I
n multicellular organisms, the expression of thousands of protein-encoding genes is subject to complex patterns of spatial and temporal regulation. The first step in transcriptional regu-lation of any gene is the integration of various signals so that they change the rate of transcription of their target gene1–3. The speci-ficity of gene expression is at least in part governed by transcrip-tional activators and repressors, many of which bind DNA in a sequence-specific manner4
. These proteins are themselves subject to regulation at different levels5
(Fig. 1).
Activators and repressors
Transcriptional activators and repressors generally consist of several functional modules with DNA-binding, activation and repression domains. On the basis of their usually highly conserved DNA-binding domains, most eukaryotic transcription factors can be
assigned to one of several classes; these include basic region-leucine zipper (bZIP) proteins, MYB-like proteins, MADS-domain proteins, helix–loop–helix proteins, zinc-finger proteins and homeo-box proteins6,7
. Within these classes, DNA-binding specificity is brought about by subtle changes in the amino acid sequence of the DNA-binding domain.
In sharp contrast to the DNA-binding domains of transcrip-tion factors, the amino acid sequences that comprise their acti-vation and repression domains are not conserved, and consensus sequences have yet to be derived from the many eukaryotic acti-vation domains that have been functionally defined to date8,9
. On the basis of their amino acid composition, activation domains can only be classified as being rich in acidic amino acids, in glutamines, prolines, or serine and threonine residues. How-ever, not all activation domains fit into these general categories, and mutational analyses (also carried out with plant tran-scription factors) have shown that those amino acids that predominate in activation do-mains are not necessarily im-portant for their function8,10,11
. It may be that the precise molecular interactions that lead to transcriptional acti-vation vary between different transcription factors in dif-ferent promoter contexts, or that mechanisms other than specific protein–protein inter-actions govern transcriptional activation.
The classification system of transcription factor repres-sion domains is poorly charac-terized9
. This may be because research on repressors is com-plicated by the fact that it is difficult to distinguish between genuine effects of a repressor protein and the effects caused by a dominant negative form of the protein. It is thought that repressors act in one of three ways:
• By binding to a cognate pro-moter site and blocking the binding of general transcrip-tion factors or activators.
The regulation of transcription factor
activity in plants
Claus Schwechheimer and Michael Bevan
The predominant mechanism for controlling gene expression in eukaryotes appears to be regulation at the transcriptional level. Transcriptional regulation of gene expression is mediated by transcription factors, which activate or repress transcription. These activators and repressors act through several mechanisms (including DNA–protein interactions, protein– protein interactions and modification of the chromatin structure) and are themselves regu-lated in a variety of ways. Using various modes, a single transcription factor can influence the transcription of different target genes. Recent research on these regulatory mechanisms in plants has provided insight into the underlying processes.
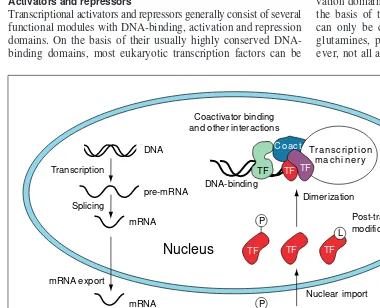
Fig. 1.Regulatory mechanisms that can play a part in the regulation of transcription factor activity. Abbreviations used TF, transcription factor; Coact, coactivator; IN, inhibitor; P, phosphate; L, ligand binding. Adapted from Ref. 5.
P
TF TF
L
TF
TF P
TF
L
TF
Post-translational modifications DNA
Transcription
Splicing
mRNA export
pre-mRNA
mRNA
mRNA
TF Translation
Inhibitor binding
Nuclear import DNA-binding
Dimerization
Dissociation Coactivator binding and other interactions
Post-translational modifications
Cytoplasm
Nucleus
IN
TF TF TF
Transcription m a c h i n e r y Coact
TF
• By blocking transcription by means of inhibitory interactions with general transcription factors or activators.
• By altering the higher-order DNA structure in a way that in-hibits transcription.
