www.elsevier.com / locate / bres
Research report
Release of synaptic zinc is substantially depressed by conventional
brain slice preparations
a,b,c b b d
Sang Won Suh
, Gorm Danscher , Morten S. Jensen , Richard Thompson ,
a a,c ,
*
Massoud Motamedi , Christopher J. Frederickson
a
Center for Biomedical Engineering, and Department of Anatomy and Neuroscience, The University of Texas Medical Branch, 625 Jennie-Sealy Hospital, Galveston, TX 77555-0456, USA
b
Department of Neurobiology, Aarhus University, Aarhus C, 8000, Denmark
c
Laboratory for Zinc Neurobiology, NeuroBioTex, Inc. 101 Christopher Columbus Blvd., Galveston TX 77550-2607, USA
d
Department of Biological Chemistry, University of Maryland, Baltimore, MD, 21201-1503, USA
Accepted 20 June 2000
Abstract
Research on synaptically-released zinc is frequently done in vitro with acute brain slice preparations. We show here the in vitro hippocampal slice preparation has two major pitfalls for zinc research. First, up to 50% of the synaptic zinc is lost during slice cutting and / or the first 10 min of slice incubation, with the losses being most pronounced on the edges of the slice. Second, the release of the remaining zinc from a slice is substantially depressed (up to 50%) at the low temperatures (328C) typically used for brain slice studies. In concert, these effects reduce zinc release about 75% in vitro, compared to in vivo. Implications for research on synaptically-released zinc are discussed. 2000 Elsevier Science B.V. All rights reserved.
Theme: Excitable membranes and synaptic transmission
Topic: Mechanisms of neurotransmitter release
Keywords: Hippocampal slice; Autometallography (AMG); Zinc staining; Temperature; Vesicular zinc; Synaptically-released zinc excitotoxicity
1. Introduction from boutons has been shown in the brain slice preparation [4,18], recent attempts to replicate and extend those early Historically, in vitro investigations of synaptically-re- findings have yielded only marginal ‘release’ of zinc leased zinc have yielded results that varied with choice of [6,20].
research paradigm. Specifically, whereas studies using In the present work we tested the hypothesis that zinc
21
pharmacologic mimicry (adding exogenous Zn ) have release might be suppressed under ‘standard’ in vitro slice consistently revealed substantial and reliable effects of the conditions. Two factors were considered (i) the potential
21
Zn [8,25,30,32], studies using pharmacologic blockade loss of vesicular zinc induced by the tissue slicing and (ii)
21
(blocking the endogenous Zn signal) have typically the potential suppression of zinc release by the relative failed to find a consistent effect of the blockade hypothermia (30–328C) that is now typically used for [6,12,13,14,28,31,32]. brain slice preparations [20,32].
One explanation of this difference could be that synaptic To measure zinc release, we used the strategy of release of endogenous zinc is absent or minimal under measuring the level of residual synaptic zinc left in a slice typical in vitro brain slice conditions. Indeed, although a after various in vitro zinc-releasing treatments. The
gas-21
temperature, Ca , and impulse-dependent release of zinc autometallographic (gas-AMG) technique [11] was used to stain vesicular zinc, and densitometry was used for quanti-fication.
Parts of this paper have been published previously in
*Corresponding author. Tel.:11-409-762-0678; fax: 413-825-0283.
E-mail address: [email protected] (C.J. Frederickson). abstract form [16].
