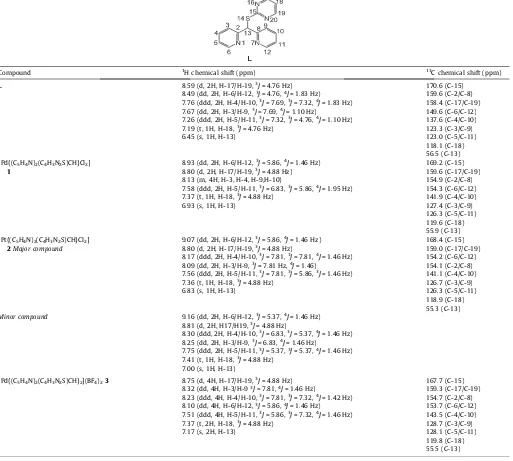
(Pd–N) at 455 and 462 cm–1for were obtained at room temperature in DMSO-d6 solutions; theassignment of the proton and carbon signals is showed inTable 1. In the1H NMR spectra of
2were observed two sets of signals of uneven intensity and have been attributed to the presence of two conformers (vide infra).
1H NMR spectra in
DMSO-d6solutions ofLand the complexes1 and3display only one set of signals, as the two pyridine rings as well as the pyrimidinic halves are magnetically equivalent,Table 1. In2, the1H spectrum showed two sets of signals in a 90:10 ratio;
each set displayed the same magnetic equivalence observed in the
1H NMR spectra of
Land1and3complexes.
In the complexes1–3the H13 proton is shifted at higher fre-quencies with respect toL, reaching a maximum of 0.72 ppm for 3. For the complexes1 and2 all aromatic protons are observed at higher frequencies with respect toL; in3only the H6/H12 pro-tons near to the metallic coordination were observed at lower fre-quencies. The observation of the pyridinic and pyrimidinic protons as averaged signals in1H spectra for the complexes
1–3suggests the formation of the six-membered chelate rings. Moreover, the Table 1
1H and13C{1H} NMR data ofL, and complexes1–3in DMSO-d
6at 20°C. The numbering scheme is showed for the ligandLmoiety in all cases.
Compound 1H chemical shift (ppm) 13C chemical shift (ppm)
L 8.59 (d, 2H, H-17/H-19,3J= 4.76 Hz)
similitude in the variation of the chemical shift (Dd) for the com-plexes 1 and 2 indicates that both complexes display a similar structure.
On the other hand, the presence of just one set of signals in the
1H spectra in
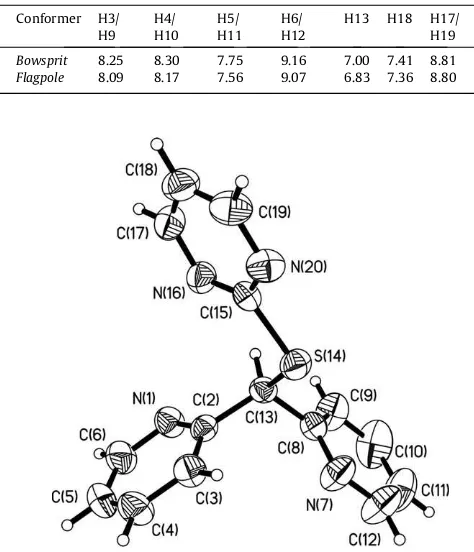
1and3suggests that in solution exists only one pre-dominant conformer; whereas in2the two signal sets of uneven intensity have been attributed to the presence in solution of the conformers inflagpoleandbowspritpositions by comparison with
other platinum complexes derived from dypiridyl ligands [24],
Fig. 2. In the complex2 the proton signals at lower frequencies
were assigned to the conformer inflagpoleposition[24](Table 2),
Fig. 2.
In the1H–1H NOESY experiments of
1,2(for the major set of signals) and3were observed correlation peaks between the methi-nic and the H3/H9 protons at 8.13, 8.09 and 8.32 ppm for com-plexes1,2major and3, respectively. These correlations support the presence of the flagpole conformer in solution as the major one for these complexes.
