www.elsevier.com / locate / bres
Research report
Stimulation of 5-HT
1Areceptors reduces apoptosis after transient
forebrain ischemia in the rat
*
Christine Schaper, Yuan Zhu, Maria Kouklei, Carsten Culmsee, Josef Krieglstein
¨ ¨
Institut f ur Pharmakologie und Toxikologie, Fachbereich Pharmazie, Philipps-Universitat Marburg, Ketzerbach 63, D-35032 Marburg, Germany Accepted 15 August 2000
Abstract
It has recently been shown that 5-HT1A receptor stimulation reduced the infarct volume after occlusion of the middle cerebral artery in rats. Since there is increasing evidence that apoptosis is involved in neurodegenerative diseases and stroke, we investigated whether the 5-HT1A agonist Bay x 3702 could protect neurons against apoptotic damage in a rat model of transient forebrain cerebral ischemia. Bay x 3702 (4mg / kg i.v.) caused a 10% reduction of neuronal damage in the hippocampal CA1 subfield. Higher doses of Bay x 3702 (40 and 12mg / kg i.v.) did not cause any neuroprotective effect, most likely because of the strong reduction of mean arterial blood pressure during the period of Bay x 3702 infusion. Bay x 3702 (4mg / kg i.v.) diminished DNA laddering in the hippocampus and striatum 4 days after 10 min forebrain ischemia. These results were confirmed by TUNEL-staining. The anti-apoptotic effect was abolished by additional treatment with the 5-HT1A receptor antagonist WAY 100635 (1 mg / kg). Taken together, the results suggest that Bay x 3702 can rescue hippocampal as well as striatal neurons from apoptotic cell death in vivo via stimulation of 5-HT1Areceptors. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
Keywords: 5-HT1Aagonist; Forebrain ischemia; WAY 100635; Apoptosis; Neuroprotection
1. Introduction receptors located on glutamatergic terminals [38,29].
Re-cently it has been shown that stimulation of 5-HT1A
It is well known that the stimulation of 5-HT1A re- receptors inhibits apoptotic cell death in primary cultures ceptors can cause neuroprotection in vitro and in vivo of embryonic chick neurons [3,43] as well as in neuronal [9,36,37,35]. Postsynaptic 5-HT1A receptors are highly HN-2-5 cells [1]. There is growing evidence that apoptotic expressed in brain regions such as hippocampus or neocor- cell death occurs under pathological conditions, such as tex [34,15]. In these regions the neurons are highly Alzheimer’s disease, Parkinson’s disease and Huntington’s sensitive to damage induced by cerebral ischemia or brain disease [18,30]. It has also been reported that delayed trauma [34,10]. The mechanism which leads to neuro- neuronal death, which is most likely apoptosis, can also protection after activation of the 5-HT1A receptors is appear after acute insults to CNS, such as cerebral thought to be due to the induction of hyperpolarisation of ischemia [27,28,19]. This demonstration of apoptosis after neuronal membranes [12,14,4]. This effect is most likely cerebral ischemia suggests an increased time window for
1
mediated by opening G protein-coupled K channels. therapeutical intervention [26]. In the light of these data it Additionally, it has been reported that 5-HT1A receptor is of interest to know whether stimulation of 5-HT1A
agonists can reduce glutamate release [39], which is most receptors could lead to reduced apoptotic cell death in likely mediated through activation of presynaptic 5-HT1A vivo. Therefore, we used the model of transient forebrain ischemia, where a maximum of apoptotic cells was de-tected at the fourth day after ischemia [51]. For 5-HT1A
*Corresponding author. Tel.:149-6421-282-1311; fax:1
49-6421-282-receptor stimulation we used the highly potent 5-HT
8918. 1A
E-mail address: [email protected] (J. Krieglstein). receptor agonist Bay x 3702 ((2 )-R-2-[4-[[(3,4-dihydro-0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
42 C. Schaper et al. / Brain Research 883 (2000) 41 –50
2H - 1 - benzopyran - 2 - yl)methyl]amino] - butyl] - 1, 2 - ben - removed, the blood was reinfused and NaHCO (50 mg /3
zisothiazol-3(2H)-one 1,1-dioxide monohydrochloride) kg, i.v.) was administered. Arterial pH, pCO , pO and2 2
[15,16]. blood pressure were measured before and after ischemia.
