A
lkaloids are low molecular weight nitrogen-containing substances with characteristic toxicity and pharmacolog-ical activity. These properties, which have traditionally been exploited by humans for hunting, execution and warfare, have also been used for the treatment of disease1. About 20% of plant species accumulate alkaloids, which are mostly derived from the amino acids, Phe, Tyr, Trp, Lys and Orn. In addition, the monoterpenoid indole alkaloids, which form a large class of com-plex compounds, are derived from Trp and terpenoid precursors. Over 12 000 different alkaloids have been described, indicating their structural and biosynthetic diversity compared to that of other secondary metabolites2(Table 1).
Our extensive knowledge about the chemistry and pharmacol-ogy of alkaloids has led to their use in a range of medical appli-cations. However, little is known about their biosynthesis or about the factors that regulate alkaloid production2–5
. Recently, intensive efforts have elucidated the basic biochemistry and molecular biol-ogy of some alkaloid pathways and, more generally, of secondary metabolism6. These discoveries are now enabling more rapid con-sensus cloning and identification of highly specific biochemical reactions involved in the production of interesting alkaloids4,7,8. These results support chemical reasoning that the diversification of secondary metabolism originated from the elaboration of a few central intermediates that could be modified through oxidation, followed by the protection of hydroxyl groups by acylation, glu-cosylation, methylation, or among others, prenylation (Fig. 1). In this context, the Arabidopsis genome, which has yet to be com-pletely sequenced, contains large gene families for each of these classes of biochemical reactions, including cytochrome P450 mono-oxygenases, dioxygenases, acyltransferases, methyltrans-ferases and glucosyltransmethyltrans-ferases6.
Additional studies have begun to illustrate that alkaloid biosyn-thesis is not a random process, but is highly ordered with respect
to plant development, controlling the expression of pathways within organs, within specific cells, or within organelles inside those cells. Here we review recent developments in our understanding of the cell and developmental biology of alkaloid biosynthesis and their biological origins.
Alkaloids of the Solanaceae
The Solanaceae produce a variety of interesting biologically active products including the steroid alkaloids solanidine, nicotine (Fig. 1) and tropane alkaloids (Fig. 2). For example, it is well known that domestication of potatoes involved the successful elimination of toxic steroid alkaloids in tubers while retaining steroid alkaloids in other plant parts for biotic defense. The tropane alkaloids, which have a colorful history of human use, have inspired the creation of several synthetic analogs. These are used to control motion sickness, as powerful bronchodilators to treat chronic bronchitis, as antimuscarinic drugs for the control of Parkinson’s disease, or as midriatics, which are used to dilate the pupil of the eye to facilitate surgery. In the case of nicotine, breeding efforts have focused on increasing the levels of this alkaloid in tobacco for smoking purposes. The continuing appeal of cigarettes to humans can be explained by the rapid absorption, transport and binding of nicotine to acetylcholine receptors in the brain and the ensuing pleasure obtained.
Root-specific scopolamine and nicotine biosynthesis
Roots have been shown to elaborate a remarkable variety of secondary metabolites9
, including alkaloids, flavonoids and terpenoids. Nicotine and tropane alkaloids, which are mainly pro-duced in roots as suggested by reciprocal grafting experiments10
, accumulate mostly within vacuoles2of plant roots and leaves. The early pathway leading to biosynthesis of both nicotine (Fig. 1) and tropane (Fig. 2) alkaloids involves the formation of a pyrrolidine ring that is derived from putrescine via the sequential action of an S-adenosyl-L-methionine-dependent
putrescine-N-methyltrans-ferase (PMT), a diamine oxidase and a spontaneous chemical re-arrangement. The presence of PMT is unique to alkaloid-producing plants and it appears to have evolved from spermidine synthase11 (SPDS), which catalyzes the transfer of the aminopropyl moiety of decarboxylated S-adenosyl-L-methionine to form spermine.
PMT enzyme activity has primarily been detected within roots of tropane alkaloid-producing Atropa belladonna, Hyoscyamus niger and Datura stramonium12, and this has been confirmed by RNA gel blot analyses for A. belladonna13
. Histochemical analysis of transgenic A. belladonna expressing b-glucuronidase behind
The cell and developmental biology
of alkaloid biosynthesis
Vincenzo De Luca and Benoit St Pierre
Plants produce unique natural products as a result of gene mutation and subsequent adap-tation of metabolic pathways to create new secondary metabolites. However, their biosynthesis and accumulation remains remarkably under the control of the biotic and abiotic environments. Alkaloid biosynthesis, which requires the adaptation of cellular activities to perform specialized metabolism without compromising general homeostasis, is accomplished by restricting product biosynthesis and accumulation to particular cells and to defined times of plant devel-opment. The cell and developmental biology of alkaloid biosynthesis, which is remarkably complex, evolved in part by recruiting pre-existing enzymes to perform new functions.