21
2. Materials and methods absence of greater numbers of competing zinc ions, Cu
21
and Fe would also be chelated by CaEDTA. 2.1. Hippocampal slice preparation
2.3. Fresh frozen hippocampal section preparation Experiments were performed on transverse hippocampal
slices taken from 5 to 6-week-old Wistar male rats. In all, For the purpose of measuring the normal concentration 40 hippocampal slices and 10 additional hippocampi taken of vesicular zinc in the hippocampal formation, ten 5- to directly from the intact animal (‘freshly frozen’ see below) 6-week-old male rats were sacrificed with halothane and were studied quantitatively. After halothane (3.5%) inhala- decapitated, and those brains were quickly removed and tion in the anesthetic box, rats were perfused with cold frozen on a CO gas freezing stage, and cut in a closed-top2
ACSF (48C, NaCl was substituted by 190 mM sucrose) by cryostat at 30 mm. The first section was collected at 3.6 intracardially for 1 min [1,26]. After perfusion, rats were mm caudal to Bregma, then every 3rd section was saved decapitated, and hippocampi were dissected out and placed until 10 were obtained. After cutting, the sections were in cold (48C) artificial cerebrospinal fluid (ACSF). Trans- thawed onto glass slides, dried, then stored (2808C) for verse slice (400mm) were cut with a vibratome (Campden subsequent exposure to gaseous H S.2
Vibraslice, model[ 752) and placed on a nylon mesh in
an incubation chamber. The ACSF temperature in the 2.4. Autometallographic zinc staining (AMG) incubation chamber was gradually increased from 48C to
208C for .30 min. The slices were kept at 208C in AMG staining was performed by the postmortem-biopsy oxygenated (95% O , 5% CO ) ACSF for 30 min before2 2 H S method [11]. Briefly, the freshly-cut sections were2
stimulation. The gas mixture was also bubbled through the thawed onto cleaned glass slides for a few seconds then
22
water bath in the chamber and vented over the chamber. returned to the298C cryostat chamber. For S exposure, The standard ACSF comprised (in mM) 130 NaCl, 3.5 the sections were transferred directly to a2178C chamber KCl, 1.25 NaH PO , 2 MgSO , 2 CaCl , 20 NaHCO , 22 4 4 2 3 which was filled with H S gas [11]. After exposing the2
1 21
ascorbic acid and 10D-glucose. The high potassium (K ) sections to H S gas for 60 min to precipitate the free Zn2
ACSF contained 23.5 mM KCl [4]. as ZnS clusters or crystallites, the slides were developed by Of the total 40 hippocampal slices (400mm) used in the the silver lactate AMG developer [9,10] for 60 min. All experiment, 10 were taken directly from the vibratome sections were counterstained with toluidine blue. After cutting bath (within 10 min of cutting, below 48C) and rinsing and dehydration, the sections were mounted using frozen for subsequent analysis of vesicular zinc. The DePex mounting medium.
remaining 30 slices were moved forward to preincubation
at 208C in normal ACSF with 5 mM CaEDTA. 2.5. Optical density measurements of zinc staining
After gas-AMG staining, sections were mounted in the
1
2.2. High K evoked zinc release microscope, transilluminated with a fixed intensity of white light (tungsten), and individual images were
cap-1
All K stimulation experiments consisted of 15 min tured by CCD camera, digitized, and stored. Every section incubation at the target temperature (268C (n510), 328C that was obtained from an individual brain slice (typically
1
(n511), or 398C (n59)), 15 min incubation with high K 10–15) was used for quantification. Two images were (23.5 mM), followed by 30 min washout in normal ACSF, captured from each section, one including the hilus of the all maintained at target temperature. CaEDTA (5 mM) was dentate gyrus and adjacent CA1 of the hippocampus, the present in the bath for blocking zinc reuptake for the entire other including CA3a (Fig. 1). The zones were digitized as hour. At the end of experiments, slices were quickly frozen follows: reference a square of stratum lacunosum-molecu-by CO gas for cutting and gas-AMG staining.2 lare just overlying the middle of the blade of the dentate The membrane impermeable disodium-calcium salt of gyrus; CA1, the same square zone, moved into s. radiatum EDTA (Ca-EDTA, Sigma, ED SC) was used to prevent2 (SR), adjacent to the reference zone; hilus, the same loss of calcium ions while allowing zinc ion chelation from square, moved top the CA3c-hilar confluence; CA3, the the extracellular fluid. The effect of the CaEDTA is same square centered over s. lucidum in CA3a–b (SL). governed by the affinities of the chelator for the divalent Optical Density was calculated conventionally (O.D.5
cations [24]: the affinity for zinc is very high (KD for (log10 [incident light / transmitted light])), with ‘incident
12
Zn-EDTA is|10–15) whereas the affinity for Ca is light’ taken as the intensity of light transmitted through the 21
lower (KD510–7.3) and that for Mg lower still (KD5 zinc-free reference zone (in lacunosum-molecular-away 10–5.4). Accordingly, when added as the calcium com- from the lateral perforant path innervation), and ‘trans-plex, CaEDTA will remove zinc (higher affinity) but not mitted light’ taken as the raw intensity reading for
in-21 21
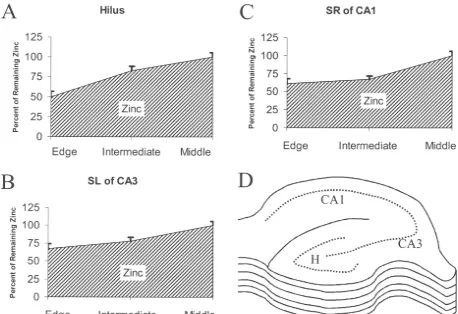
different regions lost between 45% and 60% of the total vesicular zinc by the time they were taken out of the vibratome collection tray for subsequent testing (Fig. 1). More zinc was lost from the margins of the tissue than from the core (Fig. 2).