The aim of this study was to investigate whether the The experiments were approved by the government ethics 5-HT1A agonist Bay x 3702 could cause an anti-apoptotic committee. All efforts were made to reduce the suffering effect in vivo. In addition, we attempted to find out, of the animals during the experiments.
whether this anti-apoptotic effect could be blocked by the
selective 5-HT1A receptor antagonist WAY 100635 (N-[2- 2.4. Drug administration [4-(methoxyphenyl)-piperazinyl]ethyl]-N-2-piperidinyl
cy-clohexane carboxamide) [20]. Electrophoresis and staining Bay x 3702 (40, 12 and 4mg / kg) or saline were infused with terminal deoxynucleotidyl transferase (TdT)-mediated i.v. with a perfusor (Secura, Braun, Melsungen, Germany) dUTP nick end labeling (TUNEL) were used to measure immediately after ischemia for a period of 4 h. The DNA laddering and to detect internucleosomal DNA infusion was given into the tail vein with a constant flow fragmentation in situ. rate of 1 ml / h. WAY 100635 (1 mg / kg) was injected i.p. 60 min before ischemia. The body temperature was maintained at 3760.58C with a heating lamp during the
2. Material and methods period of infusion and up to 6 h after infusion.
2.1. Animals 2.5. Histological evaluation of the neuronal damage
Male Wistar rats (Charles River, Germany) weighing For evaluation of the neuronal damage in the CA1 300–350 g were used. The animals had free access to food subfield of the hippocampus the rats (n58–12) were (Altromin, Lage, Germany) and water and were kept under anesthetized with chloral hydrate (400 mg / kg, i.p.). They standardized environmental conditions (12-h light / dark were transcardially perfused with saline followed by 4% cycle, 23618C and 5561% relative humidity). There were phosphate-buffered formalin. The brains were removed no significant differences between the mean body weight and embedded in paraffin. Coronal brain slices (5 mM, of treated and untreated animals. 23.6 mm to bregma) were taken and stained with 1% celestine blue and 1% acid fuchsin for histological
evalua-2.2. Drugs tion. Damaged neurons appeared red and shrunken,
where-as intact neurons appeared blue with round morphological The 5-HT1A agonist Bay x 3702 ((2)-R-2-[4-[[(3,4- features. Neuronal injury was blindly evaluated in the CA1 dihydro-2H-1-benzopyran- 2 -yl)methyl]amino]-butyl]-1,2 - subfield of the hippocampus 7 days after ischemia by benzisothiazol-3(2H)-one 1,1-dioxide monohydrochloride) counting all damaged and viable neurons. Neuronal injury was obtained from Bayer (Cologne, Germany). WAY was given as the percentage of the total number of 100635 (N-[2-[4-(methoxyphenyl)-piperazinyl]ethyl]-N-2- neurons.
piperidinyl cyclohexane carboxamide), a selective 5-HT1A
¨
receptor antagonist, was a gift from Wyeth (Munster, 2.6. TUNEL-staining Germany).
Male Wistar rats (n54) were treated with either Bay x 2.3. Model of transient forebrain ischemia 3702 (4 mg / kg, i.v.) or vehicle (n54) immediately after ischemia. Brains were removed 3 days after ischemia. For Forebrain ischemia was produced in male Wistar rats detection of DNA-fragmentation TUNEL staining was (300–350 g, Charles River, Germany) with some modi- performed in hippocampal and striatal coronal paraffin fications as described previously [51]. The rats were fasted sections (23.6 and 1 mm to bregma, respectively) using overnight with free access to water. They were anes- the Apop Tag Kit (Oncor, USA). Briefly, after deparaffini-thetized using a mixture of N O / O2 2 (70 / 30%) with sation slices were incubated with proteinase K (20mg / ml) halothane (1.5%). During the surgery, body and skull for 15 min at room temperature. Endogenous peroxidase temperature was maintained between 37.0 and 37.58C by a activity was quenched with 2% H O . After treatment with2 2
temperature control feedback system (TSE, Bad Homburg, equilibration-buffer, slices were incubated with terminal Germany). The skull temperature was monitored with a transferase for 1 h at 378C followed by incubation with thermocouple placed subcutaneously over the midline of stop / wash buffer. After washing with phosphate-buffered the skull, thereby supplying a stable brain temperature saline slices were treated with antidigoxigenin-peroxidase [13]. Ischemia was induced for 10 min by clamping both for 30 min, equilibrated in 0.2% H O and then exposed to2 2
common carotid arteries and reduction of the blood freshly prepared staining solution of 0.05% diaminoben-pressure to 40 mmHg. Hypotension was induced with zidine in 0.02% H O . Counterstaining was performed2 2
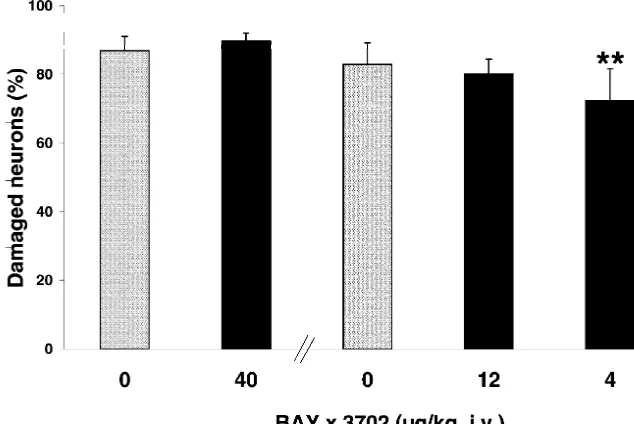
Fig. 1. Effect of Bay x 3702 on neuronal damage in the CA1 subfield of the hippocampus 7 days after transient forebrain ischemia. Rats were treated with Bay x 3702 (40, 12 and 4mg / kg, i.v.) immediately after ischemia by infusion for 4 h. The control group received vehicle in the same way. The values are given as means1S.D. of n58–12 animals. **P,0.01 compared with control (Duncan test).