Table 1. Plant secondary metabolites with known structures
Secondary metabolites Number of structures
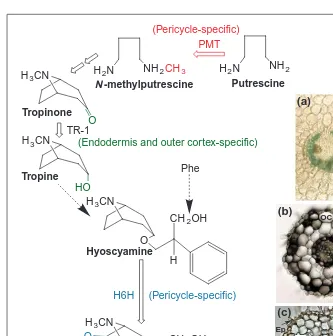
the PMT promotor shows that GUS is expressed specifically in root pericycle cells13
(Fig. 2). The pericycle-specific expression of hyoscyamine-6b -hydroxy-lase, which catalyzes the last step in scopolamine biosynthesis, has also been demonstrated by in situ hybridization stud-ies14(Fig. 2). Localization of the first and last steps in scopolamine biosynthesis in the pericycle, suggested that the rest of the pathway could also be localized there. In addition this suggests that if the root pericycle is the manufacturing centre for tropane alkaloids, its proximity to the adja-cent xylem cells probably facilitates the transport of alkaloids to leaves, where they are known to accumulate.
N-methylputrescine is converted to tropinone, through an incompletely under-stood pathway (Fig. 2) that involves the participation of phenylalanine-derived phenyllactic acid15. Tropinone is then con-verted to tropine or to pseudotropine, respectively, by tropinone reductase (TR) I or II, which catalyze opposite stereospe-cific reductions16. These enzymes are part of a short chain dehydrogenase or reduc-tase gene family, with numerous homolo-gous genes occurring in several plant species that do not make tropane alkaloids. This suggests that some plant homologs might function in other primary metabolic pathways, from which the TR enzymes were possibly recruited.
In contrast with the pericycle-specific expression observed for the first and last steps in scopolamine biosynthesis, TR-1 protein is expressed strongly only in the endodermis and outer cortex17
(Fig. 2). The different cellular compartmentation of PMT, TR-1 and H6H indicates that biosynthetic intermediates must be shuttling between the pericycle and the endodermis to complete
the biosynthetic cycle leading to the production of scopolamine. Further studies to determine the sights of PMT, TR I and H6H expression have shown that only roots express these enzyme activities and contain the proteins that react to antibodies raised against each enzyme17. Dissection of young roots into 5-mm seg-ments has revealed that the expression of each protein is restricted to a region 1–3 cm from the root apex, whereas they are absent in more differentiated root sections. In more mature roots, reactive PMT, TR I and H6H proteins are only detected in the region 1–3 cm from the root apex of the developmentally younger lateral roots. These results confirm that tropane alkaloid biosynthesis is developmentally regulated, in addition to being cell-type specific. The localization of PMT to the pericycle17, where amino acids transported through the vascular tissue are unloaded, would allow ready access to ornithine or arginine, the precursors of putrescine18
. N-methylputrescine or some biosynthetic intermedi-ate could then be transported to the endodermis for further elabo-ration into tropine. It is unclear if tropine is then further elaborated into hyoscyamine before being transported back to the pericycle for conversion into scopolamine. In this context, it would be rele-vant to identify the location of other enzymes involved in the
biosynthesis of tropane alkaloids, such as those that convert phenylalanine into phenyl-lactic acid, and those involved in the formation of the tropate ester precursor of hyoscyamine15. This information could be useful to complete our understanding of the cell-specific compartmentation of tropane alkaloid biosynthesis.