VIVIPAROUS-1, an activator and repressor of gene expression
The complexities of transcriptional regulation have been elegantly demonstrated in a series of studies on the maize transcription fac-tor Viviparous-1 (VP1). VP1 is a key regulafac-tor of seed maturation and germination and severe vp1mutations cause premature ger-mination of the seed on the maize cob12,13
(Fig. 2a). During seed maturation, VP1 acts as transcriptional activator of the (wheat)
EMgene14
and the (maize) Colourless-1(C1) genes15,16
, but at the same time it can mediate repression of (barley) a-amylase gene expression17
(Fig. 2b). All vp1 mutants are insensitive to abscisic acid (ABA), and as they do not express the C1 transcriptional regu-lator they are devoid of anthocyanin pigmentation in the seed in the dark (Fig. 2a). However, there are colourless mutants of VP1 that do not express C1, but which undergo normal seed dormancy and also express the EMgene18
. This suggests that two different mechanisms govern the activation of C1and EMgene expression during seed development.
VP1 activates C1 gene expression
The expression of C1can be activated independently by three factors: VP1 expression, ABA and light15,16
(Fig. 2a). The Sph-promoter-element of the C1promoter has been shown to be sufficient and necessary for VP1-mediated activation in transient expression studies of maize vp1embryos and suspension culture cells15,16
. A corresponding VP1 DNA-binding domain (B3 domain) was recently identified that can bind to the Sph-element in vitro19
. Transient ex-pression experiments have revealed that this DNA-binding domain is required for transcriptional activation of C1 in the absence of light20
. In addition, an activation domain (the A domain) was mapped to the highly acidic N-terminus of VP1, which when deleted abolishes transcriptional activation in transient expression assays14
. The C1 promoter element that confers a response to ABA is tightly linked to, but apparently distinct from, the VP1-responsive Sph-element16
. Light-inducibility of the C1promoter is thought to be mediated by a promoter region that comprises a light-responsive G-box el-ement located downstream of the Sph-elel-ement16
(Fig. 2b). This lat-ter finding is intriguing, because G-box binding factors (GBFs) have been suggested to be important for the expression of light-regulated genes21
. Synthesis of VP1 in organs other than seeds cannot activate C1 expression, but light is sufficient to activate C1 expression in seeds and leaves (Fig. 2a).
Fig. 2.(a) Two selfed maize ears segregating for Viviparous-1(vp1). The vp1mutant seed germinate prematurely on the maize cob. In this mutant, anthocyanin synthesis in the aleurone layer is suppressed (right cob) because of the lack of VP1; VP1 activates Colourless-1(C1) gene expression, a transcriptional activator of genes that encode for enzymes of the anthocyanin biosynthetic pathway. Light can activate C1 expres-sion independently from VP1; this accounts for the anthocyanin pigmen-tation on the viviparous kernels of the left cob where the husks of the ear have been removed to admit light. (b) VP1 participates in the acti-vation of C1 and EM and the repression of a-amylase gene expression. The schematic shows the different target genes, and factors that have a positive or negative influence on gene expression. Domains of VP1 known to be involved in the particular processes are indicated above the protein. Abbreviations: ABA, abscisic acid; GA, gibberellic acid.
EMBP1 (+ osZIP-1a)
EM
1a 1b
Sph
VP1 B2
RY
ABA
osZIP-2a osZIP-2b GT Sph G-box
VP1
A B3
ABA Light
a- A m y l a s e VP1
B1
GA ABA
?