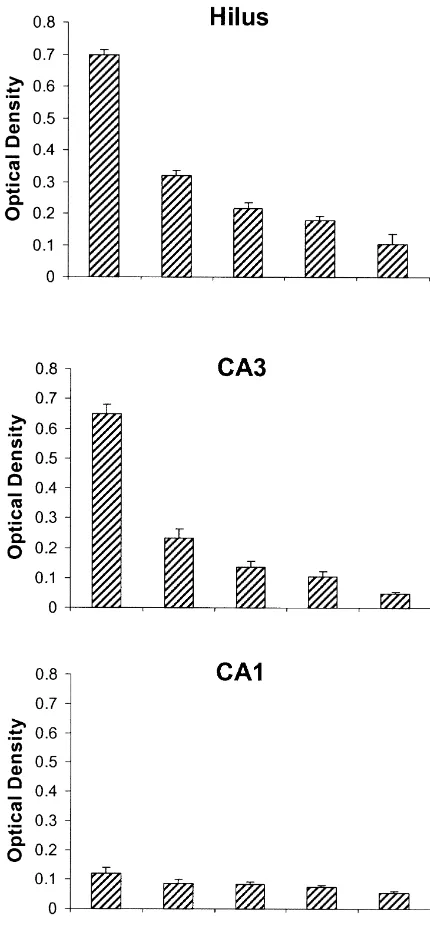
Tests of temperature effects showed that the magnitude of depolarization-induced release varied monotonically with temperature. At 268C, the mossy fiber neuropil lost 36% to 42% of the vesicular zinc, whereas at 398C roughly twice as much (67– 79%) of the vesicular zinc was released (Figs. 1, 3). To visual inspection, sections
incu-1
bated for 1 h in normal K showed no apparent loss of zinc at any temperature tested (data not shown).
4. Discussion
The use of densitometry with silver-stained tissue as a measure of vesicular zinc rests on two assumptions. The first assumption is that the silver staining actually stains vesicular zinc. This is a relatively safe assumption in the presynaptic boutons in all of the hippocampal regions under consideration [11,23].
The second assumption is that the optical density (OD) values are at least monotonically related to the actual amount of vesicular zinc in the tissue. This assumption also appears to be supportable, given that the regional OD data correlate highly with prior microfluorimetric measure-ments of regional vesicular zinc concentrations [15]. Specifically, the relative amounts of vesicular zinc found in a prior fluorimetric study (CA1, 13, Stratum lucidum 75, hilus 81) correlate highly (r50.99) with the relative abundance found in the present work by OD (CA1 0.12, stratum lucidum 0.65, hilus 0.70).
Another issue affecting the estimation of vesicular zinc concentration from the O.D. of the staining is the matter of tissue swelling, which would dilute the tissue concen-tration of vesicular zinc. The amount of slice swelling can be substantial in some conditions [2,21], but the osmolarity of the medium used in the present experiments (with 10 mM glucose) is generally associated with only a 10–15%
Fig. 1. Temperature dependent zinc release from the hippocampal slice.
increase in tissue volume (tissue water) [5]. Thus, not more
The intensity of staining of vesicular zinc (optical density) is shown for
than 15% of the reduction in O.D. of zinc staining should
sections taken directly from an intact, freshly-frozen brain (FF, n510),
from sections that were cut with vibratome for in vitro use, then collected be attributable to slice swelling.