Indole alkaloid biosynthesis in the Catharanthus roseus model system
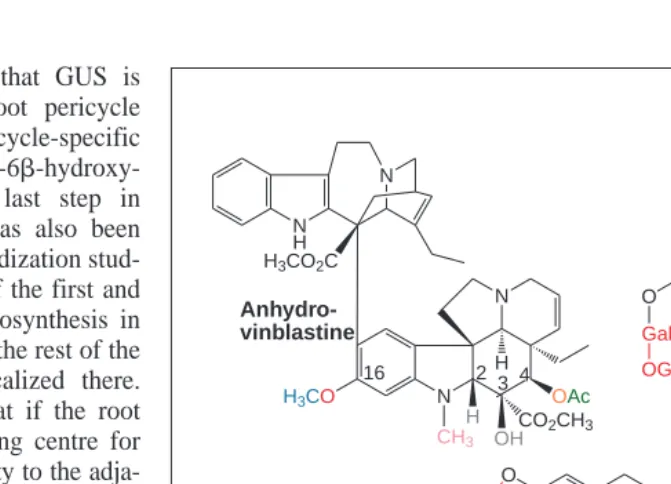
Catharanthus roseus (Madagascar periwinkle) produces monoter-penoid indole alkaloids that are derived from the shikimate and the deoxyxylulose phosphate pathways. The biosynthesis of the indole moiety requires tryptamine, which is derived from trypto-phan by the action of tryptotrypto-phan decarboxylase (TDC). The biosynthesis of the terpenoid moiety requires secologanin, which is derived from geraniol via a series of enzymatic conversions19,20. The committed step in monoterpenoid indole alkaloid biosynthe-sis involves a vacuole-specific21 strictosidine synthase (Str1), which catalyzes the stereospecific condensation of tryptamine and secologanin to yield 3-a(S)-strictosidine (Fig. 3). Several thou-sand strictosidine-derived indole alkaloids have been character-ized, a few of which are used for the treatment of hypertension and
Fig. 1. Typical substitution reactions involved in the diversification of alkaloid structures.
Anhydro-vinblastine is composed of catharanthine (upper molecule) and vindoline (lower molecule). Vindoline is derived from tabersonine by sequential aromatic hydroxylation at posi-tion 16 (red), 16-O-methylaposi-tion (blue), hydraposi-tion at posiposi-tion 2,3 (gray), N-methylaposi-tion (pink), hydroxylation at position 4 (orange) and 4-O-acetylation (green). Steroid alkaloids, such as solanidine are glycosides produced by the action of specific O-glycosyltransferases, which transfer sugars such as galactose, glucose and rhamnose (red) to the free or glycosylated aglycone. Nicotine is derived from N-methylputrescine and nicotinic acid.
Putrescine-N-methyltransferase transfers the methyl group (red) from S-adenosylmethionine. The
quinolizidine alkaloid (1)-13a-tigloyloxylupanine is produced by acylation (green) of 13-hydroxylupanine. Berberine and macarpine are isoquinoline alkaloids that accumulate in plants such as Eschscholtia californica and Papaver somniferum. Biosynthesis of berberine4
requires the participation of two flavinylated oxidases and a cytochrome P450, whereas biosynthesis of the more highly oxidized alkaloid, macarpine, involves six cytochromes P450 in addition to two other oxidases. Some of the reactions involving cytochromes P450 are highlighted in red in the berberine and macarpine molecules. Several intermediate methyl groups are highlighted in blue on the berberine and macarpine molecules. These reactions are catalyzed by substrate-specific O-methyltransferases that produce desired methylation patterns.
Trends in Plant Science
for several antineoplastic ailments22
. In particular, vinblastine and vincristine from C. roseus are valuable chemotherapeutic agents currently used in the treatment of several cancerous diseases (Fig. 1).
Indole alkaloid biosynthesis is under cell-, tissue-, development-and environment-specific control
The commercial importance of vinblastine and vincristine have led to considerable efforts to produce these chemicals in high yielding callus, cell suspension, and hairy root culture systems, but without apparent success23. The ability of these culture sys-tems to accumulate catharanthine and tabersonine, but not vindo-line, suggested that the biosynthesis of catharanthine and vindoline is differentially regulated and that vindoline biosynthesis is under a more rigid cell-, tissue-, development- and environ-ment-specific control than that of catharanthine19,20
. This hypothe-sis was further corroborated by studies that suggested that the ability to synthesize and accumulate vindoline reappeared with the regeneration of shoots and in shoot tissue cultures23.
