Differential responses of grass and a dwarf
shrub to long-term changes in soil
microbial biomass C, N and P following
factorial addition of NPK fertilizer,
fungicide and labile carbon to a heath
A N D E R S M I C H E L S E N
"
* , E N R I C O G R A G L I A
"
,
I N G E R K . S C H M I D T
"
, S V E N J O N A S S O N
"
, D A R R E N S L E E P
#
C H R I S Q U A R M B Y
#
"
Department of Plant Ecology,
University of Copenhagen,
Øster Farimagsgade 2 D,
DK-1353 Copenhagen K,
Denmark
#
Institute of Terrestrial Ecology,
Merlewood Research Station,
Grange-over-Sands,
Cumbria LA11 6JU,
UK
Received 19 October 1998 ; accepted 1 June 1999
Microbial immobilization may decrease the inorganic nutrient concentrations of the soil to the extent of affecting plant nutrient uptake and growth. We have hypothesized that graminoids with opportunistic nutrient-acquisition strategies are strongly influenced by nutrient limitation imposed by microbes, whereas growth forms such as dwarf shrubs are less affected by the mobilization–immobilization cycles in microbes. By adding NPK fertilizer, labile C (sugar) and fungicide (benomyl) over a 5 yr period in a fully factorial design, we aimed to manipulate the sink–source potential for nutrients in a non-acidic heath tundra soil. After 2 yr, N and P accumulated in the microbial biomass after fertilization with no change in microbial C, which suggests that nutrients did not limit microbial biomass growth. After 5 yr, microbial C was enhanced by 60 % in plots with addition of labile C, which points to C-limitation of the microbial biomass. Microbial biomass N and P tended to increase following addition of labile C, by 10 and 25 %, respectively. This caused decreased availability of NH
%+ and P, showing close
microbial control of nutrient availability. The most common graminoid,Festuca ovina, responded to fertilizer addition with a strong increase, and to labile C addition with a strong decrease in cover, providing the first direct field evidence that nutrient limitation imposed by immobilizing microbes can affect the growth of tundra plants. Also in support of our hypothesis, following addition of labile C the concentrations of N and K in leaves and that of N in roots ofF.ovinadecreased, whilst the demand of roots for P increased. In contrast, the most common dwarf shrub,Vaccinium uliginosum, was only slightly sensitive to changes in resource availability, showing no cover change after 4 yr addition of labile C and fertilizer, and little change in leaf nutrient concentrations. We suggest that the differential responses of the two growth forms are due to differences in storage and nutrient uptake pathways, with the dwarf shrub having large nutrient storage capacity and access to organic forms of N through its mycorrhizal association. While the fungicide had no effect on ericoid mycorrhizal colonization of roots or symbiotic function inferred from plant"&N natural abundance, it decreased microbial biomass C and N after 2 yr. Throughout the fifth season, the availability of soil NO
$−and inorganic P was decreased with no change in
microbial biomass C, N or P, suggesting a negative impact of benomyl on N and P mineralization.
Key words : arctic–alpine ecosystems, benomyl, microbial nutrient immobilization, mycorrhiza, plant"&N natural abundance.
Recent work has emphasized the potential
import-*Author for correspondence (tel 45 35322270 ; fax 45 35322321 ; e-mail andersm!bot.ku.dk).
systems not only supply nutrients to plants through mineralization of soil organic matter, but may also decrease the amounts of inorganic nutrients in the soil during periods of high microbial demand (Jonasson et al., 1996a). Although there is little experimental evidence for this causal relationship in natural ecosystems, some recent studies in the temperate region have shown that plant production
may be adversely affected by microbial
im-mobilization of nutrients from the soil inorganic pool (Schimelet al., 1992 ; Jonassonet al., 1996b).
The supply rate of inorganic nutrients in tundra soils is generally low (Nadelhoffer et al., 1992 ; Rastetter & Shaver, 1992), a feature limiting plant growth (Shaver & Chapin, 1995 ; Chapin & Shaver, 1996 ; Michelsenet al., 1996a). Furthermore, a large proportion of total soil N and P is tied up in the microbial biomass (Cheng & Virginia, 1993 ; Jonassonet al., 1996a ; Michelsenet al., 1996b). In the light of the potential effects of CO
#enhancement and warming on microbial biomass and the possible
consequences for tundra ecosystem function
(Nadelhoffer et al., 1992), it is urgent to integrate investigations of plant and microbial responses to environmental changes. For example, the current increase in the atmospheric concentration of CO
# might lead to increased rhizodeposition of labile C and therefore stimulate microbial activity and biomass. This, in turn, could affect the availability of soil nutrients and the productivity of plants in high latitude and altitude systems (Niklaus & Ko$rner, 1996).
We have previously shown that addition of labile C to tundra soils in spring leads to enhanced microbial biomass C and a decrease in the con-centration of soil inorganic N (Ni) in autumn (Jonassonet al., 1996a). We concluded that microbial biomass can periodically immobilize substantial amounts of nutrients from the inorganic pool. However, the longer-term effects on accumulation of microbial nutrients of adding labile C, and the limitations on microbial growth in general, are virtually unknown for such systems.
Recent studies have shown that nutrient im-mobilization in microbes may decrease graminoid biomass and nutrient pool sizes in monocultures potted in subarctic heath soil (Schmidtet al., 1997a, b). Furthermore, addition of labile C has previously been shown to decrease plant growth and nutrient concentrations (Shaver & Chapin, 1980 ; Marion et al., 1982 ; Yarie & Van Cleve, 1996). However, to our knowledge there is no direct field evidence for a mechanism explaining how microbial immobili-zation can aggravate plant nutrient deficiency in arctic and alpine systems. It is possible that op-portunistic, potentially fast-growing species such as graminoids are strongly sensitive to periodic nutrient limitation imposed by microbes, whereas more slow-growing, woody, ecto- or ericoid mycorrhizal plant
species are less affected by immobilization by saprotrophic microbes. This seems likely because the fungal symbionts of the dwarf shrubs can decompose organic matter and transfer N to the host species (Read, 1993 ; Michelsen et al., 1998), and these plants may have large nutrient reserves which buffer more short-lived changes in nutrient supply. This hypothesis may be tested by evaluating the longer-term effects of nutrient changes in the soil inorganic, dissolved organic and microbial pools (measured after 2 and 5 yr) for plant growth and nutrient uptake in a subarctic heath ecosystem. As model plants, two widespread plant species of different and distinct life forms were chosen : a grass,
Festuca ovina, and a dwarf shrub, Vaccinium uliginosum. By adding NPK fertilizer, labile C (sugar) and fungicide (benomyl) in a fully factorial design, we were able to manipulate the sink-source potential for nutrients in the soil. Benomyl was added in an attempt to decrease the soil microbial biomass (and therefore the immobilizing potential of the microbes) and also to affect the function of mycorrhizal fungi. Previous field experiments with benomyl have focused on its role in decreasing nutrient uptake by arbuscular mycorrhizal plants (Fitter, 1986 ; Koide
et al., 1988 ; Merryweather & Fitter, 1996) and limiting the impact of plant-pathogenic fungi (West
et al., 1993), whereas there has been less emphasis on its potential effects on mineralization and immobili-zation by microbes and the consequences for plant performance. Also, to our knowledge there have been no previous attempts to control ericoid mycor-rhizal function by benomyl addition.
