www.elsevier.com / locate / livprodsci
How does the foetal gastrointestinal tract develop in preparation
for enteral nutrition after birth?
a ,
*
b cP.T. Sangild
, A.L. Fowden , J.F. Trahair
a
˚
Division of Animal Nutrition, Royal Veterinary and Agricultural University of Copenhagen, Grønnegardsvej 3,
DK-1870 Frederiksberg C, Denmark
b
Department of Physiology, University of Cambridge, Cambridge CB2 3EG, UK
c
Department of Anatomical Sciences, University of Adelaide, Adelaide SA-5005, South Australia, Australia
Abstract
At birth, the gastrointestinal tract (GIT) must be able to cope with the shift from parenteral nutrition before birth (via the placenta) to enteral nutrition after birth (oral colostrum / milk intake). In preparation for this event, the GIT grows and matures very rapidly in the weeks before birth. A series of studies in foetal pigs and sheep have shown that both hormonal and luminal factors influence this rapid phase of GIT development in farm animals. Among the potential hormonal regulators of development, cortisol plays a pivotal role. Thus, the normal developmental increases in stomach acid and gastrin secretion, and in certain enzyme activities (chymosin, pepsin, amylase, lactase, aminopeptidases), are stimulated by circulating cortisol. Cortisol also affects the intestinal absorption of immunoglobulins at birth but has limited effects on the GIT in the postnatal period. Ingestion of amniotic fluid by the foetus and of colostrum by the neonate also modulates GIT growth and enzyme activities. These effects may be mediated via luminal actions of growth factors, hormones and nutrients present in the fluids. However, luminal influences on the developing GIT are less pronounced in the foetus than in the neonate. In conclusion, both circulating and luminal factors affect prenatal GIT development to ensure that the foetal GIT is sufficiently mature to support the dramatic changes in nutrition that occur at birth. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Lamb; Piglet; Cortisol; Foetus; Intestinal enzyme; Growth
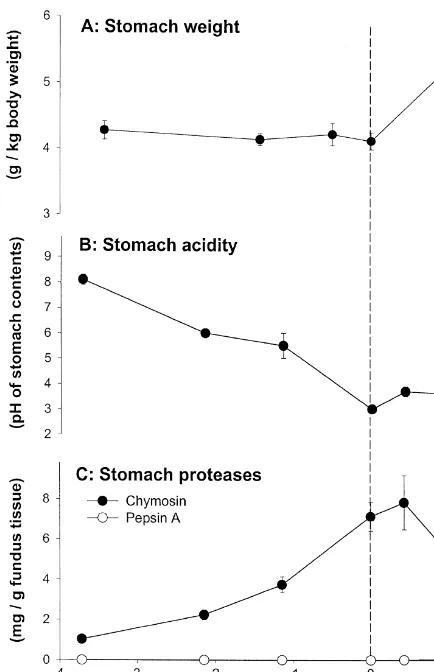
1. The prenatal stomach secretion) changes significantly during this period
(Fig. 1B and C).
In the weeks before birth, the growth rates of the In the postnatal period, gastric acid is important
porcine stomach and ovine abomasum are similar to for the activation and activity of gastric proteolytic
that of the whole body (Sangild et al., 1992,1995c) enzymes and for prevention of bacterial growth in
(Fig. 1A) but gastric function (acid and enzyme the upper gastrointestinal tract (GIT). Although
gastric acid secretion has been detected in foetal pigs (Sangild et al., 1994b) and sheep (Shulkes et al.,
*Corresponding author. Tel.:145-3528-3065; fax: 1
45-3528-1985,1987), secretagogue-stimulated acid secretion
3020.
E-mail address: [email protected] (P.T. Sangild). does not mature until some weeks after birth
develops either shortly before or just after birth (Guilloteau et al., 1983; Sangild et al., 1991,1995c) (Fig. 1C). The protease zymogens are produced mainly by the chief cells of the fundic mucosa and their activation and activity in the gastric lumen are stimulated by an acidic environment (pH 2–4). A shift in the nature of protease zymogens occurs during development in a number of species. For example, in the pig and sheep, during early life, the milk-clotting components (chymosins) are the major protease zymogens. With increasing age, they are gradually replaced by components exhibiting more general proteolytic activity (pepsins) (Foltmann, 1992) (Fig. 1C).
2. The prenatal small intestine
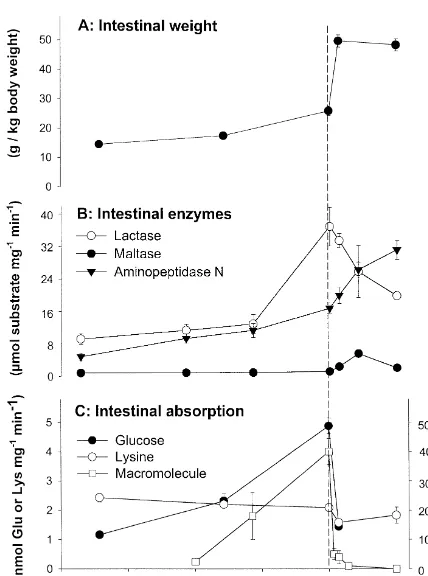
In the weeks before parturition, the pig intestine grows more rapidly than the body as a whole and its relative weight increases 70–80% over the last 3 weeks of gestation (Fig. 2A).