Compared to the free ligand, in the13C{1H} spectra of complexes
1–3, C2 is the most shielded with Dd of 5.0, 5.5 and 4.9 ppm Table 3
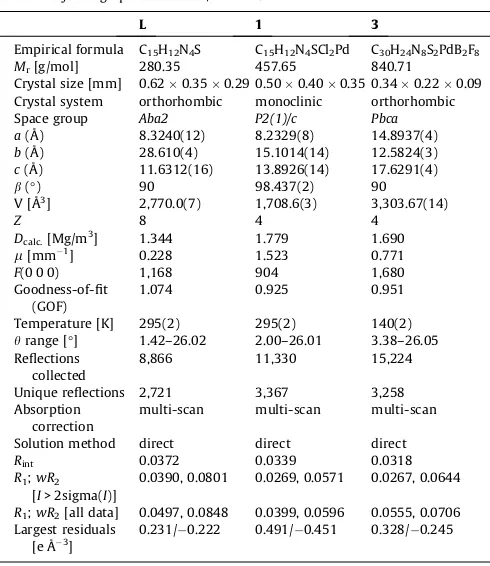
Selected crystallographic data forL,1and3.
L 1 3
Empirical formula C15H12N4S C15H12N4SCl2Pd C30H24N8S2PdB2F8 Mr[g/mol] 280.35 457.65 840.71
Crystal size [mm] 0.620.350.29 0.500.400.35 0.340.220.09
Crystal system orthorhombic monoclinic orthorhombic Space group Aba2 P2(1)/c Pbca a(Å) 8.3240(12) 8.2329(8) 14.8937(4)
b(Å) 28.610(4) 15.1014(14) 12.5824(3)
c(Å) 11.6312(16) 13.8926(14) 17.6291(4) b(°) 90 98.437(2) 90
V [Å3] 2,770.0(7) 1,708.6(3) 3,303.67(14)
Z 8 4 4
Dcalc.[Mg/m3] 1.344 1.779 1.690 l[mm 1] 0.228 1.523 0.771 F(0 0 0) 1,168 904 1,680 Goodness-of-fit
(GOF)
1.074 0.925 0.951
Temperature [K] 295(2) 295(2) 140(2) hrange [°] 1.42–26.02 2.00–26.01 3.38–26.05
Reflections collected
8,866 11,330 15,224
Unique reflections 2,721 3,367 3,258 Absorption
correction
multi-scan multi-scan multi-scan
Solution method direct direct direct
Rint 0.0372 0.0339 0.0318
R1;wR2
[I> 2sigma(I)]
0.0390, 0.0801 0.0269, 0.0571 0.0267, 0.0644
R1;wR2[all data] 0.0497, 0.0848 0.0399, 0.0596 0.0555, 0.0706
Largest residuals
[e Å 3] 0.231/ 0.222 0.491/ 0.451 0.328/ 0.245
Table 4
Selected bond lengths [Å], angles and torsion angles [°] forL,1and3.
Compound L 1 3
M–Cl(1) 2.2875(8)
M–Cl(2) 2.2840(8)
M–N1 2.052(2) 2.0192(19)
M–N7 2.045(2) 2.025(2)
C2–N1 1.345(4) 1.351(3)
C8–N7 1.346(4) 1.349(3)
C2–C13 1.524(3) 1.515(4) 1.504(3) C8–C13 1.512(3) 1.511(4) 1.509(3) S14–C13 1.824(2) 1.831(3) 1.829(2) Cl(1)–M1–Cl(2) 90.03(3)
N1–M1–N7 88.58(9) 93.19(8)
Cl(1)–M1–N1 90.43(7)
Cl(2)–M1–N1 179.01(6)
Cl(2)–M1–N7 Cl(1)–M1–N7
90.96(7) 178.95(6)
N(7)–Pd(1)–N(7)#1 180.0
N(1)–Pd(1)–N7 93.19(8)
N(1)–Pd(1)–N(7)#1 86.81(8)
C2–C13–C8 111.7(2) 111.33(19)
N16–C15–S14 120.7(2) 112.5(2) N20–C15–S14 110.9(2) 119.6(2) N1–C2–C13–C8 58.1(3) 55.8(3)
N7–C8–C13–C2 61.7(3)
N1–C2–C13–S14 151.37(16) 66.0(3) 70.3(2) N7–C8–C13–S14 53.4(2) 58.8(3) 63.7(2)
Symmetry transformations used to generate equivalent atoms: x+ 2, y+ 1, z.