Anthocyanins
Seed maturation
Seed germination
( b )
VP1 activates expression of the EM gene
In the EMpromoter, VP1 can activate transcription from several promoter elements. VP1 is sufficient to activate transcription from an Sph-like and an RY-promoter element and is necessary but not sufficient for transcriptional activation from two G-box like motifs (Em1a and Em1b)22,23
(Fig. 2b). The expression of EMfrom these G-box elements is synergistically activated by VP1 and ABA. As a tetramer, Em1a and Em1b can confer ABA-responsiveness to a minimal promoter22
. Studies using southwestern screening have identified the bZIP transcription factor EMBP1 that binds the Em1a promoter, and to a lesser extent the Em1b element23
. In contrast to its role on the C1 promoter, VP1 appears to act by facilitating the binding of EMBP1 to the Em1a and Em1b elements. Binding studies in vitrorevealed that a basic region of VP1 (the BR2 domain) possessing a weak and non-specific DNA binding capacity can greatly enhance the binding of EMBP1 to its target sites. Thus, while VP1 activates the expression of C1 through direct promoter binding and transcriptional activation, VP1 potentiates EM expres-sion by encouraging binding of the transcription factor EMBP1.
VP1 is a repressor of a-amylase gene expression
In contrast to its activating function, VP1 represses induction of a germination-specific a-amylase gene during seed maturation17
(Fig. 2b). Repression occurs even when the VP1 activation do-main has been deleted. This suggests that the VP1 dodo-main required for repression is distinct from the activation function, and that VP1 is directly repressing a-amylase gene expression rather than activating the expression of another protein that in turn represses
a-amylase gene expression. The repression domain on VP1 that is responsible for this has yet to be determined. During seed germi-nation, a-amylase production is induced by gibberellic acids, but VP1 overexpression can override hormone-induced activation dur-ing seed germination.
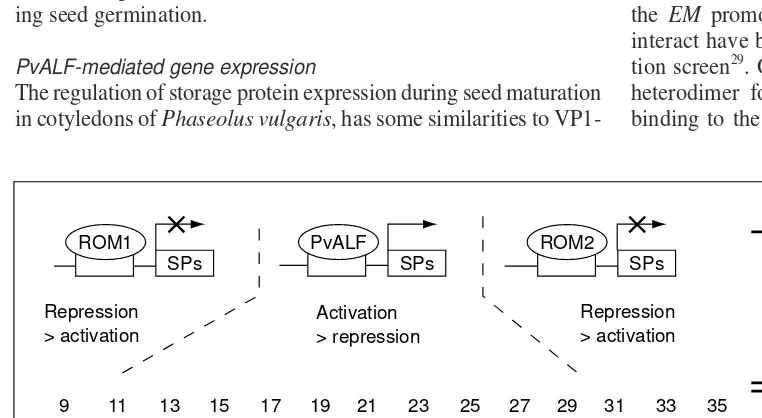
PvALF-mediated gene expression
The regulation of storage protein expression during seed maturation in cotyledons of Phaseolus vulgaris, has some similarities to
VP1-mediated expression24
. PvALF, a putative homolog of VP1, acts as a transcriptional activator of the storage proteins phytohemag-glutinin and b-phaseolin in cotyledons. In bombardment studies, it was shown that the activation potential of PvALF can be further enhanced by the addition of ABA – an effect similar to that ob-served with VP1. Transcriptional activation mediated by PvALF appears to be counteracted by the two bZIP-like repressors ROM1 and ROM2, which are expressed at different stages during cotyle-don development25,26
(Fig. 3). ROM1 represses transcription early in seed maturation but its expression declines at the onset of storage protein synthesis26
, whereas the onset of ROM2 expres-sion coincides with the decline of storage protein production25
. A reduction in storage protein concentrations has also been reported for vp1mutant maize plants27
, and repressors similar to the ones identified for PvALF may also control storage protein production in maize.
Dimerization
Transcription factor activity is also affected by interactions with other DNA-binding proteins or non DNA-binding accessory proteins28
. Dimerization increases the number of transcription factor complexes that can be formed from a smaller number of homeomeric components and can have a positive or negative in-fluence on their DNA-binding characteristics, activation poten-tial, or nuclear localization. The dimerization process is regulated by the availability of their respective partners, their affinity for each other and the affinity of the dimer for other components (such as DNA) – this sensitizes responses to minor fluctuations in effector concentration.