A similar situation is noted for foetal lambs (Trahair et al., 1986a). In foetal sheep which have been growth retarded experimentally, GIT develop-ment is very severely affected. Hence, abnormal GIT growth appears early, before the onset of growth deficiencies of any other foetal organs, and persists at least until late term (Avila et al., 1989; Trahair et
Fig. 1. Mean (6S.E.) values for some key stomach functions in
al., 1997). In the foetal lamb, the density of villi
foetal and neonatal pigs. (A) Weight of the stomach relative to 2
(villi number per mm of mucosal surface) declines
body weight, (B) the acidity (pH) of stomach contents, (C)
from 115 days of gestation to parturition, and
concentration of the zymogens for two proteases, chymosin and
pepsin A, in the fundus region of the stomach. Pepsinogen A can continues to decline after birth. Therefore, the
abso-be detected in small amounts from about 3 days after birth lute number of villi is fixed at some point during (modified from Sangild et al., 1991,1994c).
development or the rate of production of new villi is outstripped by the rate of increase in intestinal
well et al., 1984; Sangild et al., 1992). Gastric fluid diameter (and length). Crypt density increases
to-pH is 2–4 in newborn unsuckled pigs (Sangild et al., wards term and after birth. The crypt villus ratio
1994b,1995a) (Fig. 1B) whereas it is close to neutral thereby steadily increases. Since cell proliferation
in newborn sheep (Shulkes et al., 1985; Sangild et also increases towards term (Trahair et al., 1986c),
al., 1995c). In human infants, acid and intrinsic the migration rate rapidly accelerates from the long
factor secretion are positively correlated (Marino et renewal time in the foetus (up to 20 days) (Trahair et
al., 1984) and both are under the control of gastrin. al., 1986d) to the short time of the adult (2–3 days)
Likewise, in the foetal and newborn pig, the de- (Trahair and Robinson, 1986a).
velopmental increases in intrinsic factor in the gastric Hydrolysis of substrates in the foetal intestinal
fundus correlate well with changes in gastric fluid lumen is minimal (Britton and Koldovsky, 1989) and
pH (Sangild et al., 1994b). most of the digestive processes are intracellular, not
(Din-gestation in both these species, lactase specific activity decreases in lambs (Sangild et al., 1994a) and increases in piglets (Sangild et al., 1995b) (Fig. 2B) during the last weeks of gestation.
Absorption of nutrients, as measured by the uptake of monosaccharides and amino acids by the intestinal mucosa, is low during the first half of gestation but increases rapidly thereafter (Buddington and Malo, 1996). During the final weeks of gestation, there is a particularly rapid increase in glucose uptake capacity (Sangild et al., 1993; Sangild, unpublished observa-tions) while the ability of the mucosa to take up most amino acids, including lysine (Fig. 2C), remains unchanged. Just after birth, when the newborns begin to suckle, there is a dramatic decrease in the mucosal uptake of both glucose and amino acids which may be associated with colostrum-induced changes in the microvillus membrane (Zhang et al., 1997) (Fig. 2C). Nevertheless, total transport capacities along the entire length of the small intestine remain constant or even increase due to the large increase in intestinal mass (Fig. 2A) (Zhang et al., 1997).
One of the most striking features of the small intestine in newborn farm animals is its ability to
take up macromolecules, including
immuno-Fig. 2. Mean (6S.E.) values for some key intestinal functions in
globulins, and to transport them intact across the
foetal and neonatal pigs. (A) Weight of the small intestine relative
epithelium into the blood. This ability ceases within
to body weight, (B) the activity of three brush-border enzymes,
lactase-phloridzin hydrolase, maltase-glucoamylase and amino- the first day or two after birth in both piglets and peptidase N, expressed relative to intestinal weight, (C) absorption lambs by a process known as ‘‘intestinal closure’’ of nutrients and macromolecules by the small intestine; the
¨
(Westrom et al., 1984; Sangild et al., 1997a). In the
specific mucosal uptake of glucose and lysine as measured in vitro
piglet, intestinal macromolecule uptake is present in
is shown together with the proportion of bovine serum albumin
utero during the last 2 weeks of gestation but it is
(BSA, protein macromolecule marker) absorbed into the blood
stream after an oral dose of BSA1colostrum in vivo (modified markedly less in the foetus than in the neonate from Sangild et al., 1995b,1999). (Sangild et al., 1999). The prenatal developmental
increase in the ability to absorb macromolecules is
sdale and Healy, 1982; Healy, 1984). Nevertheless, illustrated in Fig. 2C by data for the capacity to take
the prenatal development of brush-border hydrolases up bovine serum albumin by non-specific
endo-is an important component in the preparation for life cytosis.