The plants’ performance was investigated by measuring responses at the plot level after 2 and 4 yr, and at the level of individual shoots and roots after 2 yr of treatment. Nutrient concentrations in tissues were analysed in early, mid and late season for F.
ovina, and at mid season forV. uliginosum. To test further for nutrient deficiencies, the absorption of "&N and$#P by excised roots was tested, with a high absorption rate interpreted as a high demand for N or P by the plants (Harrisonet al., 1991 ; Joneset al., 1991 ; Michelsen et al., 1995). Finally, the mycor-rhizal status of the plants was analysed, along with the"&N natural abundance of leaves ofV.uliginosum, in order to investigate treatment effects on ericoid mycorrhizal colonization and function. The "&N abundance of ericoid mycorrhizal species such asV.
Experimental setup
The experiment took place in a low alpine heath just above the tree limit at 450 m elevation, near Abisko in northern Swedish Lapland at 68°21«N, 18°49«E. The nearby climate station records an annual precipitation of about 300 mm, a mean annual air temperature of ®0.5°C, and a mean July tem-perature of 11°C (1961–90). The heath has a 15–20 cm deep layer of organic matter with a bulk density of 0.16 g cm−$and a pH of 7.1.Vaccinium uliginosum
L. is the most common dwarf shrub, and Festuca ovina L. the most abundant graminoid at the site. Other common species are the ericoid dwarf shrubs
Empetrum hermaphroditum Hagerup, Andromeda
polifolia L. and Rhododendron lapponicum (L.) Wahlenb., and the non-ericoid dwarf shrubsDryas octopetala L. and Betula nana L., with scattered forbs and graminoids. The climate is montane sub-arctic, with a growing season of approx. 3 months, from mid}late June to early}mid September.
The experiment was initiated in spring 1993, with the main part of the soil and plant sampling during the second season (1994), and with additional vegetation-cover analysis in 1996 and soil sampling in 1997. The experimental setup consisted of C, benomyl and fertilizer application to 1 m#plots in a fully factorial design, i.e. eight treatments altogether, replicated over six blocks.
Each year in mid-June, 1–3 wk after thawing of the soil and snow melt, the following additions were made : 250 g sugar m−#, 4 g Benlate m−#containing 50 % active substance (benomyl) dissolved in 2 l water, and a mixed NPK fertilizer (5 :1.25 :3.75 g m−#, respectively) consisting of NH
%NO$, NaH#PO% and KCl and dissolved in 2 l water. The treatments were repeated at the end of July each year.
Estimate of plant cover
The cover ofF.ovinaandV.uliginosumin each plot was estimated on 29 July 1993 (i.e. 40 d after the onset of the experiment), on 23 August 1994 (more than 14 months after the experiment was initiated), and again on 23 July 1996. A frame of 50¬50 cm was placed in a fixed position and the number of leaf interceptions of the two species in 100 points per plot noted (Jonasson, 1988).
Shoot, root and soil collection
Shoots and roots of F. ovina were sampled on 29
June, 7 August and 4 September 1994, and V.
uliginosum shoots and roots on 7 August 1994, in each plot by collection of five to ten randomly selected, individual shoots with attached roots
excavated to a depth of about 7 cm. The individual shoots and roots were bulked and brought to the laboratory. Roots were separated from the shoots, washed carefully and blotted dry. The fine roots were used in the nutrient (N and P) absorption bioassays. Live (green and yellow-green but not grey-brown) leaf material of F. ovina and green leaves of V. uliginosum were used for analysis of nutrient element concentrations.
Fine roots ofF.ovinawere either brownish-red or white, probably related to their age. As the treat-ments might affect the relative abundance of these root categories, we additionally tested whether P absorption differed between these kinds of fine roots. Additional fine roots ofF.ovinawere sampled on 4 September as above but further separated into the categories ‘ brown ’ and ‘ white ’.
Soil was collected randomly with an auger within each plot as two 10 cm deep, 4 cm diameter plugs of humus, on 30 August 1994 and again on 23 June, 29 June, 15 July, 22 July and 28 August 1997. In 1997, soil was not sampled in plots with combined treatments. After sampling the soil was kept at 4°C. In 1994 the samples from each plot were bulked and carefully sorted into fine roots, soil and other material (moss pieces, litter, coarse roots, etc.) within 1 d after collection, whereas in 1997 the soil was passed through a 2-mm sieve.
Analysis of leaf weight,area, stem weight and specific leaf area ofV. uliginosum
On 21 August 1994 the nearest dominant V.
uliginosumshoot of a branch, in a randomly selected direction from each of five randomly chosen points, was cut well below the current year’s growth. The shoots were brought to the laboratory and stored at 5°C until analysed. The leaves were separated from the stem and analysed for total leaf mass and leaf area per shoot, mean stem weight per shoot leaf area, and average specific leaf weight per shoot. Leaf area was measured with an image analyser (SI 730, Skye Instruments Ltd, Llandrindod Wells, UK) after which the leaves were oven-dried and weighed.
Analysis of$#P and"&N absorption by excised roots
The fresh, weighed roots of F. ovina were divided into two subsamples for use in the bioassay of P (Harrisonet al., 1991) and N absorption (Joneset al., 1991). The root samples ofV.uliginosumwere very small and sufficed for the P bioassay only.
For the P-deficiency bioassay, roots were placed for 30 min in 5¬10−% mol l−" CaSO
% solution to maintain cell membrane integrity and to leach out physically sorbed P in the root free space. They were then placed for 15 min in a solution of 5¬10 mol l−" CaSO
subse-quently washed for 5 min and counted in glass vials containing 15 ml water for Cerenkov radiation in a Canberra Packard 2000CA liquid scintillation spec-trometer. The roots were removed from the vials, blotted and weighed, and the vials were re-counted to correct for isotope that had not been metabolically absorbed. The activity was corrected for colour quench, background and isotope decay. Root ab-sorption of $#P was calculated as pg P mg−" fresh weight of root 15 min−".
For the N-deficiency bioassay, roots were pre-treated in 5¬10−% mol l−" CaCl
# for 30 min and transferred to 5¬10−%mol l−"CaCl
#containing 1 mg l−"N as NH
%Cl, labelled with"&N (20 % enrichment). They were left in the solution for 2 h, washed for 15 min, dried, weighed and finely cut. Samples of
c. 12 mg root material were analysed for "&N on a Roboprep, a Dumas-type continuous-flow CHN analyser, coupled to a Tracermass isotope ratio mass
spectrometer (both instruments from Europa
Scientific Ltd, Crewe, UK). Excess"&N (at %) was converted to absorption rate of N (in ng N mg−"root 2 h−"). The total N concentration in the roots was also calculated by subtraction of N that had been taken up during the bioassay.
Analysis of plant N,P and K concentration and δ"&N
Samples ofF.ovinaandV.uliginosumwere dried at 80°C to constant weight and finely ground. Leaves ofV.uliginosumwere analysed for total N, P and K and"&N natural abundance. Leaves ofF.ovinawere analysed for total N, P and K, and its roots for total N.