The use of germinating seedlings of C. roseus growing in the light or in its absence have been of great importance in elucidating how vindoline is made in the intact plant and in dissecting the unique morphogenetic- and environment-specific events involved
in activating the vindoline pathway19 . If Catharanthus seeds are germinated and grown in the absence of light, they accumu-late high levels of tabersonine, as well as small amounts of four other intermediates between tabersonine and vindoline. The growth of etiolated seedlings in the presence of light, stimulated the quantitative, large-scale turnover of tabersonine and the interme-diates of vindoline by sequential aromatic hydroxylation, O-methylation, hydration, N-methylation, hydroxylation and O-acetylation (Fig. 1). These results suggest that light treat-ment activates the late stages of vindoline biosynthesis, and that three hydroxylases, O-methyltransferase, N-methyltransferase and O-acetyltransferase are involved in the con-version of tabersonine into vindoline19,20.
Further biochemical studies have resolved that:
• Tabersonine-16-hydroxylase belongs to the class of cytochrome P450-dependent mono-oxygenases, which are usually associated with the external face of the endoplasmic reticulum24
.
• N-methyltransferase is associated with chloroplast thylakoids19
.
• Terminal desacetoxyvindoline-4-hydro-xylase (D4H)25
and deacetylvindoline-4-O-acetyltransferase (DAT)8 are cyto-solic enzymes (Fig. 1).
These results indicate that the cytosolic face of the endoplasmic reticulum, the vacuole, the chloroplast thylakoid and the cytoplasmic compartment of the cell are all involved in vindoline biosynthesis.
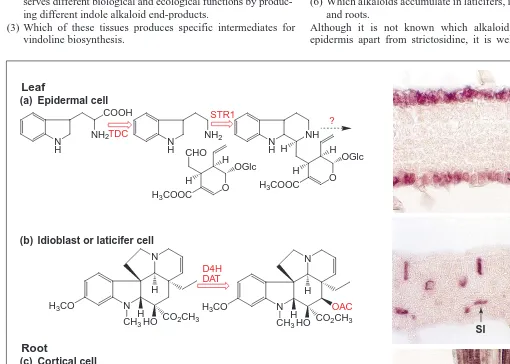
Cell-specific distribution of TDC, STR1, D4H and DAT
The genes for cytochrome P450 tabersonine-16-hydroxylase (Ref. 26), D4H (Ref. 25) and DAT (Ref. 8) have been cloned recently. Detailed immunological and molecular studies strongly suggest that D4H and DAT are only expressed in the above-ground plant parts, whereas TDC and STR1 occur throughout the plant.
C. roseus has simple, elliptical mesomorphic leaves (Box 1) composed of several cell types27
. The upper and lower epidermis are composed of thin-walled cells arranged in a single layer, whereas the mesophyll is arranged into a single layer of elongated palisade parenchyma on the adaxial side and a thicker multicellular spongy parenchyma on the abaxial side of the leaf (Fig. 2). In addition, unbranched, nonarticulated laticifers (Box 1) are associated with the veins, which are curved and diverge from the midrib at a 35–458. Branching from these are smaller veins usually composed of a tracheary element and a laticifer. The palisade and spongy mesophyll also contain idioblasts (Box 1), which are identified by their distinctive yellow autoflorescence when the leaves are illu-minated by blue light, and by their distinctively larger size than the surrounding mesophyll cells.
In situ RNA hybridization and immunocytochemistry27 have established that TDC and STR1 are localized to the epidermis of stems, leaves (Fig. 3) and flower buds, whereas they appear in most protoderm and cortical cells around the apical meristem of root tips (Fig. 3). D4H and DAT are associated with laticifers and
Fig. 2. Histochemical localization of tropane alkaloid biosynthesis in root cross-sections. (a)
Putrescine-N-methyltransferase (PMT) promotor GUS fusion expression has been detected by GUS staining in roots of Atropa belladonna. Reproduced, with permission, from Ref. 13. Immunological localization of (b) tropinone reductase (TR-1) and (c) hyoscyamine 6b -hydroxylase (H6H) in roots of Hyoscyamus niger. (b) and (c) reproduced, with permission,
from Ref. 17. The three biochemical steps leading to scopolamine biosynthesis and their
cel-lular location are depicted, respectively, in red (PMT), green (TR-1) and blue (H6H). The Phenyllactic acid component of hyoscyamine is derived from Phe. Abbreviations: Ep, epidermis; OC, outer cortex; IC, inner cortex; En, endodermis; P, pericycle.