and nutrition ex utero. In the pig and sheep, The intestinal absorption of series of proteins has
glucosidase activities (sucrase-isomaltase, maltase- also been tested in the foetal lamb at 120–125 days
glucoamylase) do not rise until postnatally, parallel- of gestation (Sangild and Trahair, unpublished
re-ing a fall in lactase-phloridzin hydrolase (b-galacto- sults). Surprisingly, these studies were unable to
sidase) activity (James et al., 1987b; Sangild et al., demonstrate transfer of intact proteins across the
1995b). Among the major brush-border enzymes, it intestine, although, by immunohistochemistry, the
is only lactase and peptidase activities that reach proteins were shown to be present in the cells lining
notable levels before birth in piglets and lambs the small intestine. These observations suggest that
(Sangild et al., 1994a,1995b) (Fig. 2B). While the ability to take up and transfer intact proteins from
process that develops close to term in both pigs and function in foetal sheep than in pigs, as abomasal
sheep. This suggestion is supported by the finding fluid pH normally remains close to neutral until birth
that the capacity for protein absorption is lower in in foetal lambs (Sangild et al., 1995c).
newborn piglets and lambs delivered prematurely In pigs and sheep, exogenous glucocorticoids
than in those born at term (Hough et al., 1990; stimulate the concentrations of chymosin and pepsin
Sangild et al., 1997a). Hence, the uptake of macro- A in the stomach (Sangild et al., 1994c,1995c) and
molecules by the newborn farm animal is not merely amylase in the pancreas (Sangild et al., 1994d,1995c)
the result of persisting intra-uterine absorptive pro- both before and immediately after birth.
Adrenalec-cesses as suggested previously (Weaver and Walker, tomy of foetal sheep or treatment of newborn pigs
1989) but also reflects a specific maturational pro- with metyrapone (an inhibitor of adrenal
glucocor-cess that is timed to maximise uptake just after birth. ticoid synthesis) reduces the activity of these
hydro-lases (Sangild, 1995; Sangild et al., 1994d,1995c). Elevations in glucocorticoid secretion during the first
3. Regulation of prenatal development by 5 weeks of postnatal life continue to induce small
glucocorticoids increases in some hydrolase activities (Sangild et al.,
1991), but these effects are unlikely to be of
Many hormones and growth factors are potential physiological importance because blood
glucocor-developmental regulators of GIT structure and func- ticoid concentrations normally decrease during this
tion. Among these, cortisol from the adrenal cortex period.
has received the most attention in relation to de- In studies on foetal lambs, exogenous infusion of
velopment of the foetal GIT and of other essential cortisol for 7 days from 108 days of gestation was
organs (e.g., lungs, liver, kidneys). In the pig and associated with a doubling of the crypt cell
prolifer-sheep, the major developmental increase in adreno- ation rate and of the enterocyte migration rate along
cortical function occurs in late gestation, such that the villus (Trahair et al., 1987b). The proliferation
glucocorticoid concentrations in foetal blood are rate was similar to that seen in the late gestation and
highest at term (Liggins, 1976; Hennessy et al., early neonatal periods, when endogenous cortisol
1982; Silver and Fowden, 1989). Postnatally, concentrations are high. However, the increase in
glucocorticoid concentrations normally decrease and cell production did not result in enhanced epithelial
show only minimal changes around the time of growth, since there were no significant changes in
weaning in pigs (Sangild et al., 1991). villus height or crypt depth. Furthermore, in
proxim-In the pig and sheep, the normal development of al regions, production of new villi declined
sig-gastric function seems to be stimulated by the nificantly. As a consequence, there was an increase
prepartum increases in adrenocortical secretion. In in total cell number per villus. The villi became
the pig foetus, the major changes in gastric acidity wider and accommodated more cells, but were
and plasma gastrin concentrations occur concomi- reduced in density per unit area of mucosal surface.
tantly with the surges in blood glucocorticoid con- To test whether enhanced growth would
eventual-centrations. In addition, treatment of foetal pigs ly occur as a result of increased cell proliferation, the
(Sangild et al., 1992,1994b) with exogenous infusion period was extended for a further 7 days (to
glucocorticoids induces precocious secretion of acid, 14 days) (Trahair et al., 1988). In these sheep
gastrin and intrinsic factor. However, elevated foetuses, the proximal villus height, and proximal
glucocorticoids have little effect on gastrin-stimu- and distal crypt depth significantly increased by up to
lated acid secretion after birth (Sangild et al., 1992) 50%. Thus, in the first 7 days of cortisol treatment,
which suggests that porcine parietal cells may be- proliferation and migration increased precociously.