About 0.1 g leaf material was digested in 5 ml of concentrated H
#SO% with 20 mg H#SeO$ and 1 ml 30 % H
#O# added. The digest was analysed for N and P with the indophenol method and the mol-ybdenum blue method, respectively (Allen, 1989), using a Hitachi U-2000 spectrophotometer. Pot-assium was analysed by atomic absorption spectro-photometry (Allen, 1989) using a Perkin Elmer AAS 4100.
Leaf material ofV.uliginosumwas analysed for N concentration and "&N natural abundance as de-scribed by Michelsen et al. (1996b, 1998). Analysis of"&N was by mass spectrometry as described above. The natural abundance of"&N was expressed asδ"&N (^)¯1000¬(Rsample®Rstandard)}RstandardwhereR
¯mass 29 } mass 28, and the standard had
previously been calibrated against atmospheric N #. Atmospheric δ"&N¯0, by definition. The SD of repeated samples was less than 0.2^.
Analyses of soil inorganic N and P, dissolved organic C and N,and microbial C, N and P
The water content of the soil was measured gravi-metrically and a part of the dried soil was ashed at
550°C for determination of loss on ignition. Fresh soil (10 g) from collection dates 30 August 1994 and 28 August 1997 was fumigated with CHCl
$for 24 h in order to release the nutrients in the soil microbial biomass, after which the soil was extracted for 1 h in 50 ml of 0.5 mol l−"K
#SO%. Another 10 g of fresh soil from all collection dates was treated as above but without fumigation in order to recover soil Niand Pi and dissolved organic C and N. The extracts were filtered through Whatman GF-D filters and frozen until their NH
%+-N content could be analysed with the indophenol method, Pi with the molybdenum blue method, and NO
$−-N with the cadmium reduction method (Allen, 1989). The latter method has a detection limit of about 0.1µg g−"soil organic matter. The remaining amount of extract was digested in H
#SO%with Se as a catalyst, and analysed for N. Dissolved organic N (DON) is calculated as the total N in digested, unfumigated samples minus the Ni in undigested, unfumigated samples. The digestion of the fumigated samples mineralizes the organic fractions of microbial N plus other sus-pended organic, nutrient-holding constituents. Hence the extractable microbial N content can be calculated by subtracting the N in digested, unfumigated extracts from that in digested, fumi-gated extracts. For microbial P, analysis of the Pi in undigested fumigated and unfumigated samples is sufficient to recover the microbial P. The C in the fumigated and unfumigated extracts was analysed with a Shimadzu TOC-5000A total organic C analyser, and microbial C was estimated as
the difference between these measurements.
The microbial N and P were calculated by assuming an extractability of 0.40, and microbial C by an extractability of 0.35. For further details see Jonassonet al. (1996a).
Analysis of fungal biomass in soil and fine roots
The roots used in the P bioassay were kept in a solution of 25 % formalin, 5 % acetic acid and 10 % ethanol, and were after $#P decay, analysed for mycorrhizal colonization by the grid-length method, after clearing in 0.5 M KOH (1 h, 80°C) and staining with Trypan Blue (10 min, 80°C) as described by Michelsenet al. (1995, 1996b).
The mixed fine roots and the soil were analysed for live fungal biomass by the ergosterol method (Antibus & Sinsabaugh, 1993 ; Capornet al., 1995 ; Michelsenet al., 1995). The soil was sieved and 3 g of sieved soil were preserved in 5 ml HPLC-grade methanol. The washed roots were blotted surface-dry. A subsample of about 150 mg was weighed and preserved in 3 ml of HPLC-grade ethanol. The samples were kept cold and dark until analysis. The roots were finely ground in ethanol, and the samples filtered through Whatman GF-A filters, injected
Spectro Monitor 3000), and their absorption measured at 282 nm.
Statistical analysis
The effects were tested by ANOVA with block, C, benomyl and fertilizer application as main effects, and their interactions. Interactions between block and other main effects were excluded from the models. Degrees of freedom were 5 for block, 1 for each of the main effects and the interactions, and 35 for the error. Plant cover 40 d after initiation of the experiment was used as covariate in the analysis of cover changes after 14 months and after four seasons. Soil inorganic nutrients measured throughout the season in 1997 were analysed with repeated-measures ANOVA using Wilks Lambda (SAS Institute, 1997). In addition, the means of single treatments were compared with that of control plots using Dunnett’s test to identify differences at specific dates. Treatment effects on mycorrhizal colonization were analysed after arc-sine transformation, whereas data on plant cover, nutrient concentrations and absorption were analysed after ln(x 1) trans-formation in order to normalize data and meet assumptions of homogeneity of variance. However, analysis using untransformed data gave nearly ident-ical results. All statistident-ical analyses were performed with SAS General Linear Model procedure (SAS Institute, 1997) using type III sums of squares. All significant (P !0.05) or near-significant (P!0.10)
effects are reported in the figures.
R E S U L T S
Inorganic N and P,dissolved organic C and N, and microbial C,N and P
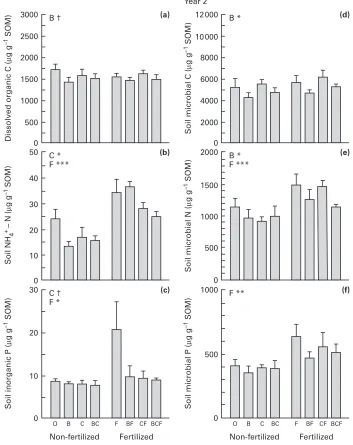
In August 1994, the concentration of microbial N was 45 times higher than that of soil NH
%+-N, whilst microbial P was 50 times higher than Pi(Fig. 1). Nitrate was undetectable in August 1994 whereas the concentration of DON was 20 times higher than that of NH
%+-N (not shown).
After two growing seasons, addition of labile C decreased the concentration of soil NH
%+-N by 20 % across treatments, and tended (P¯0.08) to decrease Pi (Fig. 1). Addition of fertilizer increased the concentration of NH
%+-N in soil by 45 %, and generally resulted in a slight increase in Pi(but more than a doubling in the fertilized-only plots). Benomyl did not generally affect the soil NH
%+-N concen-tration, although this was halved in the benomyl-only plots. The treatments did not affect DON nor was any enhancement of dissolved organic C (DOC) detected 6 wk after the last addition of labile C.
Addition of fertilizer increased microbial N and P
by 30 % across treatments, whereas benomyl
decreased microbial C by 20 % and microbial N by 15 % (Fig. 1). By contrast, although labile C decreased inorganic nutrients in soil, no increase in the soil microbial C, N or P concentration could be detected by this treatment. However, even a large microbial uptake of nutrients from the inorganic pool may remain undetected because it falls within the variability of the means of the much greater microbial nutrient pool.
After treatment over 5 yr, the concentrations of Ni (Fig. 2) were three to four times higher than the concentrations measured after two seasons (Fig. 1), most likely because the soil was sieved (and not hand-sorted) at this time which probably leads to leakage of N from damaged roots. Temporal changes in the concentrations of inorganic nutrients were moderate and most marked for NO
$−, which was detectable in year 5 although in very low amounts (Fig. 2). We cannot exclude the possibility that release of NO
$− from damaged roots resulted in detectable levels of soil nitrate in 1997. However, the mean air temperatures in June, July and August 1997 were 2°C higher than those of the summer months of 1994, which may have promoted nitri-fication. Nitrate and Pihad higher concentrations in fertilized than in unfertilized plots, and the average concentration of NH
%+was non-significantly higher at all sampling dates. Carbon addition decreased soil NH
%+ and Pi, with the greatest effect at the end of June and in July. The same pattern was observed for Piafter benomyl addition. Soil NO
$−was decreased relatively more, and more quickly, than NH
%+after benomyl addition.