The bZIP and the MADS-domain class of transcription factors are well characterized dimerizing proteins in plants. In the case of the wheat bZIP protein EMBP1, which acts together with VP1 on the EM promoter, three bZIP proteins (osZIPs) that appear to interact have been identified from rice using an in vitro interac-tion screen29
. Gel retardation experiments have revealed that the heterodimer formed by EMBP1 and osZIP-1 shows enhanced binding to the Em-1a element in the EM-promoter, but the het-erodimer formed by EMBP1 and osZIP-2a or osZIP-2b pre-vents binding of EMBP1 to its cognate promoter binding site (Fig. 2b). In addition to osZIP-1, histone H1 has also been reported to enhance bind-ing of EMBP-1 to its promoter binding sites in vitro30
. This suggests that the interplay between inhibitory and stimu-latory heterodimers is a regu-latory mechanism for EMBP-1 activity, and DNA-binding in general. However, these dif-ferent proteins originate from different species, and further research will be needed to con-firm these observations.
The activity of the Myb-like transcription factor C1 is also regulated by heterodimer-ization31
. Genetic studies and transient expression assays re-vealed that C1 is unable to acti-vate expression of its target genes (these encode enzymes
Fig. 3.The activity of PvALF, a putative bean homologue of maize Viviparous-1 (VP1), is counteracted by the repressor proteins ROM1 and ROM2, which are expressed at different stages during seed matu-ration and germination. The three proteins together regulate the synthesis of the bean storage proteins (SPs) b-phaseolin and phytohemagglutinin.
of the anthocyanin biosynthetic pathway) in the absence of the helix-loop-helix transcription factor B-Peru32–34
. Using the yeast two-hybrid system, it was found that B-Peru can interact with the DNA-binding domain of C1 (Ref. 33) and that this inter-action enhances the DNA-binding specificity of C1. However, the extent to which the putative basic helix–loop–helix DNA-binding domain of B-Peru participates in this DNA-DNA-binding is unclear.
Dimerization can also regulate nuclear localization. Evidence from genetic analysis have suggested that the activities of the hom-eotic MADS-box proteins APETALA-3 (AP3) and PISTILLATA (PI), which in Arabidopsis are responsible for the initiation of petal and pistil formation, are interdependent35
. DNA-binding stud-ies in vitrohave revealed that AP3 and PI can form a heterodimer. By transforming AP3- and PI-GUS fusion constructs into an ap3/pi mutant background, it was possible to demonstrate that the interaction between AP3 and PI is crucial for the nuclear import of the transcription factors. In these transgenic plants, GUS ac-tivity was detected solely in the cytoplasm when only one of the two MADS-box proteins was present, whereas reporter gene activity was localized to the nucleus in plants expressing both proteins.
Nuclear localization
The import and export of proteins to and from the nucleus is a highly regulated process36
. Entry of transcription factors into the nucleus can occur by diffusion or it can be a regulated process responsive to exogenous or endogenous signals. Also, as observed with AP3/PI, there may be co-transport of two or more factors. The nuclear import of large proteins is carried out by transporter proteins that shuttle between the cytoplasm and the nucleus, or by receptors at the nuclear pores. Import is mediated by nuclear lo-calization signals (NLSs), short peptide sequences rich in arginine and lysine residues, which have been found in several transcrip-tion factors36
.
Two NLSs have been identified in the transcription factor OPAQUE-2 (O2), which regulates the production of zein storage proteins in the maize endosperm37
. One of the NLS domains was mapped to the basic DNA-binding domain of O2, suggesting that the DNA-binding domain of this bZIP protein is identical to its nuclear localization domain. However, using an o2 mutant de-ficient for DNA binding, it was shown that nuclear localization and DNA binding are independent functions38
. This mutant protein still entered the nucleus but could no longer activate transcription of O2 target genes. In tobacco, a protein complex located at the nuclear pores strongly binds to the NLS of O2 and may be impli-cated in nuclear import of this transcription factor39
.