N N Cl
N N
SH
1) ∆, CH3OH
N N S N
N
+
L
2) HCl/ H2O
3) K2CO3
Scheme 1.Synthesis of the di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane (L).
N N
L 1 M= Pd
2 M= Pt S
N N
S
M
Cl Cl MCl2, ∆ CH3CN
N N
N N
Scheme 2.Synthesis of the complexes1and2.
respectively, whereas the C6 carbon was the most deshielded, with Dd of 4.7 for 1 and 4.6 ppm for 2. However, in 3, the C3 (Dd= 5.4 ppm) and C4 (Dd= 5.9 ppm) carbons were the most deshielded. The spectroscopic behavior observed in the complexes 1–3respect to the most deshielded carbon atoms (C6 for1and2)
and (C3 and C4 for3) as well as the shielding of H6/H12 in3 com-pared to1and2are all indicative of the structural differences of1 and2respect to3.
19F{1H} spectrum of
3showed a single signal at 150.1 ppm for the tetrafluoroborate anion, whereas the11B{1H} displayed a signal
at 2.2 ppm. Chemical shifts found are consistent with those re-ported for other tetrafluoroborate palladium (II) complexes[23].
3.5. X-ray crystallography
Crystalline structures in solid state forLand the complexes1 and3were determined by single crystal X-ray diffraction analyses. Compound3 is centrosymmetric. Crystallographic data are sum-marized inTable 3and selected bond lengths and angles are given
inTable 4.
The molecular structure ofLis showed inFig. 3. InLthe pyrid-inic rings are not coplanar; the same arrangement has been ob-served in other dipyridine analogs[11,12,24]. The pyridinic rings are twisted around the bridging C13 atom and the ipsocarbon bonds; taking into account the values of the N–C–C–S torsional an-gles inL, the conformation can be described asanti-synrespect to the geometrical position of the sulfur and the pyridinic nitrogen atoms [151.37(16)° and 53.4(2)° for N1–C2–C13–S14 and N7– C8–C13–S14, respectively][11,12,25]. The crystal structure is sta-bilized by weak C–H S and C–H N hydrogen bonding.
In the complexes1and3, the free rotation about the bridging C13 and theipsoC2/C8 carbon atoms of each pyridinic ring inL
3
1) Na[BF ]4, CH3CN
1
2) L, ∆ 24 h
N N S
Pd Cl Cl
N N
N
N
S
N N
S
N N N
N
Pd (BF4)2
Scheme 3.Preparation of the complex3.
flagpole
H S
Pt
N N
Cl Cl
S H
Pt
N N
Cl Cl
bowsprit
R = pyrim idin-2-yl group R
R
Fig. 2.Chelate rings in the conformers observed in solution of the complex2. (The pyridinic rings have been omitted).
Table 2
1H NMR chemical shifts (ppm) in DMSO-d6for the conformers of2.
Conformer H3/ H9
H4/ H10
H5/ H11
H6/ H12
H13 H18 H17/ H19
Bowsprit 8.25 8.30 7.75 9.16 7.00 7.41 8.81
Flagpole 8.09 8.17 7.56 9.07 6.83 7.36 8.80
Fig. 3.Molecular structure of di-(2-pyridyl)pyrimidin-2-ylsulfanylmethane (L) with displacement ellipsoids at the 50% probability level. Hydrogen atoms are shown as spheres of arbitrary radius.