There was no change in DOC throughout the 1997 season, except for a non-significant increase fol-lowing addition of labile C in mid July (Fig. 2). This is because the C added in June 1997 was not dissolved by rainfall before early July, after which it was probably quickly incorporated into microbial biomass, metabolized by microbes or leached out from the plots. The tendency towards increased DOC in fertilized plots through the season is probably due to the greater plant biomass in these plots leading to enhanced root exudation.
After 5 yr, the decrease in soil microbial C and N after benomyl addition was no longer evident, but addition of fertilizers still enhanced microbial P (Fig. 3). Furthermore, in the long term, addition of labile C led to a 60 % increase in microbial C, a tendency (P¯0.12) towards an increase in microbial P (by 25 %), and a non-significant increase in microbial N (by 10 %).
Fungal biomass in soil and fine roots
Microscopic examination of roots ofF.ovinaandV.
3000
Dissolved organic C (
l
Soil microbial C (
l
Soil microbial N (
l
Soil inorganic P (
l
Soil microbial P (
l
Fig. 1.Concentrations of (a) dissolved organic C, (b) soil NH
%+, (c) soil Pi, and (d,e,f) soil microbial C, N and
P on 30 August 1994, after two growing seasons (SOM, soil organic matter). The treatments were : O, control ; B, benomyl ; C, carbon ; F, fertilizer ; and combination of treatments in a complete factorial design ; n¯6, means³SE. Results of ANOVAs with significant effects of main factors (B, C, F) and their interactions are shown :†,P!0.1 ; *,P!0.05 ; **,P!0.01 ; ***,P!0.001.
proportion of the root length of V. uliginosum
(80–91 %) was colonized, with the highest proportion in the controls, but there were no significant treatment effects (no data presented). Likewise, there was no significant effect of treatments on the ergosterol concentration in soil, due to the large variance in this estimate of fungal biomass. The root and soil concentrations of ergosterol were 12–18 and 1.3–2.6µg g−", respectively (no data presented).
Plant cover,leaf area and weight,stem weight
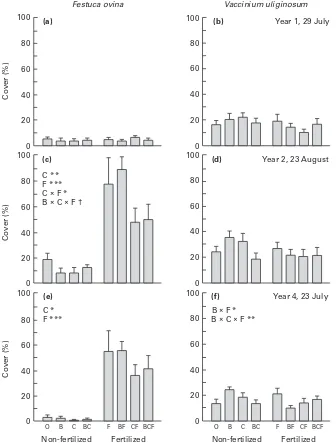
In late August 1994 the cover of F. ovina and V.
uliginosum in control plots was about three and 1.5 times that in late July of both 1993 and 1996 (Fig. 4),
a reflection of the fact that plant biomass peaks in mid-August. As expected, the plants had not responded to the treatments with changes in plant cover on 23 July 1993, 40 d after the start of the experiment. In late August of 1994 (i.e. after 14
months), however, the cover of F. ovina was
enhanced five-fold in fertilized plots, and addition of C (sugar) led to a strong decrease in cover. These effects were maintained after treatment over 4 yr. By contrast, the treatments did not have a main effect on
Soil NH
4
+ – N (
l
g g
–1 SOM)
Soil NO
3
– – N (
l
g g
–1
SOM)
150
100
50
0
(a)
Year 5
C *
(b)
2
1
0
(c)
Soil PO
4
3–
– P (
l
g g
–1 SOM)
150
50
0 100
(d)
Dissolved organic C (
l
g g
–1 SOM)
1000
0 2000 3000 4000
F * B *
F *** B † C *
F †
NPK fertilizer (F)
Control (O)
Benomyl (B) Labile C (C)
23 June 29 June 15 July 22 July 28 Aug.
Fig. 2.Variations in the concentrations of (a) NH
%+, (b) NO$−, (c) Piand (d) dissolved organic C in soil organic
matter (SOM) during the growing season of 1997, 5 yr after the experiment was initiated, in control plots (inverted triangles) and in plots treated with benomyl (B, circles), carbon (C, squares) or NPK fertilizer (F, upright triangles) ;n ¯6, means³SE. In 1997 treatments took place on 23 June and 22 July just after soil sampling, as indicated by arrows. Results of repeated measures ANOVAs presented with effects of benomyl, C and fertilizer. In addition, means of single treatments against control plots at each sampling were tested with Dunnett’s test. For both tests :†,P!0.1 ; *,P!0.05 ; **,P!0.01 ; ***,P!0.001.
Specific leaf weight and, in particular, stem weight (Fig. 5) were more sensitive parameters than cover in
V. uliginosum. Carbon addition decreased, and
8000
0
**
Soil microbial C (
l
Soil microbial N (
l
Soil microbial P (
l
Fig. 3.Concentrations of soil microbial (a) C, (b) N and (c) P on 28 August 1997, after 5 yr treatment, in control plots (O) and plots treated with benomyl (B), carbon (C) or fertilizer (F) ; n¯6, means³SE. (SOM, soil organic matter.) Means of single treatments were compared to control plots with Dunnett’s test ; **,P!0.01.
Cover (%)
Year 2, 23 August
Year 4, 23 July Vaccinium uliginosum
Cover (%)
Fig. 4. Festuca ovina (left) and Vaccinium uliginosum(right) cover on (a,b) 29 July 1993 (year 1), (c,d) 23 August 1994 (year 2), and (e,f) 23 July 1996 (year 4). The treatments were : O, control ; B, benomyl ; C, carbon ; F, fertilizer ; and combination of treatments in a complete factorial design ; n¯6, means³SE. Results of ANOVA with significant effects of main factors (B, C, F) and their interactions are shown : †,P!0.1 ;
30
20
10
0
(a)
Leaf weight per shoot (mg)
2
1
0
(c)
Leaf area per shoot (cm
2)
BC C B
O F BF CF BCF
Non-fertilized Fertilized 3
F †
30
10
0 20
10
0 15
5
(b)
(d)
BC C B
O F BF CF BCF
Non-fertilized Fertilized F **
B × C × F * C * F *
Stem weight per shoot (mg)
Specific leaf weight (mg cm
–
2)
Vaccinium uliginosum, Year 2
Fig. 5.The effect of treatments on (a) leaf weight, (b) stem weight, (c) leaf area and (d) specific leaf weight of Vaccinium uliginosumon 21 August 1994 (year 2). The treatments were : O, control ; B, benomyl ; C, carbon ; F, fertilizer ; and combination of treatments in a complete factorial design ; n¯6, means³SE. Results of ANOVAs with significant effects of main factors (B, C, F) and their interactions are shown : †, P !0.1 ;
*,P!0.05 ; **,P!0.01.