NH2 H2N
NH2CH3 H2N
(Pericycle-specific)
H3CN
O
CH2OH
H
H3CN
O
CH2OH
H
O
H6H (Pericycle-specific)
Scopolamine Hyoscyamine H3CN
O
H3CN
HO
Tropine Tropinone
PMT
TR-1
(Endodermis and outer cortex-specific)
Phe
Putrescine N -methylputrescine
OC
IC
Ep (a)
(b)
(c)
idioblast cells of leaves, stems and flower buds (Fig. 3). These results provide significant new evidence that, in addition to intra-cellular specialization involving multiple intra-cellular compartments, at least two cell types requiring intercellular translocation of a pathway intermediate are needed to allow the biosynthesis of vindoline in C. roseus.
Additional studies27
have shown that alkaloid biosynthesis only occurs transiently during early leaf, stem and root development. In situ hybridization and immunocytochemical studies have revealed that alkaloid biosynthesis follows a basipetal distribution in young expanding leaves. This expression pattern, together with the results obtained in young roots (Fig. 3), suggests that alkaloid biosynthesis is activated in young rapidly dividing cells, whereas it is down regulated in more mature tissues.
The results raise interesting questions about:
(1) The number of pathway steps occurring in the epidermis or in the root apex.
(2) Whether alkaloid biosynthesis in roots and in epidermal tissues serves different biological and ecological functions by produc-ing different indole alkaloid end-products.
(3) Which of these tissues produces specific intermediates for vindoline biosynthesis.
(4) How intermediates are mobilized into laticifers and idioblasts. (5) Why this elaborate cell-specific expression is required. (6) Which alkaloids accumulate in laticifers, idioblasts, epidermis
and roots.
Although it is not known which alkaloids are made in the epidermis apart from strictosidine, it is well known that roots
Fig. 3. Cell-specific localization of tdc, str1, d4h and dat mRNAs in developing Catharanthus leaves and roots27
. Leaf or root cross-sections were reacted with antisense digoxigenin-labeled transcripts. Hybridized transcripts were localized with antidigoxigenin-alkaline phosphatase congugate followed by BCIP/nitro tetrazolium color development. (a) Both tdc and str1 (data not shown) are expressed in the upper and lower leaf epidermis. (b) Both d4h (data not shown) and dat are expressed in leaf idioblasts and laticifers. (c) Both tdc and str1 (data not shown) are expressed in root protoderm and cortical cells around the apical meristem of root tips. Abbreviations: C, root cortex; CL, cross-connecting laticifer cells; LE, lower epidermis; PI, palisade mesophyll-associated idioblast cells; SI, spongy mesophyll-associated idioblast cell; T, tra-cheid; UE, upper epidermis. Reproduced, with permission, from Ref. 27.
N N
HO
H CO
2CH3 H3CO
CH3 H
N N
HO
H CO
2CH3 OAC H3CO
CH3 H N
H
NH2 COOH
N H
NH2
CHO
O
H3COOC
OGlc H
H
N H
NH
O
H3COOC
OGlc H
H H TDC
STR1
?
D4H DAT Leaf
Root
N H
NH2 COOH
N H
NH2
CHO
O
H3COOC
OGlc H
H
N H
NH
O
H3COOC
OGlc H
H H TDC
STR1
?
(a)
(b)
(c)
Epidermal cell
Idioblast or laticifer cell
Cortical cell
UE
LE
PI
CL
SI
T
C
Box 1. Glossary
Idioblast – specialized plant cell that contains a distinctive chemical
composition compared with surrounding cells.
Laticifer – there are two main types, both are long and narrow and
can be branched or unbranched.
Nonarticulated (non-branched) laticifer – specialized cell that
con-tains a milky, chemically complex fluid called latex. Non-articulated laticifers initiate as single cells and grow to a large size by intrusive growth (pushing their way between other cells). They continue to grow as the plant grows and become giant coenocytic cells.
Mesomorphic leaf – leaves adapted to environments where there is
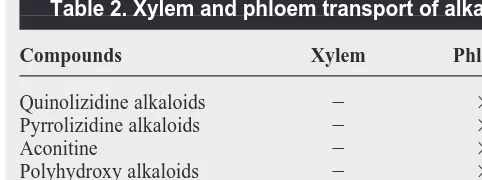
accumulate many alkaloids, including catharanthine, tabersonine and derivatives of tabersonine. Therefore it is possible that root-synthesized catharanthine and tabersonine are transported via xylem and laticifer-associated tracheids to supply these precursors to laticifers. This is plausible because there are several examples of phloem and xylem-based transport of alkaloids2,5
(Table 2). The laticifer could convert tabersonine into vindoline, which would then be coupled by a non-specific peroxidase-mediated process28
with catharanthine to yield the antineoplastic agents vinblastine and vin-cristine. Therefore the localization of the late stages of vindoline biosynthesis to the laticifer might ensure sequestration of the toxic antineoplastic agents that would be produced with the presence of catharanthine and vindoline in the same cell. However, the ability of shoot cultures lacking roots, to accumulate vindoline23
, suggests roots might not be the only source of catharanthine and tabersonine.