were considered as TUNEL-positive cells. TUNEL-posi- subsequent Duncan test. Homogeneity of errors was de-tive cells were calculated as a percentage of the total termined using Bartlett’s test. P values of ,0.05 were number of neurons in the CA1 subfield of the hippocam- considered to be significant.
pus.
2.7. Analysis of DNA fragmentation 3. Results
Rat brains were removed 4 days after ischemia and the 3.1. Neuroprotection of Bay x 3702 demonstrated in a tissue of hippocampus and striatum was quickly taken and rat model of transient forebrain ischemia
stored at2708C. DNA from the hippocampus and striatum
was individually extracted using the QIAamp Tissue Kit The histology of the slices demonstrated severe damage (Qiagen, Hilden, Germany) according to the manufactur- of pyramidal neurons in the CA1 subfield of the hippocam-er’s protocol. Same amounts of total DNA were loaded and
Table 1 separated in a 1.5% agarose gel containing silver-gold
Physiological parameters of Bay x 3702 (40 mg / kg, i.v.) (B) and using electrophoresis for a period of 2.5 h.
vehicle-treated animals (C) during the period of drug infusion after In one series of experiments rats (n54) were infused a
transient forebrain ischemia with Bay x 3702 (4mg / kg) or vehicle for 4 h directly after
Hours of infusion ischemia. In another series of experiments animals (n53)
1 2 3 4
were divided into four groups. The first group received
Bay x 3702 (4mg / kg, i.v.) for a period of 4 h directly after Glucose (mg / dl)
C 110.6616.6 110.0612.3 109.3613.0 108.4614.3 ischemia, the second group received the vehicle only. The
B 113.8619.1 114.0619.1 113.8623.5 114.1623.0 third group received Way 100635 (1 mg / kg, i.p.) 60 min
before ischemia with a following infusion of vehicle for 4 PH
h. Animals of the fourth group were injected with Way C 7.2460.11 7.2960.03 7.2860.04 7.2860.04 100635 intraperitoneally (1 mg / kg) 60 min before is- B 7.3960.01 7.4160.03 7.4260.03 7.4160.04 chemia followed by an intravenous infusion of Bay x 3702
pO (mmHg)2 The values for the neuronal damage are presented as
B 39.562.7 38.363.1 36.764.0 36.963.0 means6S.D of n experiments. Multiple comparisons were
a
44 C. Schaper et al. / Brain Research 883 (2000) 41 –50
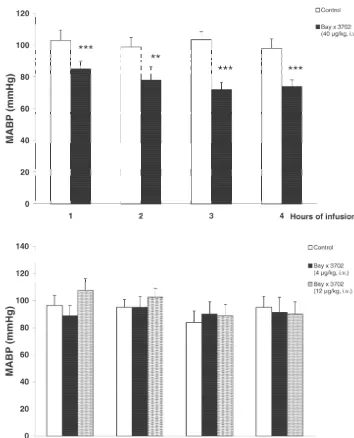
pus in both drug- and vehicle-treated animals. However, Infusion of 4 and 12 mg / kg Bay x 3702 exhibited no the histological quantification 7 days after global ischemia reduction in mean arterial blood pressure (Fig. 2B). resulted in a 10% reduction of damaged neurons in the Bay
x 3702 (4 mg / kg, i.v.)-treated group (72.469.2%) com- 3.2. Bay x 3702 prevents DNA degradation measured by pared to the control group (82.966.3%) (Fig. 1). Animals TUNEL-staining and gel electrophoresis
treated with higher doses of Bay x 3702 (40 and 12mg / kg,
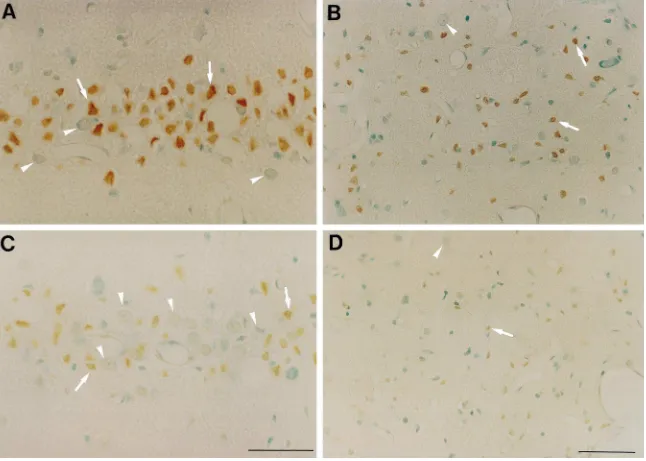