0
(d)
BC C B
O F BF CF BCF
Non-fertilized Fertilized C *
B × C *
P uptake (pg mg
–1
root 15 min
–1
)
200 800 1200
400 600 1000
1400 (e)
BC C B
O F BF CF BCF
Non-fertilized Fertilized C †
F ***
(f)
BC C B
O F BF CF BCF
Non-fertilized Fertilized F †
(a)
29 June B † F *
N uptake (ng mg
–1
root 2 h
–1
)
0 100
50 150
200 7 August (b)
C † C × F *
(c)
4 September B *
Festuca ovina, Year 2
0
Leaf K concentration (%)
1.00
Leaf P concentration (%)
0.15
Leaf N concentration (%)
1
Vaccinium uliginosum, Year 2
©fertilizer d15Nª
Fig. 7. Concentrations inVaccinium uliginosumleaves of (a) N, (b) P and (c) K, (d) P absorption by excised roots, and (e) leaf"&N natural abundance on 7 August 1994 (year 2). The treatments were : O, control ; B, benomyl ; C, carbon ; F, fertilizer ; and combination of treatments in a complete factorial design ; n¯6, means³SE. Results of ANOVAs with significant effects of main factors (B, C, F) and their interactions are shown :†,P!0.1 ; *,P!0.05 ;
**,P 0.01 ; ***,P 0.001.
decreased the specific leaf weight of these shoots, as this treatment tended to enhance the leaf area per shoot relatively more than the leaf weight per shoot (Fig. 5).
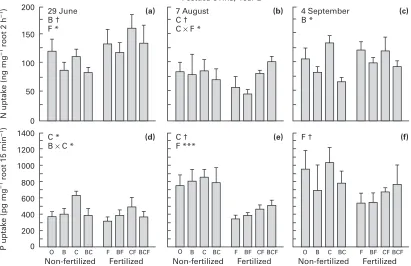
"&N and$#P absorption by excised roots
Absorption of P by roots ofF.ovinain control plots steadily increased from the end of June to the beginning of September (Fig. 6), suggesting con-tinuously increasing sink strength for P towards the end of the season. Seasonal changes in N absorption were less obvious ; a small decrease was found at the beginning of August, suggesting decreased sink strength for N
iat that time. The seasonal changes in the demand of roots for N and P are consistent (negatively correlated) with the soil inorganic nu-trient pools observed through the season 3 yr later (Fig. 2), suggesting that the sink strength reflected variations in the availability of N
iand Piduring the growing season.
Festuca ovinaresponded to addition of labile C by an increased root absorption of P in June, and tended to do so in August but not in September (Fig. 6). There was no P absorption response to benomyl appli-cation. In August the P demand by F. ovinaroots was halved by fertilizer addition, and there was also a non-significantly (P ¯0.10) decreased absorption in September, although the sensitivity of the test was low due to high variances within treatments.
Absorption of N byF.ovinaroots after treatments (Fig. 6) followed a different pattern to that of the P demand. Surprisingly, fertilizer addition enhanced absorption of N by roots in June, probably because fertilized plants started growing earlier and therefore had higher demand for N than non-fertilized plants early in the season. Addition of labile C tended to increase the absorption in August but had no effect in early and late season. There was a tendency towards an interaction with fertilizer in August caused by high absorption in the combined treat-ments. Benomyl caused a decrease in N demand by roots both in June (tendency only) and September. There was no difference in absorption of P between brown (presumably old) and white (more recently formed) fine roots of F. ovina. The ab-sorption was 922³204 pg P mg−" brown roots 15 min−", 1010³182 pg P mg−"white roots 15 min−", and 941³232 pg P mg−"normal (mixed brown and white) roots 15 min−" for roots taken from control plots at 4 September. Hence the absorption of P appears unrelated to root age.
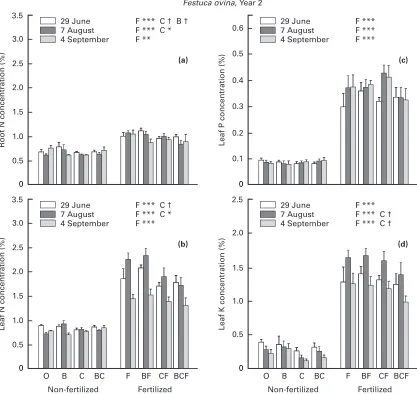
29 June
Root N concentration (%)
1.5
Leaf N concentration (%)
1.5
Leaf P concentration (%)
0.3
Leaf K concentration (%)
1.5
Festuca ovina, Year 2
Fig. 8.Concentrations of (a) root N, (b) leaf N, (c) leaf P and (d) leaf K ofFestuca ovinaon 29 June (white), 7 August (dark grey) and 4 September (mid-grey) 1994 (year 2). The treatments were : O, control ; B, benomyl ; C, carbon ; F, fertilizer ; and combination of treatments in a complete factorial design ; n¯6, means³SE. For each of the dates, results of ANOVAs with significant effects of main factors (B, C, F) and their interactions are shown :†,P!0.1 ; *P,!0.05 ; **,P!0.01 ; ***,P!0.001.
Tissue nutrient concentrations
The concentrations of K in leaves of non-fertilized
F.ovinawere lower in September than in June and August, probably due to on average older leaf biomass, leaching losses from yellowing leaves and translocation to roots. Fertilized F. ovina showed peak concentrations of leaf N and K in August whereas seasonal changes were less for the concen-trations of leaf P and root N (Fig. 8).
Root N and leaf N, P and K were all strongly increased by fertilizer application at all sampling times (Fig. 8). The proportional enhancement was greatest for leaf K, followed by leaf P, leaf N and root N. Benomyl tended to enhance the concentration of N in roots sampled in June (Fig. 8), coincident with low absorption of N in excised roots (Fig. 6). Carbon addition tended to decrease both leaf and root N in June (P¯0.08 and 0.07, respectively) and leaf K in August and September (P¯0.09 and 0.05,
re-spectively), and significantly decreased N in both pools in August (Fig. 8).
Concentrations of N, P and K in leaves of V.
uliginosum were enhanced by addition of fertilizer (Fig. 7), although less strongly than for leaves ofF.
ovina. The concentrations of P increased pro-portionally more than those of K and N. Application of labile C decreased the concentrations of N and K in leaves ofV. uliginosum.
"&N ofV. uliginosumleaves and added fertilizer
Non-fertilizedV.uliginosumhad aδ"&N ofc.®5.5^
(Fig. 7). Fertilizer application enhanced the value of δ"&N to about ®3.5^, or 2^ higher δ"&N than unfertilized plants, approaching the δ"&N signature of the applied fertilizer which was 0.46³0.06^. The fertilizer NH
%+-N "&N was determined to
®0.92^ and the NO
plant δ"&N was notably lower in fertilized than in non-fertilized plants. There was no effect of addition of labile C or benomyl (Fig. 7).
Aims
The main aim of our manipulations was to in-vestigate whether changes in microbial nutrient pool sizes in situ would affect the availability of soil nutrients and, in turn, the performance of plants of contrasting growth forms. It was possible to change the size of the microbial biomass and its N and P content after 1 (Jonassonet al., 1996a), 2 and 5 yr of treatment, with particular strong effects of fertilizer addition during the first 2 yr and of addition of labile C after 5 yr. Furthermore, as predicted, nutrient immobilization had different consequences for the cover of the most common graminoid and dwarf shrub in the community.