The chloroplast as the site of quinolizidine alkaloid biosynthesis
Biosynthesis of lysine-derived quinolizidine alkaloids, which are found in many legumes, such as lupins (Lupinus), appears to occur within the mesophyll chloroplasts of green leaves5. A biochemical-localization study has been performed with two acyltransferases that catalyze terminal acylations leading to the production, respec-tively, of (1) p-coumaroylepilupinine and (2) 13a tigloyloxy-multiflorine29(Fig. 1). This study in Lupinus albus demonstrated that one acylation occurred within the cytoplasm and the other in mitochondria, but not within chloroplasts. These findings suggest that although chloroplasts might be responsible for the formation of the quinolizidine skeleton5, further modifications must be occurring after intracellular transport to the cytosol and to the mitochondria. The need for tigloylation to occur within the mito-chondria can be explained by the fact that the acyl donor, tigloyl-CoA, which is derived via a three-step process from isoleucine, is produced within mitochondria in animal systems. Therefore an analogous pathway might be present in plants. The endproducts of biosynthesis are thought to accumulate particularly within the vac-uoles of the epidermis of lupin plants, where their defensive roles can be most effectively exploited by the plant2,5
. This adds the mitochondria to the growing list of cellular organelles that might participate in alkaloid biosynthesis. These studies also illustrate how plants have been opportunistic to adapt primary metabolic processes, as well as the organelles in which they evolved, to permit the diversification of secondary metabolism.
Isoquinoline alkaloid biosynthesis
The biosynthesis of isoquinoline alkaloids3,4,30
proceeds via the decarboxylation of tyrosine or DOPA to yield the respective amine precursors. Dopamine and p-hydroxyphenylacetaldehyde are then combined by the action of (S)-norcoclaurine synthase to
yield (S)-norcoclaurine, which is a central precursor to several thousand isoquinoline alkaloids. In situ-hybridization studies in opium poppy (Papaver) with tyrosine/DOPA decarboxylase (TYDC) have revealed that the expression of two subgroups of differentially expressed genes are restricted to the metaphloem and to the protoxylem of vascular tissues in mature stems and roots31
. Opium poppy alkaloids accumulate within laticifers, which are derived from the metaphloem. The restriction of TYDC expression to these particular cells illustrates the strict control to which this gene family is subject.
Further biochemical localization studies30
have been performed using Berberis wilsoniae cell cultures. Berberine bridge enzyme (BBE) and (S)-tetrahydroberberine oxidase, which catalyze the third to last and final steps of berberine (Fig. 1) biosynthesis, are localized to a membrane fraction with a different specific gravity from those of the endoplasmic reticulum and plant vacuoles. The biochemical localization results were in part corroborated when the berberine bridge enzyme was cloned32and it was shown to contain a 22 amino acid signal peptide directing it to the endo-plasmic reticulum. Additional studies in the Catharanthus model system for indole alkaloid biosynthesis have also shown that Str1 contains a putative signal peptide that is cleaved from the mature protein33
, which appears to be located within plant vacuoles21 . These results suggest that unique specialized cellular compartments in plants serve as scaffolds for alkaloid biosynthesis.