i.v.) showed no significant reduction in the CA1 hippocam- The results of TUNEL-staining were demonstrated in pal damage (40 mg / kg, 89.962.2% compared to control Fig. 3. Control slices from animals which were subjected 86.964.2%; 12 mg / kg, 80.164.3 compared to control to 10 min of forebrain ischemia (n54) showed marked 82.966,3%). During the period of Bay x 3702 (40mg / kg) TUNEL-staining 3 days after ischemia. Positively stained infusion, blood glucose, pO and pCO2 2 levels were not neurons were localized in the CA1 subfield of the hip-changed compared with vehicle-treated animals (Table 1). pocampus and in the dorsolateral region of the striatum However, the mean arterial blood pressure was signifi- (Fig. 4A,B). Staining with celestine and acid fuchsin cantly reduced (Fig. 2A) during the whole period of 40 revealed that most of the dying neurons in the
hippocam-mg / kg Bay x 3702 infusion (P,0.01 and P,0.001). pus (¯90%) were TUNEL-positive. The number of
extent of DNA degradation in rats which received WAY 100635, Bay x 3702 plus WAY 100635 or vehicle, respectively.
4. Discussion
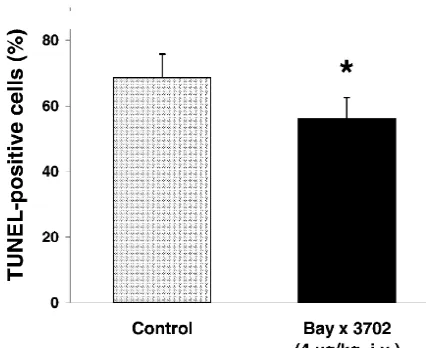
The data from this study demonstrated that Bay x 3702, a highly potent and selective 5-HT1A receptor agonist, reduced the number of damaged neurons in the CA1 pyramidal cell layer of the hippocampus 7 days after forebrain ischemia. In addition, our results provide evi-dence that DNA laddering induced by global ischemia can be reduced by Bay x 3702. This effect was reversed by the 5-HT1A receptor antagonist WAY 100635. Neuroprotective potency was demonstrated for 5-HT receptor agonists in Fig. 3. Quantification of TUNEL-positive cells in the CA1 subfield of the 1A
vivo and in vitro [8,9,36,37]. Recently, pronounced neuro-hippocampus 3 days after transient forebrain ischemia. Rats were treated
immediately after ischemia with Bay x 3702 (4mg / kg, i.v.) or vehicle by protective properties of Bay x 3702 were reported in infusion into the tail vein for 4 h. Values are given as means1S.D. of n54 models of transient and permanent middle cerebral artery animals. Control, 68.667.2%; Bay x 3702, 56.967.4%. *P,0.05,
occlusion and of traumatic brain injury [16,23,24,40]. In compared with control (Duncan test).
the present study we demonstrated that Bay x 3702 (4
mg / kg, i.v.) showed a 10% reduction of damaged neurons TUNEL-stained neurons in Bay x 3702 (4 mg / kg)-treated in the CA1 subfield of the hippocampus. However, infu-rats (n54) was reduced in the hippocampus and striatum sion with higher dosages (12 and 40mg / kg) did not show compared with control animals (Fig. 4). TUNEL-positive any neuroprotective effect. Decreased mean arterial blood neurons in the CA1 region of the hippocampus were pressure was found in the animals that were treated with reduced by 10% in the Bay x 3702-treated group 40mg / kg Bay x 3702. It is suggested, that the decreased (56.266.4%) compared with the control-group blood pressure is able to antagonize protective effects after
(68.667.2%) (Fig. 3). cerebral ischemia [33].