come unresponsive to cortisol once acid secretion Over the next 7 days of treatment, the increased cell
has been initiated. In foetal sheep, gastric fluid production resulted in hypertrophy of the epithelial
acidity is not affected by exogenous cortisol infusion surface. Furthermore, in this mid-gestation period,
or by adrenalectomy (Sangild et al., 1995c). This proximal regions of the small intestine responded
the distal regions. However, increases in cell prolif- increased cell replacement in the intestinal mucosa
eration and migration did increase in distal regions (Trahair, 1989; Henning et al., 1994). Consistent
with significant crypt growth after 14 days of cortisol with this, Bate et al. (1991) observed histological
treatment which suggest that hypertrophy may occur, changes and a decreased intestinal uptake of bovine
even distally, after more prolonged treatment. immunoglobulin G in newborn piglets from sows
Abolition of the endogenous cortisol surge near with elevated blood cortisol concentrations in late
term by bilateral foetal adrenalectomy prevents the gestation, but in this study it was not known how
increase in villus height that normally occurs along foetal blood glucocorticoid concentrations had been
the entire small intestine as parturition approaches affected. Several lines of evidence suggest that the
(Trahair et al., 1987a). Crypts were also shorter in cellular mechanisms of closure and the role of
proximal regions of the foetal small intestine after cortisol in intestinal closure differ between rodents
adrenalectomy. Indeed, very little villus growth and the large farm animals. Firstly, the limiting
occurred during late gestation in the absence of factor for macromolecule uptake in pigs is the
cortisol. The observation that cortisol has little effect transmission of these molecules into the blood
on villus growth early in gestation but is essential for (lasting 12–36 h) and not the uptake into the
the normal ontogenetic increase in villus height vacuolated cells of the intestinal mucosa (lasting
indicates that there are critical periods during de- about 3 weeks) (Clarke and Hardy, 1971; Smith and
velopment when the GIT is glucocorticoid sensitive. Jarvis, 1978). Secondly, cell migration rate does not
The developmental rise in lactase and amino- normally change during the first days of postnatal
peptidase activities in the prenatal pig is, at least in life in pigs (Smith and Jarvis, 1978) or after
part, regulated by cortisol because infusion of cor- glucocorticoid treatment (James et al., 1987a).
Third-tisol into immature foetuses stimulates the activity of ly, neonatal caesarean-delivered piglets and lambs
these enzymes (Sangild et al., 1995b). The lower show severely depressed immunoglobulin G uptake
cortisol concentration observed in piglets after into plasma after metyrapone treatment (inhibition of
caesarean delivery (as opposed to spontaneous vagi- cortisol synthesis) (Patt and Eberhart, 1976; Hough
nal birth) is also associated with altered GIT enzymic et al., 1990; Sangild et al., 1993). Finally,
compro-development in the newborn pig (Sangild et al., mised newborn pigs with elevated cortisol
concen-1994e,1996c). Similarly, in the GIT of the sheep trations, such as vaginally-delivered premature pigs
foetus, the normal prepartum rise in aminopeptidase or growth-retarded newborn pigs (‘‘runts’’), have
N activity and decrease in lactase activity can be higher macromolecule uptake than the corresponding
stimulated precociously by exogenous cortisol and caesarean-delivered pigs or normally-sized pigs
prevented by foetal adrenalectomy (Sangild et al., (Svendsen et al., 1990; Sangild et al., 1997a). Hence,
1994a). However, the effects of glucocorticoids on the literature indicates that, in the farm animal
the enzymic development of the small intestine and species, cortisol stimulates (rather than inhibits)
other regions of the foetal GIT (Sangild, 1995; macromolecule transport across the small intestine.
Sangild et al., 1994c,d,1995b,c) are variable and The potential effects of glucocorticoids on prenatal
dependent on the region of the GIT and the specific development of the porcine GIT are summarised in
enzyme. These variable effects may be due to Fig. 3.
regional differences in the number and activity of the glucocorticoid receptors in the epithelial cells and / or
to differences in the molecular mechanisms by which 4. Regulation of prenatal development by
the glucocorticoids act to alter transcription and luminal fluids
translation in these cells.
Studies in rats and mice have shown that glucocor- Major developmental changes take place in the gut
ticoids stimulate the normal advancement of intesti- in foetal sheep following the onset of foetal
swallow-nal closure which occur at 2 to 3 weeks of age in ing (Trahair and Robinson, 1986b) and in neonatal
these species (Daniels et al., 1973; Moog, 1979). piglets in response to colostrum intake (Widdowson
Fig. 3. Summary of the effects of endocrine factors (e.g., foetal cortisol secretion) and luminal factors (e.g., intake of amniotic fluid by the foetus and of colostrum by the neonate) on GIT development in the perinatal period (1, increase; 0, no change; 2, decrease; ?, not investigated). Most effects are similar between pigs and lambs, which are the species for which most experiments have been done. Nevertheless, species differences do exist, and the effects of cortisol, amniotic fluid and colostrum on individual functions may also be highly dependent on foetal and postnatal age. See text for references.