Several vacuolar homologs of BBE (Accession no. Al080254) and Str1 (Accession no. P92976) have been identified in Arabidop-sis recently, which is not known to make alkaloids. The presence of these homologs could indicate that the BBE and Str1 reactions occur in the vacuole because these genes evolved from common ancestors that had other vacuolar functions. Therefore, the complex intracellu-lar compartmentation of alkaloid biosynthesis might have occurred as a consequence of adapting compartmented reactions of primary metabolism to participate in alkaloid biosynthesis. The recent cloning of homospermidine synthase34
, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, clearly showed that it had evolved from deoxyhypusine synthase (DHS). DHS is highly conserved among eukaryotes and archaebacteria where it catalyzes the first step in the activation of translation initiation factor 5A, which is essential for eukaryotic cell proliferation. This is a further example of recruiting a gene involved in a primary process to perform a pathway-specific function in alkaloid biosynthesis
Conclusions
Recently, rapid progress has been made in our understanding of the biochemistry, molecular biology and cell biology of alkaloid biosynthesis in plants. This fundamental understanding is expected to lead to rapid progress in the isolation of almost any desired gene. The availability of these tools have been used to study the cell and developmental processes leading to the synthe-sis and accumulation of alkaloids that are often toxic to the host as well as to potential predators. The data from several different alkaloid-producing plants suggests that their biosynthesis and accumulation involve a highly regulated process that includes cell-, tissue-, development- and environment-specific controls. The evolution of alkaloid pathways together with their cellular compartmentation appears to be closely associated with the pri-mary reactions from which they have evolved. With the avail-ability of an increasing number of genes involved in alkaloid biosynthesis, increasing efforts will be made to identify the regu-lators35
that are associated with the development of specialized cell-types that accommodate alkaloid biosynthesis and accumu-lation. Continuing studies promise to yield exciting new discoveries about the biological origins of alkaloid biosynthesis in plants. Table 2. Xylem and phloem transport of alkaloidsa
Compounds Xylem Phloem
Quinolizidine alkaloids 2 3
Pyrrolizidine alkaloids 2 3
Aconitine 2 3
Polyhydroxy alkaloids 2 3
Nicotine alkaloids 3 2
Tropane alkaloids 3 2
aReproduced, with permission, from Ref. 5.
Acknowledgements
We thank Takashi Hashimoto for the photographs used in Figure 2. We also thank Michael Wink for sharing unpublished reviews. This work was supported by a grant to V.D.L. from the Natural Sciences and Engineering Council of Canada.
References
1 Mann, J. (1992) Murder, Magic and Medicine, Oxford University Press 2 Wink, M. (1999) Plant secondary metabolites: biochemistry, function and
biotechnology. In Biochemistry of Plant Secondary Metabolism (Annual Plant Reviews) (Vol. 2) (Wink, M., ed.), pp. 1–16, Sheffield
Academic Press
3 Kutchan, T.M. (1995) Alkaloid biosynthesis – the basis for metabolic engineering of medicinal plants. Plant Cell 7, 1059–1070
4 Chou, W.M. and Kutchan, T.M. (1998) Enzymatic oxidations in the biosynthesis of complex alkaloids. Plant J. 15, 289–300
5 Wink, M. and Roberts, M.F. (1998) Compartmentation of alkaloid biosynthesis, transport and storage. In Alkaloids: Biochemistry, Ecology and Medicinal Applications (Roberts, M.F. and Wink, M., eds), pp. 239–262, Plenum Press
6 Facchini, P. (1999) Plant secondary metabolism: out of the evolutionary abyss. Trends Plant Sci. 4, 382–384
7 De Carolis, E. and De Luca, V. (1994) 2-oxoglutarate-dependent dioxygenases and related enzymes: biochemical characterization. Phytochemistry 36, 1093–1107 8 St-Pierre, B. et al. (1998) The terminal O-acetyltransferase involved in
vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J. 14, 703–713
9 Flores, H.E. et al. (1999) ‘Radicle’ biochemistry: the biology of root-specific metabolism. Trends Plant Sci. 4, 220–226
10 Waller, G.R. and Nowacki, E.K. (1979) Alkaloid Biology and Metabolism in Plants, Plenum Press
11 Hibi, N. et al. (1994) Gene expression in tobacco low nicotine mutants. Plant Cell 6, 723–735
12 Hibi, N. et al. (1992) Putrescine-N-methyltransferase in cultured roots of Hyoscyamus albus. Plant Physiol. 100, 826–835