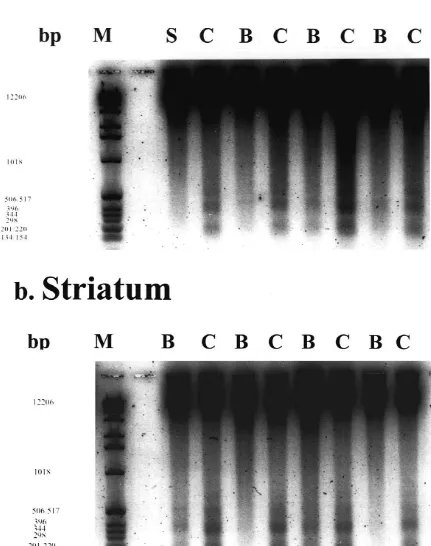
The data from DNA laddering are based on four animals Recent reports suggest that apoptosis is involved in in the control and Bay x 3702-treated group, respectively delayed neuronal death in the CA1 subfield of the hip-(Fig. 5). Extracted DNA from tissue of rat brains subjected pocampus after transient global ischemia [32,48]. Increase
21
to 10 min of transient forebrain ischemia followed by 4 in the intracellular free Ca level, induced by global
21 21
days of recovery demonstrated a typical DNA ladder ischemia, can activate Ca / Mg -dependent endonu-pattern with oligonucleosome-sized fragments of DNA of clease [5,44,41]. This leads to fragmentation of nuclear approximately 180 bp in the hippocampus and striatum DNA into 180–360 bp and finally into oligonucleosomal (Fig. 5a,b). In contrast, animals which were treated with cleavage products. Using gel electrophoresis, these prod-Bay x 3702 (4mg / kg) revealed a clear reduction of DNA ucts can be detected as DNA laddering, which is consid-fragmentation in the hippocampus and in the striatum (Fig. ered as a biochemical hallmark of apoptosis [22,28]. In our 5a,b). One Bay x 3702-treated animal failed to prevent experiments, time courses of DNA fragmentation in the DNA laddering in the striatum. Sham-operated animals did hippocampus and striatum revealed a typical DNA ladder-not show the typical DNA fragmentation (Fig. 5a). ing pattern which peaked at 4 days after onset of ischemia (data not shown). We demonstrated that the neuroprotective dose of the 5-HT1Areceptor agonist Bay x 3702 (4mg / kg) 3.3. The 5-HT1 A receptor antagonist WAY 100635 strongly reduced DNA laddering in the hippocampus and
abolished the antiapoptotic effect of Bay x 3702 striatum after global ischemia. Furthermore, Bay x 3702 (4
46
C
.
Schaper
et
al
.
/
Brain
Research
883
(2000
)
41
–
50
48 C. Schaper et al. / Brain Research 883 (2000) 41 –50
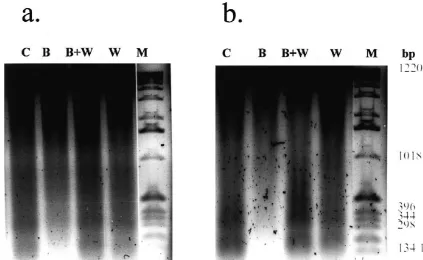
Fig. 6. WAY 100635 blocks the effect of Bay x 3702 on DNA fragmentation in the hippocampus (a) and striatum (b) 4 days after transient global ischemia. 0.88mg (a) and 0.64mg (b) DNA per lane were added to a 1.5% agarose gel. Bay x 3702 (4mg / kg) or vehicle were infused for a period of 4 h. Way 100635 (1 mg / kg) was injected 60 min before ischemia intraperitoneally. Lanes were representative for three animals per group. Lanes: (B) Bay x 3702-treated; (C) vehicle-treated; (W) WAY 100635-treated; (B,W) WAY 100635 and Bay x 3702-treated; (M) marker.