GIT lumen appear to be important in maintaining foetal lambs have not been associated with notable
and stimulating the normal sequence of mucosal changes in body weight, studies in pigs show that
differentiation during the perinatal period. Foetal foetuses prevented from swallowing amniotic fluid in
lambs, in which swallowing of amniotic fluid has utero are growth retarded at birth.
been prevented by ligating the oesophagus at 90 days While luminal fluid has an important role in the
of gestation, showed both reduced growth and al- structural development of the small intestine, its
tered differentiation of the intestine by late gestation effects on the functional development of the GIT is
(Trahair et al., 1986b; Avila and Harding, 1991; more equivocal (Trahair and Sangild, 1997). In
Trahair and Harding, 1992,1995). Similar effects foetal piglets, amniotic fluid-infused animals have
were observed in foetal lambs or piglets after more significantly higher aminopeptidase activities than
short-term oesophageal ligation in late gestation foetuses without any luminal input, but this effect is
(Trahair and Sangild, 1999). When luminal fluid only present in the distal small intestine (Sangild et
input was restored after oesophageal ligation, gut al., 1997b). Lack of swallowed fluid has no apparent
development was partly reversed (Trahair and Hard- effect on the ability to absorp glucose, amino acids
ing, 1995). Lack of foetal ingestion had the most and dipeptides, and it actually increases the activity
pronounced effect on intestinal diameter which is a of lactase in the small intestine (Sangild et al.,
central index of intestinal growth and correlates well 1997b). Because enterocyte turnover is slower in the
with most of the other parameters of gut growth foetal intestine than in the postnatal intestine, the
(Trahair and Harding, 1992,1995; Trahair and San- modulating effects of luminal factors on foetal GIT
become expressed. Only long term changes in the In long gestation species such as the pig and
amount or quality of swallowed amniotic fluid may sheep, the time of intestinal closure to
macromole-have effects on intestinal function. The effects that cule transport may be influenced more by luminal
luminal factors may have on early GIT development factors than by endocrine factors such as
glucocor-¨
are summarised in Fig. 3. ticoids. Studies in neonatal (Westrom et al., 1985)
Some evidence from studies on both the pig and and foetal pigs (Sangild et al., 1999) indicate that
sheep suggests that the ingestion of amniotic fluid in colostrum itself plays the most important role in the
the foetus and of colostrum in the neonate may induction of intestinal closure. When colostrum whey
stimulate the acid secretory function in the stomach. was infused into the foetal pig intestine at 101–107
Experiments on pig foetuses (87–94% of gestation) days of gestation, the uptake of macromolecules
and sheep foetuses (82–87% of gestation) showed declined from 104 days onwards (Sangild et al.,
increased plasma gastrin concentrations after 6–7 1999). In contrast, foetuses infused with amniotic
days of intra-gastric infusion of either amniotic fluid, fluid or milk whey remained fully capable of taking
milk whey or colostrum whey (Sangild et al., up macromolecules by pinocytosis during the entire
1996a,b). The gastrin-releasing effects of these three period of infusion. In these studies, some foetuses
fluids were similar despite the large differences in had significantly elevated plasma cortisol (probably
their protein and growth factor content. However, in response to foetal surgery or treatment) but this
both the absolute concentrations and the relative did not shorten the time available for macromolecule
increases in plasma gastrin over the infusion period absorption in these foetuses.
were smaller in the foetal sheep than in the foetal Studies in both foetuses and neonates have shown
pig. Foetuses which received no fluid at all because that colostrum ingestion is associated not only with
of oesophageal ligation showed decreased plasma rapid growth and enhanced pinocytotic activity of the
gastrin concentrations (Sangild et al., 1996a,b). Both intestinal mucosa but also with changes in the
the lack of luminal nutrients (proteins, peptides) and activity of brush-border enzymes. Colostrum
inges-gastrin-releasing substances in amniotic fluid may be tion has been reported to either increase or decrease
responsible for the decrease in gastrin secretion in the specific activity of lactase (expressed per
intesti-such foetuses. In the pig, the infusions of amniotic nal weight, protein or DNA) while consistently
fluid, milk whey or colostrum whey also decreased increasing the specific activities of enzymes such as
gastric fluid pH at birth (Sangild et al., 1996b) which maltase and aminopeptidases (Sangild et al., 1996c;
indicates that luminal fluids may enhance maturation Wang and Xu, 1996; Zhang et al., 1997; Fig. 2B).
of immature parietal cells. The suggestion that Exposure of the neonatal intestine to colostrum is
enteral input is necessary for the normal develop- associated with profound changes, both in mucosal
ment of acid secretory function in the pig during structure and in the biochemical events which lead to
perinatal period is consistent with previous findings the insertion of functional enzymes into the
brush-in the foetal rabbit (Mulvihill et al., 1985). border membrane (Dudley et al., 1996). The nature
In foetal pigs and sheep, the increases in blood of the nutrient components or regulatory factors in
gastrin concentrations observed in response to colostrum that causes these structural and functional
changes in enteral stimuli (Sangild et al., 1996a,b) changes remains unknown.
were relatively small compared with the response to exogenous cortisol administration (Sangild et al.,
1994b) or to the normal rise in gastrin that occurs 5. Perspectives
with advancing gestational age (Shulkes et al., 1985;
Sangild et al., 1994b). Glucocorticoids appear more Perinatal mortality is very high in farm animals,
important in regulating gastrin secretion in the pig including pigs and sheep (e.g., 10–20%). A large
and sheep during the perinatal period than the part of this mortality may arise because the offspring
presence in the gastric lumen of amniotic fluid before of these species are born at a time in gestation when
birth or colostrum after birth. The same may be true they are only just able to survive. Thus, the final
or-of abomasal secretion in the milk-fed lamb. Can. J. Anim. Sci.
gans, including the GIT, plays a critical role in
64 (Suppl.), 95–96.
determining neonatal viability. Improving neonatal
Daniels, V.G., Hardy, R.N., Malinowska, K.W., Nathanielsz, P.W.,
viability therefore depends on identifying the precise 1973. The influence of exogenous steroids on macromolecule
nature of the systemic and luminal factors that uptake by the small intestine of the new-born rat. J. Physiol.