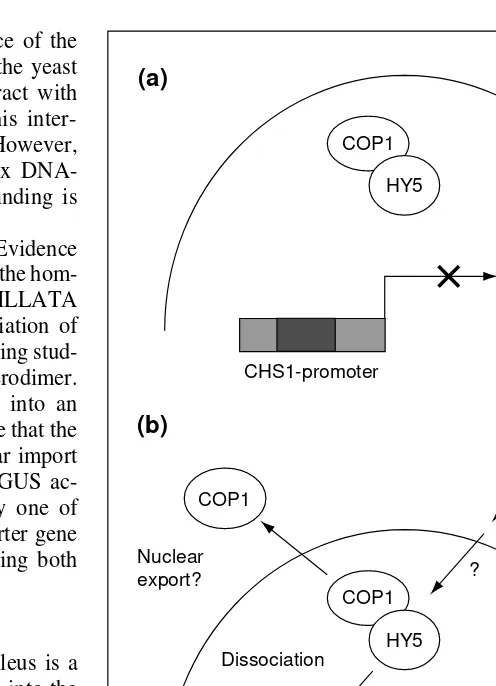
An example of the control of transcription by dimerization and nuclear localization is the interaction between COP1 and the bZIP transcription factor HY5. The pleiotropic Arabidopsis cop1 mu-tants have the phenotype of light-grown seedlings when grown in the dark. The COP1 protein has therefore been assigned the role of a repressor of photomorphogenesis. By contrast, on the basis of the light-insensitivity of hy5seedlings, HY5 has been defined as a positive regulator of photomorphogenesis. Using COP1-GUS fusions, it has been shown that COP1 protein is localized in the nucleus in the dark but that it leaves the nucleus after light irradi-ation40
. Genetical evidence had already suggested that COP1 and HY5 interact directly, and a direct interaction between the two has now been established in vitro and in vivo41–43
. This led to the development of a model in which COP1 interacts with HY5 in the dark to keep it in an inactive state. In the light, COP1 releases HY5 and leaves the nucleus, allowing subsequent activation of target genes by HY5 (Ref. 42; Fig. 4).
Post-translational events
Post-translational modification by protein phosphorylation acti-vates the nuclear import of transcription factors and stimulates or represses the DNA-binding affinity or the activation potential of many transcription factors44
. Different phosphorylation events are not mutually exclusive, as phosphorylation events at several sites could play a part in different aspects of the regulation of tran-scription factor activity. In plants, the predominant kinase used in phosphorylation studies is casein kinase II (CKII)45,46
. The bZIP transcription factor GBF1 from Arabidopsiscan be phosphorylated by purified CKII from broccoli46
and by extracts from nuclei of
Arabidopsis45
. This modification greatly enhances its DNA-binding activity.
Phosphorylation is also crucial for regulation of the DNA-binding activity of O2 (Ref. 47). Up to seven distinct phosphoryl-ated forms of O2 can be identified in protein extracts from maize endosperm separated on two-dimensional protein gels. Only the unphosphorylated O2 forms bind DNA with high affinity, and the phosphorylated forms only bind DNA after dephosphorylation by phosphatase treatment. The phosphorylation pattern of O2 is sub-ject to diurnal changes: hypophosphorylated forms accumulate by day and hyperphosphorylated forms in the dark. This pattern sug-gests a temporal regulation of O2 activity ensuring that storage pro-teins are synthesized at times of high energy supply. The specific kinases that participate in this process have yet to be identified.
Fig. 4.The activity of the ArabidopsisbZIP transcription factor HY5 is regulated by COP1. (a) In the dark, COP1 is localized to the nucleus where it binds HY5. (b) After light irradiation, COP1 releases HY5 and leaves the nucleus such that HY5 can then acti-vate its target genes.
CHS1-promoter (a)
COP1
CHS1-promoter HY5
Light signal (b)
COP1
HY5 COP1
HY5
Nuclear export?
Nucleus ?
?