mediated by 5-HT1A receptors. These data support previ- ment [46,45]. This neurotrophic effect of serotonin seems ous findings that 5-HT1A receptor agonists can inhibit to be mediated by stimulation of 5-HT1A receptors on neuronal apoptosis in vitro [3,43]. Although the density of astrocytes and subsequent release of S-100b protein
21
5-HT1A receptors in the striatum under physiological [6,47,50]. S-100b is a Ca -binding protein which is conditions seemed to be low [25] we found a neuroprotec- produced predominantly in astrocytes [17]. Barger et al. tive effect of Bay x 3702 in this brain region after [7] reported that low concentrations of S-100b protein ischemia. Singh et al. [42] reported a dramatic increase in (0.3–10 ng / ml) could protect hippocampal neurons from the 5-HT1A receptor density of neuronal cell cultures glucose deprivation-induced damage by modulation of during stress and degeneration. However, how the expres- intracellular calcium homeostasis. Furthermore, there is sion of 5-HT1A receptors changes in brain tissue after evidence that in this low concentration S-100b is able to
ischemia is unclear. protect cultured neurons against staurosporine-induced
There are several possible mechanisms which could be apoptosis [2]. In contrast, high concentrations of S-100b
involved in the neuroprotective effect of 5-HT1A receptor protein (1–2 mM) are thought to induce cell death [21]. agonists. The protective potency of Bay x 3702 in vivo However, as we have already demonstrated in a previous could be explained by hyperpolarization of neuronal study [2] that Bay x 3702 can induce S100b protein in membranes and increased resistance to excitotoxicity. brain tissue of rats we suggest that the anti-apoptotic effect Hyperpolarization of neurons combined with the inhibition of Bay x 3702 can also be mediated by the induction of
21
of voltage-dependent Ca -influx in neurons [39] results in S-100b protein.
21
Huntington’s disease striatum and Alzheimer’s disease temporal
Acknowledgements
lobes, Neuroreport 6 (1995) 1053–1057.
[19] C. Du, R. Hu, C.A. Csernansky, C.Y. Hsu, D.W. Choi, Very delayed We thank Alexander Ravati for his valuable discussion. infarction after mild focal cerebral ischemia: a role for apoptosis?, J. We also thank Sandra Engel and Emma Esser for their Cereb. Blood Flow Metab. 16 (1996) 195–201.
skillful technical assistance. [20] E.A. Forster, I.A. Cliffe, D.J. Bill, G.M. Dover, D. Jones, Y. Reilly, A. Fletcher, A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635, Eur. J. Pharmacol. 281 (1995) 81–88.
[21] S. Fulle, M.A. Mariggio, S. Belia, I. Nicoletti, G. Fano, Nerve
References
growth factor inhibits apoptosis induced by S-100 binding in neuronal PC12 cells, Neuroscience 76 (1997) 159–166.
[1] T. Adayev, Y. El-Sherif, M. Barua, N.J. Penington, P. Banerjee, [22] A. Heron, H. Pollard, F. Dessi, J. Moreau, F. Lasbennes, Y. Ben Ari, Agonist stimulation of the serotonin1A receptor causes suppression C. Charriaut-Marlangue, Regional variability in DNA fragmentation of anoxia-induced apoptosis via mitogen-activated protein kinase in after global ischemia evidenced by combined histological and gel neuronal HN2-5 cells, J. Neurochem. 72 (1999) 1489–1496. electrophoresis observations in the rat brain, J. Neurochem. 61 [2] B. Ahlemeyer, H. Beier, I. Semkova, C. Schaper, J. Krieglstein, (1993) 1973–1976.
S-100b protects cultured neurons against glutamate- and stauros- [23] E. Horvath, K.H. Augstein, Neuroprotection by the novel 5-HT1A
porine- induced damage and is involved in the antiapoptotic action receptor agonist Bay x 3702 in the rat model of acute subdural of the 5-HT1A-receptor agonist, Bay x 3702, Brain Res. 858 (2000) hematoma, J. Neurotrauma 14 (1997) Abstr. 170.
121–128. [24] E. Horvath, K.H. Augstein, R. Wittka, Neuroprotective effect of the [3] B. Ahlemeyer, J. Krieglstein, Stimulation of 5-HT1A receptor novel 5-HT1A receptor agonist Bay x 3702 in rat model of inhibits apoptosis induced by serum deprivation in cultured neurons permanent focal cerebral ischemia and traumatic brain injury, Soc. from chick embryo, Brain Res. 777 (1997) 179–186. Neurosci. (1997) Abstr. 745.9.
[4] R. Andrade, Electrophysiology of 5-HT1A receptors in the rat [25] M.P. Kung, Z.P. Zhuang, D. Frederick, H.F. Kung, In vivo binding
123
hippocampus and cortex, Drug. Dev. Res. 26 (1992) 275–286. of [ I]4-(29-methoxyphenyl)-1-[29-(N-29 -pyridinyl)-p-iodoben-[5] M.J. Arends, R.G. Morris, A.H. Wyllie, Apoptosis: the role of the zamido-]ethyl-piperazine, p-MPPI, to 5-HT1A receptors in rat brain,
endonuclease, Am. J. Pathol. 136 (1990) 593–608. Synapse 18 (1994) 359–366.