229, 681–695.
influence GIT development during the critical period
Dinsdale, D., Healy, P.J., 1982. Enzymes involved in protein
just before birth. Such information will help to
transmission by the intestine of the newborn lamb. Histochem.
optimise the clinical and nutritional care of newborn J. 14, 811–821.
farm animals. Dudley, M.A., Burrin, D.G., Quaroni, A., Rosenberger, J., Cook,
G., Nichols, B.L., Reeds, P.J., 1996. Lactase phlorhizin hydro-lase turnover in vivo in water-fed and colostrum-fed newborn pigs. Biochem. J. 15, 735–743.
Acknowledgements Foltmann, B., 1992. Chymosin: a short review on foetal and
neonatal gastric proteases. Scand. J. Clin. Lab. Invest. 52 (Suppl. 210), 65–79.
The authors are indebted to the now deceased Dr.
Guilloteau, P., Corring, T., Garnot, P., Martin, P., Toullec, R.,
Marian Silver, University of Cambridge, who played
Durand, G., 1983. Effects of age and weaning on enzyme
an active role in many of the animal experiments activities of abomasum and pancreas of the lamb. J. Dairy Sci.
required to study the GIT in foetal pigs and lambs. 66, 2373–2385.
We thank our colleagues and the technical staff that Healy, P.J., 1984. Lysosomal enzymes in the intestine of the newborn lamb. Biol. Neonate 46, 186–191.
made it possible to analyse many different
parame-Hennessy, D.P., Coghlan, J.P., Hardy, K.J., Wintour, E.M., 1982.
ters of gut structure and function: Mette Schmidt, Jan
Development of the pituitary–adrenal axis in chronically
Elnif, Merete Stubgaard, Anni Mehlsen, Randall cannulated ovine fetus. J. Dev. Physiol. 4, 339–352.
¨ ¨
Buddington, Bjorn Westrom, Malcolm Bloomfield, Henning, S.J., Rubin, D.C., Shulman, R.J., 1994. Ontogeny of the
Inger Mattsson, Bent Foltmann and Linda Hilsted. intestinal mucosa. In: Johnson, L.R. (Ed.), Physiology of the
Gastrointestinal Tract, 3rd Edition. Raven Press, New York, pp.
The work was supported by the Danish Agricultural
571–610.
and Veterinary Research Council, The Wellcome
Hough, R.L., McCarthy, F.D., Thatcher, C.D., Kent, H.D.,
Ever-Trust, UK and the National Health and Medical sole, D.E., 1990. Influence of glucocorticoid on
macromolecu-Research Council of Australia. lar absorption and passive immunity in neonatal lambs. J.
Anim. Sci. 68, 2459–2464.
James, P.S., Smith, M.W., Tivey, D.R., Wilson, T.J.G., 1987a. Dexamethasone selectively increases sodium-dependent alanine
References transport across neonatal piglet intestine. J. Physiol. 393, 569–
582.
Avila, C.G., Harding, R., 1991. The development of the gastroin- James, P.S., Smith, M.W., Tivey, D.R., Wilson, T.J.G., 1987b. testinal system in fetal sheep in the absence of ingestion. J. Epidermal growth factor selectively increases maltase and Pediatr. Gastroenterol. Nutr. 12, 96–104. sucrase activities in neonatal piglet intestine. J. Physiol. 393, Avila, C.G., Harding, F., Rees, S., Robinson, P.M., 1989. Small 583–594.
intestinal development in growth-retarded fetal sheep. J. Liggins, G.C., 1976. Adrenocortical-related maturational events in Pediatr. Gastroenterol. Nutr. 8, 507–515. the fetus. Am. J. Obstet. Gynecol. 126, 931–941.
Bate, L.A., Ireland, W., Connell, B.J., Grimmelt, B., 1991. Marino, L.R., Bacon, B.R., Hines, J.D., Halpin, T.C., 1984. Development of the small intestine of piglets in response to Parietal cell function of full-term and premature infants: prenatal elevation of glucocorticoids. Histol. Histopathol. 6, unstimulated gastric acid and intrinsic factor secretion. J.
207–216. Pediatr. Gastroenterol. Nutr. 3, 23–27.