Carbon and nutrient limitation of microbial growth
The strong increase in microbial C after 5 yr labile C addition (Fig. 3) suggests C limitation of the microbial biomass in the longer term. Such C limitation of soil microbial biomass is common in natural ecosystems, although there is also field evidence of N limitation, or C and N co-limitation of microbes (Clein & Schimel, 1995 ; Niklaus & Ko$rner, 1996 ; Zhang & Zak, 1998). In the second year, nutrient accumulation in the microbial biomass occurred after nutrient addition, with no concurrent increase in microbial biomass C even when labile C was added. The lack of a response in microbial biomass C suggests that if there was nutrient or C limitation to microbial biomass formation during the second season, this could not be detected at the time of soil sampling in late August (Fig. 1). This lack of C limitation on microbial growth after 2 yr might seem surprising, considering that C limitation oc-curred both after one season (Jonassonet al., 1996a) and after 5 yr at the same site. However, the rainfall pattern might affect the rate at which microbes acquire and metabolize added labile C. In years 1 and 5, rainfall was very low for the first 2 wk after treatment application in July, whereas rainfall was higher during this period in year 2, possibly resulting in faster turnover of added C. In addition, at an adjacent heath site, low responses of the microbial biomass to long-term additions of nutrients and to warming were probably due to intensified feeding on bacteria and fungi by nematodes, which are abundant in these soils and almost doubled in number in response to these treatments (Ruess et al., 1999). The pool size of microbial biomass C, N and P in tundra soils may therefore be strongly influenced by fluctuations in the population size of grazers induced,
for example, by changes in climate, resulting in time-specific responses to manipulations.
Nutrient availability and microbial immobilization
The seasonally stable pool of inorganic nutrients observed during the growing season (Fig. 2) suggests that there is no pronounced pulse of inorganic nutrients released from early spring freeze–thaw cycles or from lysed cells of microbes that have died during winter. This is consistent with the moderate fluctuations in the concentrations of soil inorganic nutrients observed at a nearby heath and fell field (Michelsenet al., 1996a). However, it is not possible to exclude an early spring pulse of nutrients (Brooks
et al., 1996) that could have taken place before our first spring sampling.
The mean concentrations of NH
%+ and Pi were consistently higher in fertilized plots, and lower in plots treated with labile C, both after 2 yr (Fig. 1) and throughout the fifth growing season (Fig. 2), although these differences were not always stat-istically significant. However, after 5 yr the con-centration of NH
%+ in fertilized plots approached that in the controls, suggesting that the N-sink strength of the plant community as a whole was probably higher at 5 yr than at 2 yr, because after 5 yr all species had had the time needed to respond to fertilizer addition by increased meristem formation. Hence, in the long term, soil nutrient availability appeared to be controlled partially by plant-sink strength, confirming observations from nearby tundras (Jonasson et al., 1999). However, in cor-respondence with earlier findings (Chapin et al., 1986 ; Nadelhoffer et al., 1992 ; Clein & Schimel, 1995 ; Schmidtet al., 1997a,b), our data suggest that the availability of soil nutrients is also under microbial control. After 5 yr, addition of labile C tended to enhance microbial biomass, and decreased the availability of NH
%+ and Pi more strongly than after 2 yr.
Plant-growth responses to soil nutrient enrichment and depletion
We hypothesized that the opportunistic graminoid would be strongly influenced by nutrient limitation imposed by microbes, whereas the slower-growing dwarf shrub would be less affected by nutrient immobilization in saprotrophic microbes. Indeed,F.
ovinaresponded to fertilizer addition with a strong increase in cover, and to addition of labile C with a decrease in cover, after both 2 and 4 yr of treatment (Fig. 4), providing the first direct field evidence that
intensified nutrient limitation imposed by
uliginosum: most pronounced was the increase in current-year stem production after fertilizer ad-dition, and the opposite response after addition of labile C (Fig. 5). As the most widespread of the few opportunistic species with potentially high growth rates in the community, the grass exploited the benefits of enhanced resource level, but at the same time was very sensitive to a decrease in resource level below that normally found. By contrast, the dwarf shrub, the most common plant species in this mixed community, was only slightly sensitive either to increase or decrease in resource levels. This is consistent with the moderate responses of V.
uliginosum to 8 yr of fertilizer addition observed in nearby non-acidic graminoid tundra (Jonasson, 1992), and in dwarf shrub tundra (Graglia et al., 1997), but differs from the tendency towards a stronger response in subarctic forest (Press et al., 1998). Our data suggest that this species will respond relatively slowly to changes in soil nutrient supply driven by environmental changes such as enhanced summer temperature and CO
# concentration.
Seasonal changes in plant nutrient demand : effects of nutrient enrichment and depletion
The low and similar capacity to absorb P in excised roots of fertilized and non-fertilizedF.ovinaearly in the season (Fig. 6), despite the strongly different leaf P concentrations in those treatments (Fig. 8), suggests that available soil Piwas sufficient to meet the demand just after the first leaves emerge, except after microbial P immobilization when labile C was added. This immobilization led to increased root P absorption (demand), supporting our hypothesis that graminoids are highly sensitive to immobilization of nutrients by microbes. The decreased concentrations of N in roots and of N and K in leaves throughout most of the growing season, in response to additions of labile C, also confirm our hypothesis.
The lower absorption rate of P in roots of fertilized
F. ovinain August and September reflects the low demand of plants whose concentration of P in leaves had increased three- to four-fold by addition of fertilizer. In contrast, addition of fertilizer did not decrease the rate of absorption of N, and the concentration of N in shoots increased relatively little, suggesting that the N-sink strength remained high in fertilized plants, although the concentration of N had increased in the leaves. In the early season, nutrient addition even enhanced root N demand, most probably because fertilized plants initiated leafing earlier than non-fertilized plants. The weaker response of N, relative to that of P, following fertilization was most likely because the N}P ratio of the added fertilizer was lower than the tissue N}P ratio.
The continued capacity ofF. ovinato take up N and P even during early senescence (Fig. 6) confirms
earlier observations on tundra graminoids (Chapin & Bloom, 1976). High root absorption capacity reflects high above-ground sink strength depleting the roots for nutrients, so the increase in N and P absorption between 7 August and 4 September suggests that the graminoid had not yet initiated net downward translocation of N and P by early August. This is in contrast to graminoids from the middle arctic coastal tundra, which have a shorter growing season and start net downward translocation of P by late July (Chapin & Bloom, 1976).