[6] E.C. Azmitia, K. Dolan, P.M. Whitaker-Azmitia, S-100B but not [26] M.D. Linnik, Programmed cell death in cerebral ischemia: therapeu-NGF, EGF, insulin or calmodulin is a CNS serotonergic growth tic implication, CNS Drugs 3 (1995) 239–244.
factor, Brain Res. 516 (1990) 354–356. [27] M.D. Linnik, R.H. Zobrist, M.D. Hatfield, Evidence supporting a [7] S.W. Barger, L.J. Van Eldik, M.P. Mattson, S100 beta protects role for programmed cell death in focal cerebral ischemia in rats,
hippocampal neurons from damage induced by glucose deprivation, Stroke 24 (1993) 2002–2008.
Brain Res. 677 (1995) 167–170. [28] J.P. MacManus, A.M. Buchan, I.E. Hill, I. Rasquinha, E. Preston, [8] G.W. Bielenberg, M. Burkhardt, 5-hydroxytryptamine1A agonists, A Global ischemia can cause DNA fragmentation indicative of
apop-new therapeutic principle for stroke treatment, Stroke 21 (1990) tosis in rat brain, Neurosci. Lett. 164 (1993) 89–92.
IV161–163. [29] S. Matsuyama, K. Nei, C. Tanaka, Regulation of glutamate release [9] K.M. Bode-Greuel, J. Klisch, E. Horvath, T. Glaser, J. Traber, via NMDA and 5-HT1A receptors in guinea pig dentate gyrus, Brain
Effects of 5-hydroxytryptamine1A-receptor agonists on hippocampal Res. 728 (1996) 175–180.
damage after transient forebrain ischemia in the Mongolian gerbil, [30] H. Mochizuki, H. Mori, Y. Mizuno, Apoptosis in neurodegenerative Stroke 21 (1990) IV164–166. disorders, J. Neural Transm. Suppl. 50 (1997) 125–140.
[10] D.T. Chalmers, S.J. Watson, Comparative anatomical distribution of [31] K. Murase, M. Randic, T. Shirasaki, T. Nakagawa, N. Akaike, 5-HT1A receptor mRNA and 5-HT1A binding in rat brain: a Serotonin suppresses N-methyl-D-aspartate responses in acutely
combined in situ hybridisation / in vitro receptor autoradiographic isolated spinal dorsal horn neurons of the rat, Brain Res. 525 (1990)
study, Brain Res. 561 (1991) 51–60. 84–91.
[11] C. Charriaut-Marlangue, Y. Ben Ari, A cautionary note on the use of [32] T. Nitatori, N. Sato, S. Waguri, Y. Karasawa, H. Araki, K. Shibanai, the TUNEL stain to determine apoptosis, Neuroreport 7 (1995) E. Kominami, Y. Uchiyama, Delayed neuronal death in the CA1 61–64. pyramidal cell layer of the gerbil hippocampus following transient [12] A. Colino, J.V. Halliwell, Differential modulation of three separate ischemia is apoptosis, J. Neurosci. 15 (1995) 1001–1011.
K-conductances in hippocampal CA1 neurons by serotonin, Nature [33] K.A. Osborne, T. Shigeno, A.M. Balarsky, I. Ford, J. McCulloch, 328 (1987) 73–77. G.M. Teasdale, D.I. Graham, Quantitative assessment of early brain [13] D. Corbett, S.J. Evans, S.M. Nurse, Impaired acquisition of the damage in a rat model of focal cerebral ischaemia, J. Neurol.
Morris water maze following global ischemic damage in the gerbil, Neurosurg. Psychiatry 50 (1987) 402–410.
Neuroreport 3 (1992) 204–206. [34] A. Pazos, J.M. Palacios, Quantitative autoradiographic mapping of [14] M.F. Davies, R.A. Deisz, D.A. Prince, S.J. Peroutka, Two distinct serotonin receptors in the rat brain: I. Serotonin-1 receptors, Brain
effects of 5-hydroxytryptamine on single cortical neurons, Brain Res. 346 (1985) 205–230.
Res. 423 (1987) 347–352. [35] M.J. Piera, M. Beaughard, M.T. Michelin, R. Massingham, Effects [15] J. De Vry, 5-HT1A receptor agonists: recent developments and of the 5-hydroxytryptamine1A receptor agonists, 8-OH-DPAT, controversial issues, Psychopharmacology (Berlin) 121 (1995) 1– buspirone and flesinoxan, upon brain damage induced by transient
26. global cerebral ischaemia in gerbils, Arch. Int. Pharmacodyn. Ther.
[16] J. De Vry, H. Dietrich, T. Glaser, H.G. Heine, E. Horvath, R. Jork, 329 (1995) 347–359.