13 Suzuki, K. et al. (1999) Expression of Atropa belladonna putrescine N-methyltransferase in root pericycle. Plant Cell Physiol. 40, 289–297 14 Suzuki, K. et al. (1999) An Atropa belladonna hyoscyamine 6a-hydroxylase
gene is differentially expressed in the root pericycle and anthers. Plant Mol. Biol. 40, 141–152
15 Zabetakis, I. et al. (1998) The biosynthetic relationship between littorine and hyoscyamine in transformed roots of Datura stramonium. Plant Cell Rep. 18, 341–345
16 Nakajima, K. et al. (1993) Two tropinone reductases with different stereospecificities are short chain dehydrogenases evolved from a common ancestor. Proc. Natl. Acad. Sci. U. S. A. 90, 9591–9595
17 Nakajima, K. and Hashimoto, T. (1999) Two tropinone reductases, that catalyse opposite stereospecific reductions in tropane alkaloid biosynthesis, are localized in plant root with different cell-specific patterns. Plant Cell Physiol. 40, 1099–1107
18 Hashimoto, T. and Yamada, Y. (1994) Alkaloid biogenesis: molecular aspects. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45, 257–285
19 De Luca, V. (1993) Indole alkaloid biosynthesis. In Methods in Plant Biochemistry. Enzymes of Secondary Metabolism (Vol. 9) (Lea, P., ed.), pp. 345–368, Academic Press
20 Meijer, A.M. et al. (1993) Regulation of enzymes and genes involved in terpenoid indole alkaloid biosyntheis in Catharanthus roseus. J. Plant Res. (Special Issue) 3, 145–164
21 McKnight, T.D. et al. (1991) Expression of enzymatically active and correctly targeted strictosidine synthase in transgenic tobacco plants. Planta 185, 148–152
22 Van Tellingen, O. et al. (1992) Pharmacology, bio-analysis and pharmacokinetics of the vinca alkaloids and semi-synthetic derivatives. Anticancer Res. 12, 1699–1715
23 Van der Heijden, R. et al. (1989) Cell and tissue cultures of Catharanthus roseus (L.) G. Don: a literature survey. Plant Cell, Tissue Organ Cult. 18, 231–280
24 St-Pierre, B. and De Luca, V. (1995) A cytochrome P450 monooxygenase catalyses the first step in the conversion of tabersonine to vindoline in Catharanthus roseus. Plant Physiol. 109, 131–139
25 Vazquez-Flota, F. et al. (1997) Molecular cloning and characterization of desacetoxyvindoline-4-hydroxylase, a 2-oxoglutarate-dependent dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus. Plant Mol. Biol. 34, 935–948
26 Schroder, G. et al. (1999) Light-induced cytochrome P450-dependent enzyme in indole alkaloid biosynthesis: tabersonine 16-hydroxylase. FEBS Lett. 458, 97–102
27 St-Pierre, B. et al. (1999) Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11, 887–900
28 Sottomayor, M. et al. (1998) Purification and characterization of a-39,49-anhydrovinblastine synthase (peroxidase-like) from Catharanthus roseus (L.) G. Don. FEBS Lett. 428, 299–303
29 Suzuki, H. et al. (1996) Subcellular localization of acyltransferases for quinolizidine alkaloid biosynthesis in Lupinus. Phytochemistry 42, 1557–1562 30 Kutchan, T.M. and Zenk, M. (1993) Enzymology and molecular biology of
benzophenanthridine alkaloid biosynthesis. J. Plant Res. (Special issue) 3, 165–173 31 Facchini, P.J. and De Luca, V. (1995) Phloem-specific expression of
tyrosine/dopa decarboxylase genes and the biosynthesis of isoquinoline alkaloids in opium poppy. Plant Cell 7, 1811–1821
32 Dittrich, H. and Kutchan, T.M. (1991) Molecular cloning, expression and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response to pathogen attack. Proc. Natl. Acad. Sci. U. S. A. 88, 9969–9973
33 McKnight, T.D. et al. (1990) Nucleotide sequence of a cDNA encoding the vacuolar protein strictosidine synthase from Catharanthus roseus. Nucleic Acids Res. 18, 4939
34 Ober, D. and Hartmann, T. (1999) Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. U. S. A. 96, 14777–14782 35 Menke, F.L. et al. (1999) A novel jasmonate- and elicitor-responsive element in
the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate- and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO J. 18, 4455–4463
Vincenzo De Luca*is at Novartis Agribusiness Biotechnology Research Inc., 3054 Cornwallis Road, Research Triangle Park, NC 27709-2257, USA; Benoit St Pierre is at Laboratoire de Physiologie Végétale, EA 2106, UFR des Sciences et Techniques, Université de Tours, Parc de Grandmont, 37200 TOURS, France (tel 133 247 367101; fax 133 247 367042;
e-mail [email protected]).
*Author for correspondence (tel 11 919 541 8575;
fax 11 919 541 8585; e-mail [email protected]).
Students
Did you know that you are entitled to a 50% discount on a subscription to
Trends in Plant Science?