Dissociation
Cytosol Nucleus
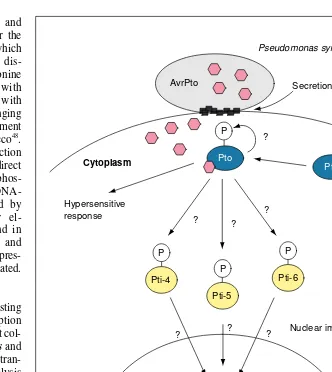
A link between a receptor kinase and transcription has been established for the plant disease resistance gene, Pto, which confers resistance to bacterial speck dis-ease in tomato. The Pto serine/threonine kinase can interact directly not only with the pathogenic elicitor avrPto, but also with at least three transcription factors belonging to the class of ethylene-responsive element binding proteins (EREBP) from tobacco48
. The kinase activity of Pto and its interaction with transcription factors suggests a direct regulation of their activity through phos-phorylation (Fig. 5), although their DNA-binding affinity was not influenced by phosphorylation. Cognate promoter el-ements for EREBPs have been found in several pathogenesis-related proteins and upon infection with the pathogen the expres-sion of some of these genes is upregulated.
Outlook
We have summarized some interesting examples of the regulation of transcription factors. The availability of large mutant col-lections, in particular from Arabidopsisand maize, will lead to the isolation of more tran-scription factor mutants. Mutant analysis will provide an important tool for the study of these transcription factors. The inclusion of genomics approaches in several plant species combined with classical genetics and improved in vitro techniques should allow a more mechanistic understanding of how plant transcription factors work. In this respect, the development of reliable and reproducible in vitrotranscription systems is an important objective49
. In conjunction, these tools will help us to decipher the plant-specific regulatory networks and so understand the process from signal trans-duction to transcriptional regulation.
Acknowledgements
We thank Roderick Card for critical reading of the manuscript. Our work is supported by the Ministry of Agriculture, Fisheries and Food (MAFF).
References
1Pugh, B.F. (1996) Mechanisms of transcription complex assembly, Curr. Opin. Cell Biol.8, 303–311
2Orphanides, G., Lagrange, T. and Reinberg, D. (1996) The general transcription factors of RNA polymerase II, Genes Dev. 10, 2657–2683 3Ranish, J.A. and Hahn, S. (1996) Transcription: basal factors and activation,
Curr. Opin. Genet. Dev. 6, 151–158
4Cowell, I.G. (1994) Repression versus activation in the control of gene transcription, Trends Biochem. Sci.19, 38–42
5Calkhoven, C.F. and Geert, A.B. (1996) Multiple steps in the regulation of transcription-factor level and activity, Biochem. J. 317, 329–342 6Meshi, T. and Iwabuchi, M. (1995) Plant transcription factors, Plant Cell
Physiol.36, 1405–1420
7Pabo, C.O. and Sauer, R.T. (1992) Transcription factors: structural families and principles of DNA recognition, Annu. Rev. Biochem. 61, 1053–1095
8Triezenberg, S.J. (1995) Structure and function of transcriptional activation domains, Curr. Opin. Genet. Dev5, 190–196
9Hanna-Rose, W. and Hansen, U. (1996) Active repression
mechanisms of eukaryotic transcription repressors, Trends Genet.12, 229–234
10Sainz, M.B., Goff, S.A. and Chandler, V.L. (1997) Extensive mutagenesis of a transcriptional activation domain identifies single hydophobic and acidic amino acids important for activation in vivo, Mol. Cell Biol. 17, 115–122
11Uesugi, M. et al.(1997) Induced a-helix in the VP16 activation domain upon binding to a human TAF, Science277, 1310–1313
12McCarty, D.R. and Carson, C.B. (1990) The molecular genetics of seed maturation in maize, Physiol. Plant.81, 267–272
13McCarty, D.R. (1995) Genetic control and integration of maturation and germination pathways in seed development, Annu. Rev. Plant Physiol. Plant Mol. Biol.46, 71–93
14McCarty, D.R. et al. (1991) The Viviparous-1developmental gene of maize encodes a novel transcriptional activator, Cell66, 895–905
15Hattori, T. et al.(1992) The Viviparous-1gene and abscisic acid activate the C1regulatory gene for anthocyanin biosynthesis during seed maturation in maize, Genes Dev. 6, 609–618
Fig. 5.The cytoplasmic receptor Pto-kinase interacts with the elicitor of Pseudomonas syringae, AvrPto, and subsequently phosphorylates several Pti transcription factors belonging to the class of ethylene-responsive element binding proteins (EREBPs). EREBP-promoter binding sites have been found in several promoters of pathogenicity-related proteins. The role of phosphorylation in regulating the activity of these transcription factors remains unclear. Abbreviation: P, phosphate.