Britton, J.R., Koldovsky, O., 1989. Development of luminal Moog, F., 1979. Endocrine influences on the functional differen-digestion: Implications for biologically active dietary poly- tiation of the small intestine. J. Anim. Sci. 49, 239–249. peptides. J. Pediatr. Gastroenterol. Nutr. 9, 144–162. Mulvihill, S.J., Stone, M.M., Debas, H.T., Fonkalsrud, E.W., 1985. Buddington, R.K., Malo, C., 1996. Intestinal brush-border mem- The role of amniotic fluid in fetal nutrition. J. Pediatr. Surg. 20,
brane enzyme activities and transport functions during the 668–672.
prenatal development of pigs. J. Pediatr. Gastroenterol. Nutr. Patt, J.A.J., Eberhart, R.J., 1976. Effects of metyrapone and 23, 51–64. ACTH on intestinal absorption of immunoreactive bovine IgG Clarke, R.M., Hardy, R.N., 1971. Histological changes in the in cesarean-derived pigs. Am. J. Vet. Res. 37, 1409–1413.
Sangild, P.T., Foltmann, B., Cranwell, P.D., 1991. Development of Shulkes, A., Chick, P., Hardy, K.J., 1987. Nature of the gastric gastric proteases in fetal pigs and pigs from birth to thirty six gastrin-acid feedback loop in the fetal sheep. Clin. Exp. Pharm. days of age. The effect of adrenocorticotropin (ACTH). J. Dev. Physiol. 14, 503–512.
Physiol. 16, 229–238. Shulkes, A., Chick, P., Hardy, K.J., Robinson, P., Trahair, J.F., Sangild, P.T., Cranwell, P.D., Hilsted, L., 1992. Ontogeny of 1985. Ontogeny of gastric acidity in the ovine fetus. J. Dev.
gastric function in the pig: acid secretion and the synthesis and Physiol. 7, 195–206.
secretion of gastrin. Biol. Neonate 62, 363–372. Silver, M., Fowden, A.L., 1989. Pituitary–adrenocortical activity Sangild, P.T., Diernæs, L., Christiansen, I.J., Skadhauge, E., 1993. in the fetal pig in the last third of gestation. Q. J. Exp. Physiol.
Intestinal transport of sodium, glucose and immunoglobulin in 74, 197–206.
neonatal pigs. Effect of glucocorticoids. Exp. Physiol. 78, Smith, M.W., Jarvis, L.G., 1978. Growth and cell replacement in
485–497. the new-born pig intestine. Proc. Royal Soc. London 203,
Sangild, P.T., Fowden, A.L., Silver, M., 1994. Development and 69–89.
glucocorticoid regulation of brushborder hydrolases in the Svendsen, L.S., Westrom, B.R., Svendsen, J., Olsson, A.C., small intestine of the ovine fetus. Proceedings of the Thorburn Karlsson, B.W., 1990. Intestinal macromolecular transmission Symposium, Hamilton Island, abstract. in underprivileged and unaffected newborn pigs: implication Sangild, P.T., Hilsted, L., Bjørnskov-Bartholdy, L., Holst, J.J., for survival of underprivileged pigs. Res. Vet. Sci. 48, 184–
Trahair, J.F., 1996a. Gastrin release in the fetal lamb in 189.
response to luminal infusion of amniotic fluid, milk or GRP. Trahair, J.F., 1989. Remodelling of the rat small intestinal mucosa Reg. Peptides 64, 167. during the suckling period. J. Pediatr. Gastroenterol. Nutr. 9, Sangild, P.T., Hilsted, L., Nexø, E., Fowden, A.L., Silver, M., 232–237.
1994b. Secretion of acid, gastrin and cobalamin-binding pro- Trahair, J.J., Avila, G.C., Robinson, P.M., 1986a. Growth of the teins by the fetal pig stomach: developmental regulation by fetal sheep small intestine. Growth 50, 201–216.
cortisol. Exp. Physiol. 79, 135–146. Trahair, J.F., DeBarro, T.N., Robinson, J.S., Owens, J.A., 1997. Sangild, P.T., Hilsted, L., Nexø, E., Fowden, A.L., Silver, M., Restriction of nutrition in utero selectively inhibits
gastroin-1995a. Vaginal birth versus elective caesarean section: effects testinal growth in fetal sheep. J. Nutr. 127, 637–641. on gastric function in the neonate. Exp. Physiol. 80, 147–157. Trahair, J.F., Harding, R., 1992. Ultrastructural anomalies in the Sangild, P.T., Hilsted, L., Silver, M., Fowden, A.L., 1996b. fetal small intestine indicate that fetal swallowing is important Gastrin secretion in response to amniotic fluid or milk in the for normal development: an experimental study. Virchows. fetal pig GI tract. Reg. Peptides 64, 167. Arch. [A] 420, 305–312.
Sangild, P.T., Holtug, K., Diernæs, L., Schmidt, M., Skadhauge, Trahair, J.F., Harding, R., 1995. Restitution of fetal swallowing E., 1997a. Birth and prematurity influence intestinal function in restores intestinal growth after mid-gestation oesophageal the newborn pig. Comp. Biochem. Physiol. 118A, 359–362. obstruction. J. Pediatr. Gastroenterol. Nutr. 20, 156–161. Sangild, P.T., Silver, M., Fowden, A.L., Turvey, A., Foltman, B., Trahair, J.F., Harding, R., Bocking, A.D., Silver, M., Robinson,
1994c. Adrenocortical stimulation of stomach development in P.M., 1986b. The role of ingestion in the development of the the prenatal pig. Biol. Neonate 65, 378–389. small intestine in fetal sheep. Q. J. Exp. Physiol. 71, 99–104. Sangild, P.T., Silver, M., Schmidt, M., Fowden, A.L., 1996c. The Trahair, J.F., Perry, R.A., Silver, M., Robinson, P.M., 1986c.