immediately and frozen (0 min, n510), and from sections that were Accepting OD levels as indicative of vesicular zinc stimulated in vitro with elevated potassium (23.5 mM) at 26 (n510), 32
levels (115%) leads to the conclusion that very
little-(n511), and 39 (n59)8C bath temperature for 15 min. Hilus,
hippocam-perhaps only 1 / 4th or less of the normal vasicular zinc can
pal hilus mossy fiber area; SR of CA1, Stratum Radiatum of Cornu
be released from presynaptic boutons in a conventional,
Ammonis area 1; SL of CA3, Stratum Lucidum of Cornu Ammonis area
3. Bars show one S.E. 328C acute brain slice preparation. This may explain, for
example, why Budde and colleagues [6,20] could only
3. Results obtain modest and inconsistent synaptic release of zinc in their prior work, whereas studies done at normal rat body Hippocampal slices showed a substantial loss of vesicu- temperature either in vivo [3,7,19] or in vitro [17,18,27] lar zinc staining immediately after cutting and collecting obtained consistent and vigorous release.
Fig. 2. Zinc Loss From Slice Edges. The percent intensity of staining of vesicular zinc (optical density) is shown at 3 different layers. Hippocampal slices were cut with vibratome for in vitro use, then collected immediately and frozen (30mm, within 10 min). Slices were cut into 8–13 sections. Total OD determinations have been done from 381 slice images. ‘Edge’ refers to the first 2 sections on the top of the slices and the last 2 on the bottom of the slices, which were averaged together (Hilus, n547; CA1, n546; CA3, n540). ‘Intermediate’ refers to the next 2 sections from the top and the next 2 sections from the bottom of the slices, which were averaged together (Hilus, n547; CA1, n546; CA3, n540). The ‘middle’ refers to the remaining section’s average on the core of the slice (Hilus, n537; CA1, n539; CA3, n539). Intensity of zinc staining in edge is lower than middle of slices about 33–50%. All of the values are relative to 100% for ‘middle’. A, Hilus, hippocampal hilus mossy fiber area; B, SL of CA3, Stratum Lucidum of Cornu Ammonis area 3; C, SR of CA1, Stratum Radiatum of Cornu Ammonis area 1. D represents schematic drawing of OD measured layers; e, ‘edge’, i, ‘intermediate’ and m, ‘middle’. Bars show one S.E.
spotty and inconsistent in the typical acute brain slice inconsistent and transitory effects on synaptic transmission paradigm (as shown here), this may explain prior failures [6].
to find a role for synaptically-released zinc with the brain The present results suggest that acute brain slice prepa-slice preparation. If little or no endogenous zinc is released rations may be poorly suited for research on roles of in the brain slice paradigm, then blocking that signal with a endogenous synaptic zinc. Possibly, incubating slices in chelator would be expected to have little effect. This is media in which the free zinc is held constant by a suitable what Easley et al. [13,14] found when using DEDTC or zinc buffer would allow the slices to replenish vesicular TPEN to chelate zinc in slices maintained at 30–328C. zinc pools by the use of demonstrated zinc uptake [18,29] Although an effect of chelation on high-frequency synaptic and intravesicular zinc pumping [22] processes. However, driving was inferred, essentially no effect whatever was another strategy might be to use organotypic cultured brain observed on single-pulse monosynaptic transmission at slices, which, if collected from suitably mature rat pups zinc-containing synapses. Doller and Crawford likewise [33] will contain richly-laden zinc-containing boutons and found essentially no effect of zinc chelation (dithizone) on will release zinc vigorously [17,27].
synaptic transmission in hippocampal slices [12]. Smart and colleagues have also had mixed success in
demon-strating effects of zinc-signal blockade in vitro. Some zinc Acknowledgements
binding agents have produced effects on post-synaptic
ous zinc from the hippocampal mossy fibers in situ, Brain Res. 404 (1987) 58–64.
[4] S.Y. Assaf, S.H. Chung, Release of endogenous zinc from brain tissue during activity, Nature 308 (1984) 734736.
[5] B. Brahma, R.E. Forman, E.E. Stewart, C. Nicholson, M.E. Rice, Ascorbate inhibits edema in brain slices, J. Neurochem. 74 (2000) 1263–1270.