P AvrPto
Pseudomonas syringae
Secretion
Pto Pto
Hypersensitive response
P
Pti-4
P
Pti-6 P
Pti-5
P
Pti
PR-box Cytoplasm
Nucleus
? ? ?
?
16 Kao, C-Y. et al.(1996) Localization and interaction of the cis-acting elements for abscisic acid, VIVIPAROUS1, and light activation of the C1gene of maize, Plant Cell8, 1171–1179
17 Hoecker, U., Vasil, I.K. and McCarty, D.R. (1995) Integrated control of seed maturation and germination programs by activator and repressor functions of Viviparous-1 of maize, Genes Dev.9, 2459–2469
18 McCarty, D.R.S. et al.(1989) Molecular analysis of vivparous-1: an abscisic acid-insensitive mutant of maize, Plant Cell1, 523–532
19 Suzuki, M., Kao, C-Y. and McCarty, D.R. (1997) The conserved B3 domain of Viviparous-1 has a cooperative DNA binding activity, Plant Cell9, 799–807
20 Carson, C.B. et al.(1997) The quiescent/colourless alleles of viviparous1 show that the conserved B3 domain of VP1 is not essential for ABA-regulated gene expression in the seed, Plant J.12, 1231–1240
21 Menkens, A.E., Schindler, U. and Cashmore, A.R. (1995) The G-box: a ubiquitous regulatory DNA element in plants bound by the GBF family of bZIP proteins, Trends Biochem. Sci. 20, 506–510
22 Vasil, V. et al.(1995) Overlap of Viviparous1 (VP1) and abscisic acid response elements in the Empromoter: G-box elements are sufficient but not necessary for VP1 transactivation, Plant Cell7, 1511–1518
23 Guiltinan, M.J., Marcotte, W.R., Jr and Quatrano, R.S. (1990) A plant leucine zipper that recognizes an abscisic acid response element, Science250, 268–271
24 Bobb, A.J., Eiben, H.G. and Bustos, M.M. (1995) PvAlf, an embryo-specific acidic transcriptional activator enhances gene expression from phaseolin and phytohemagglutinin promoters, Plant J.8, 331–343
25 Chern, M-S., Eiben, H.G. and Bustos, M.M. (1996) The developmentally regulated bZIP factor ROM1 modulates transcription from lectin and storage protein genes in bean embryos, Plant J.10, 135–148
26 Chern, M-S., Bobb, A.J. and Bustos, M.M. (1996) The regulator of MAT2 (ROM2) protein binds to early maturation promoters and represses PvALF-activated transcription, Plant Cell8, 305–321
27 Kriz, A.L., Wallace, M.S. and Paiva, R. (1990) Globulin gene expression in embryos of maize vivparousmutants, Plant Physiol.92, 538–542 28 Lamb, P. and McKnight, S.L. (1991) Diversity and specificity in
transcriptional regulation: the benefits of heterotypic dimerization, Trends Biochem. Sci.16, 417–422
29 Nantel, A. and Quatrano, R.S. (1996) Characterization of three rice basic/leucine zipper factors, including two inhibitors of EmBP-1 DNA-binding activity, J. Biol. Chem.271, 31296–31305
30 Schultz, T.F., Spiker, S. and Quatrano, R.S. (1996) Histone H1 enhances the DNA binding activity of the transcription factor EmBP-1, J. Mol. Biol. 271, 25742–25745
31 Martin, C. (1996) Transcription factors and the manipulation of plant traits, Curr. Opin. Biotech.7, 130–138
32 Goff, S.A., Cone, K.C. and Fromm, M.E. (1991) Identification of functional domains in the maize transcriptional activator C1: comparison of wild-type and dominant inhibitor proteins, Genes Dev.5, 298–309