In Vaccinium, addition of NPK fertilizer did not affect cover (Fig. 4), but led to a slight growth response at shoot level only (Fig. 5) and to ac-cumulation of N, P and K in leaf tissue (Fig. 7). This promotion of nutrient status was also reflected in the low rate of absorption of P by excised roots. In combination with the moderate effects caused by addition of labile C and the consequent microbial immobilization of nutrients (leading, for example, to slight decreases in the concentrations of N and K in leaves and in current-year stem biomass only), our data suggest that this species may be more limited by other factors, such as growing-season length and temperature, than by nutrients at this subarctic heath. This behaviour is similar to that of other dwarf shrubs without a main arctic–alpine dis-tribution (Gragliaet al., 1997). The independence of inorganic nutrient supply may be due to large nutrient stores in the stems which serve as a buffer against shorter-term fluctuations in the availability of soil nutrients, and also to the intense colonization of roots by ericoid mycorrhizal fungi which promote nutrient uptake in organic form. That variance in leaf "&N abundance was notably higher in non-fertilized than in non-fertilized plants (Fig. 7) might suggest thatV.uliginosumis exploiting a range of soil N forms (with different δ"&N)in situ. However, the clear shift in leaf "&N abundance of plants from fertilized plots towards that of the fertilizer δ"&N demonstrates thatV.uliginosumtook up a significant part of its N in inorganic form when fertilizer was added, despite its ability to acquire organic N through the mycorrhizas (Michelsen et al., 1998). Ericoid mycorrhizal colonization was unaffected by fertilizer addition, a response similar to that of heather in a temperate heathland (Caporn et al., 1995).
Effects of benomyl on availability of soil nutrients,
soil microbes,plant performance and mycorrhizal function
resulted in approx. 20 % lower concentrations of microbial C and N in the soil compared to un-amended plots, whereas there was no detectable effect on N and P availability (Fig. 1). By contrast, after 5 yr the availability of soil NO
$− and Pi was decreased (Fig. 2) while microbial C, N and P were unaffected (Fig. 3). As the fungicide selectively inhibits the growth of some but not all fungi found in rhizosphere soil (Newsham et al., 1995), shifts in the composition of the microbial community are likely. In the fifth season, benomyl addition decreased the rate of mineralization (i.e. microbial activity) as availability of N and P was lower than in plots without added benomyl while microbial biomass C, N and P were unchanged.
The lack of an increase in the concentration of soil nutrients after two seasons, despite decrease of microbial C and N following addition of benomyl, could be due to plant uptake of nutrients released from dying microbes. This hypothesis is supported by the decreased absorption of N by excised roots of the non-mycorrhizal F. ovina in benomyl-treated plots in June and August (Fig. 6), and by the tendency towards enhanced concentration of N in roots of F. ovinain June (Fig. 8). Note that direct toxic effects of benomyl on plants are unlikely : Paul
et al. (1989) did not find any detrimental side effects on growth of any of 19 herbaceous wild plants tested. Benomyl affected plant P concentration and ab-sorption in some instances when labile C was also added, as shown by the significant interaction between these two factors for absorption of P by F.
ovinain June (Fig. 6), and for P concentration and absorption in V. uliginosum in August (Fig. 7). Through its decrease of soil microbial biomass, benomyl seemed to relieve the plants from the P deficiency imposed by the addition of labile C. For the graminoid, there was a main effect on root N absorption and concentration that was independent of the addition of labile C.
Our data show that benomyl can affect nutrient uptake by plants and the soil nutrient source}sink relationship, even in organogenic soils that strongly adsorb the compound (Liu & Hsiang, 1994). Effects of benomyl on the concentrations of inorganic nutrients in soil have been measured in only a few instances (e.g. Fitter & Nichols, 1988 ; Merryweather & Fitter, 1996) and no influence of benomyl on soil nutrients was detected in these studies, perhaps because the fungicide targeted a microbial biomass that was presumably much lower than in the system described here. However, consistent with our results after two seasons, Jamieson & Killham (1994) showed that a decrease of active fungal hyphae by addition of benomyl to pots with intact forest soil profiles led to an increase in N uptake by Sitka spruce.
The ericoid mycorrhizal colonization of V.
uliginosum roots was unchanged by benomyl
ap-plication, which suggests that mycorrhizal fungi were less affected than fungal saprotrophs. Although benomyl often decreases arbuscular mycorrhizal function more than its colonization of roots (Larsen
et al., 1996), no evidence was found for an impact on ericoid mycorrhizal function. The isotopic com-position of leaf N (δ"&N) of V. uliginosum was unchanged by benomyl addition (Fig. 7), whereas the expected response to impaired function of the associated ericoid mycorrhizal fungi would have been a shift towards higher (less negative) plant δ"&N. This is firstly because less organic N (with low δ"&N) and relatively more Ni(with highδ"&N) would be taken up, and secondly because discrimination against"&N during transfer of N from fungus to plant would probably decrease if mycorrhizal function was impaired (Michelsenet al., 1996b, 1998).
By adding fertilizer and labile C over 5 yr, we were able to alter the plant and soil microbial nutrient demand in a subarctic heath and to confirm that the concentrations of soil inorganic nutrients in heath tundra are under both plant and microbial control. After 5 yr, the microbial biomass C was enhanced by addition of labile C which led to a non-significant increase in microbial immobilization of N and P, and to a strong decrease of soil inorganic N and P. Together with the graminoid response showing decreased cover and tissue nutrient concentrations after ad-dition of labile C, this provides the first direct field evidence that nutrient limitation imposed by im-mobilizing microbes can affect the growth of tundra plants. In contrast to the strong graminoid response, the dwarf shrub was only slightly sensitive to changes in the resource level. We suggest that the differential responses of the two growth forms are due to differences in storage and nutrient uptake pathways, with the dwarf shrub having large nutrient storage capacity and access to organic N forms through its mycorrhizal association.
The effects of benomyl show (i) that the compound can decrease soil microbial biomass C and N, nutrient mineralization and nutrient availability ; (ii) that plants may respond to those changes by decreased nutrient absorption and enhanced tissue concentrations of nutrients without simultaneous effects on plant cover, shoot and leaf production ; and (iii) that the compound does not necessarily affect ericoid mycorrhizal function. These findings call for cautious interpretation of the effects of fungicides in natural systems.
Natural Science Research Council, Grant Nos 11–0611–1, 11–0421–1 and 95–01046, and the Swedish Environmental Protection Board, Grant No. 127402. We are much indebted to Esben Nielsen, Karna Heinsen, Karin Larsen, Malgorzata Afshar, Peter Sandbach and the staff at Abisko Scientific Research Station for assistance in this work, and to two anonymous referees for constructive comments.
Allen SE. 1989.Chemical analysis of ecological material,2nd edn. Oxford, UK : Blackwell Scientific Publications.
Antibus RK, Sinsabaugh RL. 1993. The extraction and quantification of ergosterol from ectomycorrhizal fungi and roots.Mycorrhiza3: 137–144.
Brooks PD, Williams MW, Schmidt SK. 1996. Microbial activity under alpine snowpacks, Niwot Ridge, Colorado. Biogeochemistry32: 93–113.
Caporn SJM, Song W, Read DJ, Lee JA. 1995.The effect of repeated nitrogen fertilization on mycorrhizal infection in heather [Calluna vulgaris (L.) Hull]. New Phytologist 129: 605–609.
Chapin III FS, Bloom A. 1976.Phosphate absorbtion : adaptation of tundra graminoids to a low temperature, low phosphorus environment.Oikos26: 111–121.
Chapin III FS, Shaver GR. 1996. Physiological and growth responses of arctic plants to a field experiment simulating climatic change.Ecology77: 822–840.
Chapin III FS, Vitousek PM, Van Cleve K. 1986.The nature of nutrient limitation in plant communities.American Naturalist 127: 48–58.
Cheng W, Virginia RA. 1993. Measurements of microbial biomass in arctic tundra soils using fumigation-extraction and substrate-induced respiration procedures. Soil Biology and Biochemistry25: 135–141.