[6] T. Budde, A. Minta, J.A. White, A.R. Kay, Imaging free zinc in synaptic terminals in live hippocampal slices, Neurosci. 79 (1997) 347–358.
[7] G. Charton, C. Rovira, Y. BenAri, V. Leviel, Spontaneous and evoked release of endogenous zinc in the hippocampal mossy fibers zone of the rat in situ, Exp. Brain Res. 58 (1985) 202–205. [8] T. Defazio, J.J. Hablitz, Zinc and zolpidem modulate mlPSPs in rat
neocortical pyramidal neurons, J. Neurophysiol. 80 (1988) 1670– 1677.
[9] G. Danscher, Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electron microscopy, Histochemistry 71 (1981) 1–16. [10] G. Danscher, G. Howell, J. PerezClausell, N. Hertel, The dithizone,
Timm’s sulphide silver and the selenium methods demonstrate a chelatable pool of zinc in CNS, Histochemistry 83 (1985) 419–422. [11] G. Danscher, S. Juhl, M. Stoltenberg, B. Krunderup, H.D. Schroder, A. Andreasen, Autometallographic silver enhancement of zinc sulfide crystals created in cryostat sections from human brain biopsies: a new technique that makes it feasible to demonstrate zinc ions in tissue sections from biopsies and early autopsy material, J. Histochem. Cytochem. 45 (1997) 1503–1510.
[12] H.J. Doller, I.L. Crawford, The effest of dithizone on neuron activity in the hippocampal preparation, Neurol. Neurobiol. 11 (1984) 163– 176.
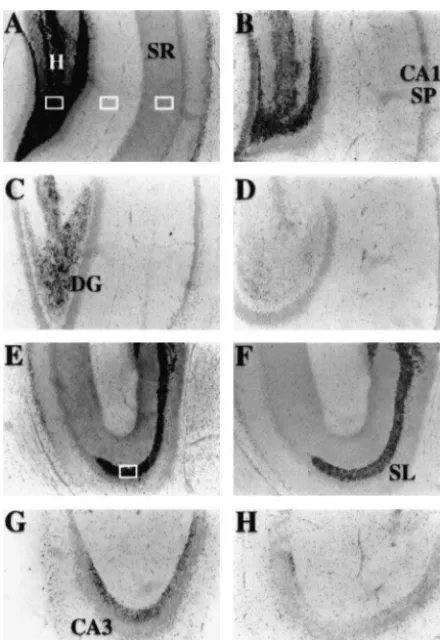
[13] J.D. Easley, D.W. Moncrieff, D.L. Cassel, S.H. Juo, C.J. Fre-derickson, Hippocampal synaptic function after chelation of vesicu-Fig. 3. Light microscopic images of temperature dependent zinc release lar zinc, Soc. Neurosci. Abstr. 19 (1993) 194.
from the hippocampal slice. A–D. The zinc staining of the dentate gyrus [14] J.D. Easley, A. Lee, E. Fleming, C.J. Frederickson, A selective role (DG) and field CA1 of the hippocampus. Note that the mossy fiber for vesicular zinc during fast neuronal firing, Soc. Neurosci. Abstr. neuropil in the Hilus of the DG is darkest (due to the zinc in this region. 21 (1995) 614.
A is from a freshly-frozen brain; B–D are Hilus area from slices that have [15] C.J. Frederickson, E.J. Kasarskis, D. Ringo, R.E. Frederickson, A 1
been stimulated in vitro with elevated K (23.5 mM) at 26 (B), 30 (C), quinoline fluorescence method for visualizing and assaying the or 39 (D) degrees C. E–H. Zinc staining in the mossy fiber neuropil of histochemically reactive zinc (bouton zinc) in the brain, J. Neurosci. Stratum Lucidum (SL) of CA3 is shown for freshly-frozen slices (E) and Meth. 20 (1987) 91–103.
slices previously stimulated at 26 (F), 30 (G), and 39 (H) degrees C. Zinc [16] C.J. Frederickson, S.W. Suh, M.S. Jensen, D.J. Hayes, G. Danscher, staining is gas AMG method; all 30 mm frozen sections. Scale bar Temperature dependent release and translocation of zinc in hip-represents 500 mm. The four rectangles show measurement areas for pocampus in vitro and in vivo, Soc. for Neurosci. Abst. 24 (1998) Lacunosum-Moleculare, Hilus, SL of CA3, and SR of CA1. 234.