T. Maerins, F. Mauler, W. Opitz, D. Scherling, R. Schohe-Loop, T. [36] J.H. Prehn, C. Backhauss, C. Karkoutly, J. Nuglisch, B. Peruche, C. Schwarz, Bay x 3702, Drugs of the Future 22 (1997) 341–349. Rossberg, J. Krieglstein, Neuroprotective properties of 5-HT1A [17] R. Donato, Perspectives in S-100 protein biology, Cell Calcium 12 receptor agonists in rodent models of focal and global cerebral
(1991) 713–726, (Review article). ischemia, Eur. J. Pharmacol. 203 (1991) 213–222.
[37] J.H. Prehn, M. Welsch, C. Backhauss, J. Nuglisch, F. Ausmeier, C. [18] M. Dragunow, R.L. Faull, P. Lawlor, E.J. Beilharz, K. Singleton,
50 C. Schaper et al. / Brain Research 883 (2000) 41 –50
perimental brain ischemia: evidence for a protective role of [45] S. Ueda, X.P. Hou, P.M. Whitaker-Azmitia, E.C. Azmitia, Neuro-serotonin in cerebral ischemia, Brain Res. 630 (1993) 10–20. glial neurotrophic interaction in the S-100 beta retarded mutant [38] M. Raiteri, G. Maura, A. Barzizza, Activation of presynaptic 5- mouse (Polydactyly Nagoya). II. Co-cultures study, Brain Res. 633
hydroxytryptamine1-like receptors on glutamatergic terminals inhib- (1994) 284–288.
its N-methyl-D-aspartate-induced cyclic GMP production in rat [46] P.M. Whitaker-Azmitia, E.C. Azmitia, Autoregulation of fetal cerebellar slices, J. Pharmacol. Exp. Ther. 257 (1991) 1184–1188. serotonergic neuronal development: role of high affinity serotonin [39] K. Rupalla, R. Jakob, J. Krieglstein, Effects of 5-HT1A receptor receptors, Neurosci. Lett. 67 (1986) 307–312.
agonists on glutamate induced rise in cytosolic calcium concen- [47] P.M. Whitaker-Azmitia, R. Murphy, E.C. Azmitia, Stimulation of tration as well as on calcium and potassium currents in hippocampal astroglial 5-HT1A receptors releases the serotonergic growth factor, neurons, in: J. Krieglstein, H. Oberpichler-Schwenk (Eds.), Pharma- protein S-100, and alters astroglial morphology, Brain Res. 528 cology of Cerebral Ischemia, Wissenschaftliche Verlagsgesellschaft, (1990) 155–158.
Stuttgart, 1994, pp. 89–94. [48] C. Wiessner, P. Vogel, T. Neumann-Haefelin, K.A. Hossmann, [40] I. Semkova, P. Wolz, J. Krieglstein, Neuroprotective effect of 5- Molecular correlates of delayed neuronal death following transient HT1A receptor agonist, Bay X 3702, demonstrated in vitro and in forebrain ischemia in the rat, Acta Neurochir. Suppl. (Wien) 66 vivo, Eur. J. Pharmacol. 359 (1998) 251–260. (1996) 1–7.
[41] B.K. Siesjo, K. Katsura, Q. Zhao, J. Folbergrova, K. Pahlmark, P. [49] J.H. Wijsman, R.R. Jonker, R. Keijzer, C.J. van de Velde, C.J. Siesjo, M.L. Smith, Mechanisms of secondary brain damage in Cornelisse, J.H. van Dierendonck, A new method to detect apoptosis global and focal ischemia: a speculative synthesis, J. Neurotrauma in paraffin sections: in situ end-labeling of fragmented DNA, J.
12 (1995) 943–956. Histochem. Cytochem. 41 (1993) 7–12.
[42] J.K. Singh, B.A. Chromy, M.J. Boyers, G. Dawson, P. Banerjee, [50] W. Yan, C.C. Wilson, J.H. Haring, 5-HT1A receptors mediate Induction of the serotonin1A receptor in neuronal cells during neurotrophic effect of serotonin on developing dentate granule cells, prolonged stress and degeneration, J. Neurochem. 66 (1996) 2361– Dev. Brain Res. 98 (1995) 185–190.
2372. [51] Y. Zhu, C. Culmsee, I. Semkova, J. Krieglstein, Stimulation of [43] B. Suchanek, H. Struppeck, T. Fahrig, The 5-HT1A receptor agonist beta2-adrenoceptors inhibits apoptosis in rat brain after transient BAY33702 prevents staurosporine-induced apoptosis, Eur. J. Phar- forebrain ischemia, J. Cereb. Blood Flow Metab. 18 (1998) 1032–
macol. 355 (1998) 95–101. 1039.