33 Goff, S.A., Cone, K.C. and Chandler, V.L. (1992) Functional analysis of the transcriptional activator encoded by the maize Bgene: evidence for a direct functional interaction between two classes of regulatory proteins, Genes Dev. 6, 864–875
34 Sauer, F. and Tjian, R. (1997) Mechanisms of transcriptional activation: differences and similarities between yeast, Drosophila, and man, Curr. Opin. Genet. Dev.7, 176–181
35 Goto, K. and Meyerowitz, E.M. (1994) Function and regulation of the Arabidopsisfloral homeotic gene PISTILLATA, Genes Dev. 8, 1548–1560 36 Raikhel, N. (1992) Nuclear targeting in plants, Plant Physiol.100, 1627–1632 37 Varagona, M.J., Schmidt, R.J. and Raikhel, N.V. (1992) Nuclear localization
signal(s) required for nuclear targeting of the maize regulatory protein Opaque-2, Plant Cell4, 1213–1227
38 Varagona, M.J. and Raikhel, N.V. (1994) The basic domain in the bZIP regulatory protein Opaque2 serves two independent functions: DNA binding and nuclear localization, Plant J.5, 207–214
39 Hicks, G.R. and Raikhel, N.V. (1995) Nuclear localization signal binding proteins in higher plant nuclei, Proc. Natl. Acad. Sci. U. S. A.92, 734–738 40 von Arnim, A.G. and Deng, X-W. (1994) Light inactivation of Arabidopsis
photomorphogenic repressor COP1 involves a cell-specific regulation of its nucleocytoplasmic partitioning, Cell79, 1035–1045
41 Ang, L-H. and Deng, X-W. (1994) Regulatory hierarchy of photomorphogenic loci: allele-specific and light-dependent interaction between the HY5 and COP1 loci, Plant Cell6, 613–628
42 Ang, L-H. et al.(1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsisdevelopment,Mol. Cell1, 213–222
43 Chattopadyay, S. et al.(1998) ArabidopsisbZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression, Plant Cell10, 673–683
44 Karin, M. and Hunter, T. (1995) Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus, Curr. Biol. 5, 747–757
45 Klimczak, L.J. et al.(1995) Reconstitution of Arabidopsiscasein kinase II from recombinant subunits and phosphorylation of transcription factor GBF1, Plant Cell7, 105–115
46 Klimczak, L.J., Schindler, U. and Cashmore, A.R. (1992) DNA binding activity of the ArabidopsisG-box binding factor GBF1 is stimulated by phosphorylation by casein kinase II from broccoli, Plant Cell4, 87–98 47 Ciceri, P. et al.(1997) Phosphorylation of Opaque2 changes diurnally and
impacts its DNA binding activity, Plant Cell9, 97–108
48 Zhou, J., Tang, X. and Martin, G.B. (1997) The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind a cis-element of pathogenesis-related genes, EMBO J.16, 3207–3218 49 Sugiura, M. (1997) Plant in vitrotranscription systems, Annu. Rev. Plant
Physiol. Plant Mol. Biol.48, 383–398
Claus Schwechheimer and Michael Bevan*are at the Molecular Genetics Dept, John Innes Centre, Norwich, Norfolk, UK NR4 7UH; Claus Schwechheimer is presently at the Dept for Molecular, Cellular and Developmental Biology, Yale University, New Haven, CT 06520-8140, USA.
*Author for correspondence (tel +44 1603 452571; fax +44 1603 505725; e-mail [email protected]).