[17] C.J. Frederickson, S.W. Suh, D. Silva, C.J. Frederickson, C.J. Frederickson, R.B. Thompson, Importance of zinc in the central nervous system: The zinc containing neuron, J. Nutr. 130 (5 Suppl.)
and Cathy J. Frederickson. This study was supported by (2000) 1471S–1483S.
[18] G.A. Howell, M.G. Welch, C.J. Frederickson, Stimulation-induced
the Danish Medical Research Academy (SWS, GD) and by
uptake and release of zinc in hippocampal slices, Nature 308 (1984)
NeuroBioTex, Inc.
736–738.
[19] T. Itoh, T. Saito, M. Fujimura, S. Watanbe, K. Saito, Restraint stress-induced changes in endogenous zinc release from the rat hippocampus, Brain Res. 618 (1993) 318–322.
References [20] A.R. Kay, T. Budde, A. Minta, J.A. White, C.J. Frederickson,
Imaging zinc release from mossy fiber terminals, Soc. Neurosci. [1] G.K. Aghajanian, K. Rasmussen, Intracellular studies in the facial Abstr. 21 (1995) 1521.
nucleus illustrating a simple new method for obtaining viable [21] G.C. Newman, F.E. Hospod, H. Qi, H. Patel, Effects of dextran on motoneurons in adult rat brain slice, Synapse 3 (1989) 331–338. hippocampal brain slice water, extracellular space, calcium kinetics [2] P.G. Aitken, G.R. Breese, F.F. Dudek, F. Edwards, M.T. Espanol, and histology, J. Neurosci. Meth. 61 (1995) 33–46.
P.M. Larkman, P. Lipton, G.C. Newman, T.S. Nowak, K.L. Paniz- [22] R.D. Palmiter, T.B. Cole, C.J. Quaife, S.D. Findley, ZnT-3, a zon, K.M. Raley-Susman, K.H. Reid, M.E. Rice, J.M. Sarvey, M. putative transporter of zinc into synaptic vesicles, Proc. Natl. Acad. Schoepp, D.D. Segal, C.P. Taylor, T.J. Teyler, P.J. Voulalas, Prepara- Sci. 93 (1996) 14934–14939.
[24] D.D. Perrin, in: Stability Constants of Metal-ion Complexes: Or- [29] J. Wensink, A.J. Molenaar, U.D. Woroniecka, C.J. Van den Hamer, ganic Ligands, Pergamon Press, Oxford, 1979, pp. 399–405. Zinc uptake into synaptosomes, J. Neurochem. 50 (1988) 782–789. 11 [25] S. Peters, J. Koh, D.W. Choi, Zinc selectively blocks the action of [30] G.L. Westbrook, M.L. Mayer, Micromolar concentration of Zn
N-methyl-D-aspartate on cortical neurons, Science 236 (1987) 589– antagonize NMDA and GABA responses of hippocampal neurons,
593. Nature 328 (1987) 640–643.
[26] G.B. Richerson, C. Messer, Effect of composition of experimental [31] X.M. Xie, T.G. Smart, A physiological role for endogenous zinc in solutions on neuronal survival during rat brain slicing, Exp. Neurol. rat hippocampal synaptic neurotransmission, Nature 349 (1991)
131 (1995) 133–143. 521–524.
[27] R.B. Thompson, W.O. Whetsell Jr., B.P. Maliwal, C.A. Fierke, C.J. [32] X. Xie, R.C. Hider, T.G. Smart, Modulation of GABA-mediated Frederickson, Fluorescence microscopy of stimulated Zn(II) release synaptic transmission by endogenous zinc in the immature hip-from organotypic cultures of mammalian hippocampus using a pocampus in vitro, J. Physiol. 478 (1994) 75–86.
carbonic anhydrase-based biosensor system, J. Neurosci. Meth. 96 [33] J. Zimmer, B.H. Gahwiler, Cellular and connective organization of (2000) 35–45. slice cultures of the rat hippocampus and fascia dentata, J. Comp. [28] K. Vogt, J. Mellor, G. Tong, R. Nicoll, The actions of synaptically Neurol. 228 (1984) 432–446.