3
perinatal pig in pediatric gastroenterology. In: Tumbleson, Autoradiographic localization of [ H]thymidine incorporation M.E., Schnook, L. (Eds.), Advances in Swine in Biomedical in the small intestinal epithelium of fetal sheep. J. Pediatr. Research. Plenum Press, New York, pp. 745–756. Gastroenterol. Nutr. 5, 648–654.
¨ ¨ ´
Sangild, P.T., Sjostrom, H., Noren, O., Fowden, A.L., Silver, M., Trahair, J.F., Perry, R.A., Silver, M., Robinson, P.M., 1986d. 1995b. The prenatal development and glucocorticoid control of Enterocyte migration in the foetal sheep small intestine. Biol. brush-border hydrolases in the pig small intestine. Pediatr. Res. Neonate 50, 214–220.
37, 207–212. Trahair, J.F., Perry, R.A., Silver, M., Robinson, P.M., 1987a. Sangild, P.T., Trahair, J.F., Loftager, M.K., Fowden, A.L., 1999. Studies on the maturation of the small intestine of the fetal Intestinal macromolecule absorption in the fetal pig after sheep. I. The effects of bilateral adrenalectomy. Q. J. Exp. infusion of colostrum in utero. Pediatr. Res. 45, 595–602. Physiol. 72, 61–69.
Sangild, P.T., Trahair, J.F., Silver, M., Fowden, A.L., 1997b. Trahair, J.F., Perry, R.A., Silver, M., Robinson, P.M., 1987b. Luminal fluids affect intestinal enzyme activities in prenatal Studies on the maturation of the small intestine in the fetal pigs. Eur. Ass. Anim. Prod. 88, 194–197. sheep. II. The effects of exogenous cortisol. Q. J. Exp. Physiol.
¨
Sangild, P.T., Westrom, B.R., Fowden, A.L., Silver, M., 1994d. 72, 71–79.
Developmental regulation of the porcine exocrine pancreas by Trahair, J.F., Robinson, P.M., 1986a. Perinatal development of the glucocorticoids. J. Pediatr. Gastroenterol. Nutr. 19, 204–212. small intestine of the sheep. Rep. Nutr. Dev. 26, 1255–1263.
¨
Sangild, P.T., Westrom, B.R., Fowden, A.L., Silver, M., 1994e. Trahair, J.F., Robinson, P.M., 1986b. The development of the Effects of delivery type on gut enzyme development in the ovine small intestine. Anat. Rec. 214, 294–303.
neonate. Eur. Ass. Anim. Prod. 80, 220–222. Trahair, J.F., Sangild, P.T., 1997. Systemic and luminal influences ¨
Sangild, P.T., Westrom, B.R., Silver, M., Fowden, A.L., 1995c. on the perinatal development of the gut. Equine Vet. J. 24 Maturational effects of cortisol on the exocrine abomasum and (Suppl.), 40–50.
¨
development of the GIT and other fetal organs. Gastroenterolo- Westrom, B.R., Svendsen, J., Ohlsson, B.G., Tagesson, C., gy 116, A582. Karlsson, B.W., 1984. Intestinal transmission of macromole-Trahair, J.F., Silver, M., Robinson, P.M., 1988. The role of the cules (BSA- and FITC-labelled dextrans) in the neonatal pig.
adrenal in small intestinal development in the sheep. In: Jones, Biol. Neonate 46, 20–26.
C.T. (Ed.), Research in Perinatal Medicine (VII) – Fetal and Widdowson, E.M., Colombo, V.E., Artavanis, C.A., 1976. Changes Neonatal Development. Perinatalogy Press, Ithaca, NY, pp. in the organs of pigs in response to feeding for the first 24 h 468–471. after birth. II. The digestive tract. Biol. Neonate 28, 272–281. Weaver, L.T., Walker, W.A., 1989. Uptake of macromolecules in Wang, T., Xu, R.J., 1996. Effects of colostrum feeding on the neonate. In: Lebenthal, E. (Ed.), Human Gastrointestinal intestinal development in newborn pigs. Biol. Neonate 70, Development. Raven Press, New York, pp. 731–748. 339–348.
¨
Westrom, B.R., Ohlsson, B.G., Svendsen, J., Tagesson, C., Zhang, H., Malo, C., Buddington, R.K., 1997. Suckling induces Karlsson, B.W., 1985. Intestinal transmission of macromole- rapid intestinal growth and changes in brush border digestive cules (BSA- and FITC-dextran) in the neonatal pig: enhancing functions of newborn pigs. J. Nutr. 127, 418–426.