Clein JS, Schimel JP. 1995.Nitrogen turnover and availability during succession from alder to poplar in Alaskan taiga forests. Soil Biology and Biochemistry27: 743–752.
Fitter AH. 1986. Effect of benomyl on leaf phosphorus con-centration in alpine grasslands : a test of mycorrhizal benefit. New Phytologist103: 767–776.
Fitter AH, Nichols R. 1988. The use of benomyl to control infection by vesicular–arbuscular mycorrhizal fungi. New Phytologist110: 201–206.
Graglia E, Jonasson S, Michelsen A, Schmidt IK. 1997.
Effects of shading, nutrient application and warming on leaf growth and shoot densities of dwarf shrubs in two arctic–alpine plant communities.Ecoscience4: 191–198.
Harrison AF, Taylor K, Hatton JC, Dighton J, Howard DM. 1991.Potential of a root bioassay for determining P-deficiency in high altitude grasslands. Journal of Applied Ecology 28: 277–289.
Harte J, Kinzig AP. 1993.Mutualism and competition between plants and decomposers : implications for nutrient allocation in ecosystems.American Naturalist141: 829–846.
Jamieson N, Killham K. 1994.Biocide manipulation of N flow to investigate root}microbe competition in forest soil.Plant and Soil159: 283–290.
Jonasson S. 1988.Evaluation of the point intercept method for the estimation of plant biomass.Oikos52: 101–106.
Jonasson, S. 1992.Plant responses to fertilization and species removal in tundra related to community structure and clonality. Oikos63: 420–429.
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV, Callaghan TV. 1996a.Microbial biomass C, N and P in two arctic soils and responses to addition of NPK fertilizer and sugar : implications for plant nutrient uptake.Oecologia 106: 507–515.
Jonasson S, Vestergaard P, Jensen M, Michelsen A. 1996b.
Effects of carbohydrate amendments on nutrient partitioning, plant and microbial performance of a grassland–shrub eco-system.Oikos75: 220–226.
Jonasson S, Michelsen A, Schmidt IK, Nielsen EV. 1999.
Responses in soil microbes and plants to changed temperature, nutrient and light regimes in the Arctic.Ecology80: 1828–1843.
Jones HE, Quarmby C, Harrison AF. 1991.A root bioassay test for nitrogen deficiency in forest trees. Forest Ecology and Management42: 267–282.
Koide RT, Huenneke LF, Hamburg SP, Mooney HA. 1988.
Effects of application of fungicide, phosphorus and nitrogen on the structure and productivity of an annual serpentine plant community.Functional Ecology2: 335–344.
Larsen J, Thingstrup I, Jakobsen I, Rosendahl S. 1996.
Benomyl inhibits phosphorus transport but not fungal alkaline phosphatase activity in a Glomus–cucumber symbiosis. New Phytologist132: 127–133.
Liu LX, Hsiang T. 1994.Bioassays for benomyl adsorption and persistence in soil.Soil Biology and Biochemistry26: 317–324.
Marion GM, Miller PC, Kummerow J, Oechel WC. 1982.
Competition for nitrogen in a tussock tundra ecosystem.Plant and Soil66: 317–327.
Merryweather J, Fitter A. 1996.Phosphorus nutrition of an obligately mycorrhizal plant treated with the fungicide benomyl in the field.New Phytologist132: 307–311.
Michelsen A, Schmidt IK, Jonasson S, Dighton J, Jones HE, Callaghan TV. 1995. Inhibition of growth, and effects on nutrient uptake of arctic graminoids by leaf extracts – allelopathy or resource competition between plants and microbes ?Oecologia103: 407–418.
Michelsen A, Jonasson S, Sleep D, Havstro$m M, Callaghan TV. 1996a. Shoot biomass, δ"$C, nitrogen and chlorophyll responses of two arctic dwarf shrubs toin situshading, nutrient application and warming simulating climatic change.Oecologia 105: 1–12.
Michelsen A, Schmidt IK, Jonasson S, Quarmby C, Sleep D. 1996b.Leaf"&N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non- and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia105: 53–63.
Michelsen A, Quarmby C, Sleep D, Jonasson S. 1998.
Vascular plant "&N natural abundance in heath and forest tundra is closely correlated with presence and type of mycor-rhizal fungi in roots.Oecologia115: 406–418.
Nadelhoffer KJ, Giblin AE, Shaver GR, Linkins AE. 1992.
Microbial processes and plant nutrient availability in arctic soils. In : Chapin III FS, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J, eds.Arctic ecosystems in a changing climate.An ecophysiological perspective. San Diego, CA, USA : Academic Press, 281–300.
Newsham KK, Watkinson AR, Fitter AH. 1995.Rhizosphere and root-infecting fungi and the design of ecological field experiments.Oecologia102: 230–237.
Niklaus PA, Ko$rner C. 1996.Responses of soil microbiota of a late successional alpine grassland to long term CO
#enrichment.
Plant and Soil184: 219–229.
Paul ND, Ayres PG, Wyness LE. 1989.On the use of fungicides for experimentation in natural vegetation.Functional Ecology3: 759–769.
Press MC, Potter JA, Burke MJW, Callaghan TV, Lee JA. 1998. Responses of a subarctic dwarf shrub community to simulated environmental change. Journal of Ecology 86: 315–327.
Rastetter EB, Shaver GR. 1992.A model of multiple-element limitation for acclimating vegetation.Ecology73: 1157–1174.
Read DJ. 1993. Plant–microbe mutualisms and community structure. In : Schulze ED, Mooney HA, eds.Biodiversity and ecosystem function. Ecological Studies 99. Berlin, Germany : Springer, 181–209.
Ruess L, Michelsen A, Schmidt IK, Jonasson S. 1999.
Simulated climate change affecting microorganisms, nematode density and biodiversity in subarctic soils. Plant and Soil. (In press.)
Schimel JP, Helfer S, Alexander IJ. 1992. Effects of starch addition in Sitka spruce forest floor. Plant and Soil 139: 139–143.
Schmidt IK, Michelsen A, Jonasson S. 1997a.Effects on plant production after addition of labile carbon to arctic}alpine soils. Oecologia112: 305–313.
Shaver GR, Chapin III FS. 1980.Response to fertilization by various plant growth forms in an Alaskan tundra : nutrient accumulation and growth.Ecology61: 662–675.
Shaver GR, Chapin III FS. 1995. Long-term responses to factorial, NPK fertilizer treatment by Alaskan wet and moist tundra sedge species.Ecography18: 259–275.
SAS Institute. 1997. SAS}STAT Users Guide, Release6.12. Cary, NC, USA : Statistical Analysis Systems Institute.
Walbridge MR. 1991. Phosphorus availability in acid organic soils of the lower North Carolina coastal plain. Ecology 72: 2083–2100.
West HM, Fitter AH, Watkinson AR. 1993.The influence of three biocides on the fungal associates of the roots ofVulpia ciliatassp.ambiguaunder natural conditions.Journal of Ecology 81: 345–350.
Yarie J, Van Cleve K. 1996.Effects of carbon, fertilizer, and drought on foliar chemistry of tree species in interior Alaska. Ecological Applications6: 815–827.