Fig. 4.Molecular structure of1with displacement ellipsoids at the 50% probability level. Hydrogen atoms are shown as spheres of arbitrary radius.
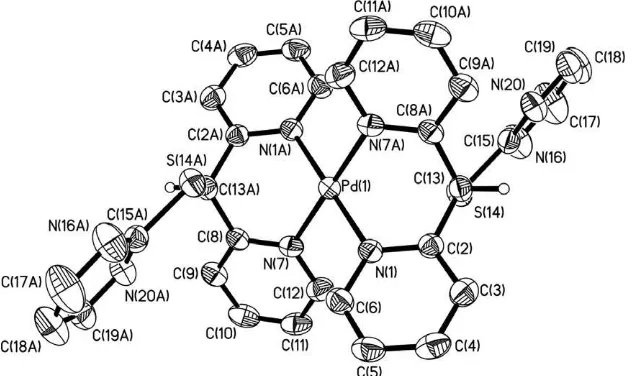
allows to the ligand display a bidentate coordination mode toward the palladium atom, forming a six-membered chelate ring and dis-playing a boat conformation,Figs. 4 and 5.
The local geometry at palladium center in1and3is distorted square planar. In1the N7–Pd–N1 and Cl(1)–Pd–Cl(2) angles are 88.58(9)° and 90.03(3)°; while in 3 the N7–Pd–N1 angles are 93.19(8)°and 86.81(8)°, these angles are similar to other cationic complexes of palladium [26,27]. The distances N–Pd in 1 [2.045(2) and 2.052(2) Å] and3 [2.0192(19) and 2.025(2) Å] are similar to other previously reported for dipyridinic complexes con-taining N–Pd bonds[11,12,19,27,28]. The torsional angles in1are 66.0(3)°and 58.8(3)°, whereas in3 are 70.3(2)°and 63.7(2)°, both complexes display asyn–synconformation in solid state.
In1and3the –S–C4H3N2group is in aflagpoleposition with
intramolecular interactions Pd S of 3.1539(8) Å in 1 and 3.1667(7) Å in3; these distances are shorter than their van der Waals radii sum [PrvdW(Pd, S) = 3.43 Å] but longer than the
cova-lent radii sum [Prcov(Pd, S) = 2.33 Å][11,12,29]. These interactions
enhance theflagpoleconformation and give structural evidence for the situation observed in solution by means of1H NMR for the
complexes1–3; the same short distances have been observed in other palladium complexes.
Acknowledgements
GRG fully acknowledges a scholarship for supporting her stud-ies. NAL fully acknowledges the financial support of the CONACyT (Project 81003) for these investigations.
Appendix A. Supplementary data
CCDC 807805, 807807, and 807806 contain the supplementary crystallographic data forL,1and3, respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/
retrieving.html, or from the Cambridge Crystallographic Data
Cen-tre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or email: [email protected].
References
[1] B. Milani, G. Mestroni, E. Zangrando, Croat. Chem. Acta 74 (2001) 851.
[2] M.E. Wright, C.K. Lowe-Ma, Organometallics 9 (1990) 347.
[3] C. Nájera, J. Gil-Moltó, S. Karlström, L.R. Falvello, Org. Lett. 5 (2003) 1451. [4] J.M. Hoover, A. DiPasquale, J.M. Mayer, F.E. Michael, J. Am. Chem. Soc. 132
(2010) 5043.
[5] J. Yorke, C. Dent, A. Decken, A. Xia, Inorg. Chem. Commun. 13 (2010) 54. [6] J. Grewe, A. Hagenbach, B. Stromburg, R. Alberto, E. Vazquez-Lopez, U. Abram,
Z. Anorg. Allg. Chem. 629 (2003) 303.
[7] M. Bakir, O. Brown, J. Mol. Struct. 641 (2002) 183.
[8] C. Xu-Dong, D. Miao, H. Feng, C. Xiao-Ming, C.W.M. Thomas, Polyhedron 24 (2005) 1047.
[9] M. Lutz, A.L. Spek, P.C.A. Bruijnincx, R.J.M.K. Gebbink, G. van Koten, Acta Crystallogr. E61 (2005) m1813.
[10] Y. Wang, N. Okabe, Inorg. Chim. Acta 358 (2005) 3407.
[11] N. Andrade-López, J.G. Alvarado-Rodríguez, S. González-Montiel, M.G. Rodríguez-Méndez, M.E. Páez-Hernández, C.A. Galán-Vidal, Polyhedron 26 (2007) 4825.
[12] N. Andrade-López, J.G. Alvarado-Rodríguez, S. González-Montiel, M.E. Páez-Hernández, C.A. Galán-Vidal, A. Jiménez-Pérez, Arkivoc (2008) 43.
[13] M.J. Rauterkus, S. Fakih, C. Mock, I. Puscasu, B. Krebs, Inorg. Chim. Acta 350 (2003) 355.
[14] C. Godard, S.B. Duckett, S. Parsons, R.N. Perutz, Chem. Comm. (2003) 2332. [15] F. Zhang, E.M. Prokopchuk, M.E. Broczkowski, M.C. Jennings, R.J. Puddephatt,
Organometallics 25 (2006) 1583.
[16] F. Zhang, M.C. Jennings, R.J. Puddephatt, Organometallics 23 (2004) 1396. [17] C. Tu, X. Wu, Q. Liu, X. Wang, Q. Xu, Z. Guo, Inorg. Chim. Acta 357 (2004) 95. [18] H. Mansuri-Torshizi, T.S. Srivastava, H.K. Parekh, M.P. Chitnis, J. Inorg.
Biochem. 45 (1992) 135.
[19] C. Mock, I. Puscasu, M.J. Rauterkus, G. Tallen, J.E.A. Wolff, B. Krebs, Inorg. Chim. Acta 319 (2001) 109.
[20] N. Andrade-López, T.A. Hanna, J.G. Alvarado-Rodríguez, A. Luqueño-Reyes, B.A. Martínez-Ortega, D. Mendoza-Espinosa, Polyhedron 29 (2010) 2304. [21] Oxford Diffraction (2009) CrysAlis software system, version 1.171.33.31.
Oxford Diffraction Ltd., Abingdon, UK.
[22] Bruker Analytical X-ray Systems. SMART: Bruker Molecular Analysis Research Tool V. 5 .057 c (1997–98); Bruker Analytical X-ray Systems. SAINT + NT Version 6.01, 1999; Bruker Analytical X ray Systems. SHELXTL-NT Version 5.10, 1999.
[23] V. Montoya, J. Pons, J. Garcia-Antón, X. Solans, M. Font-Bardia, J. Ros, Inorg. Chim. Acta 360 (2007) 625.
[24] J. Wilson, J. Fedoce Lopes, S.J. Lippard, Inorg. Chem. 49 (2010) 5303. [25] B. Flores-Chávez, B.A. Martínez-Ortega, J.G. Alvarado-Rodríguez, N.
Andrade-López, J. Chem. Cryst. 35 (2005) 451.
[26] F. Nicoló, G. Bruno, G. Tresoldi, Acta Cryst. C52 (1996) 2188.
[27] E. Guney, V.T. Yilmaz, O. Buyukgungor, Inorg. Chim. Acta 363 (2010) 2416. [28] T. Schareina, G. Hillebrand, H. Fuhrmann, R. Kempe, Eur. J. Inorg. Chem. (2001)
2421.
[29] W.W. Porterfield, Inorganic Chemistry: A Unified Approach, 2nd ed., Academic Press, Inc., USA, 1993. pp. 214.
Fig. 5.Molecular structure of3with displacement ellipsoids at 40% probability level. Hydrogen atoms are shown as spheres of arbitrary radius. Aromatic hydrogens as well as the BF4anions have been omitted for clarity.
G. Rodríguez-García et al. / Polyhedron 30 (2011) 1324–1329