Postmenopausal Women before and after Estrogen
Replacement Therapy
John E. Piletz and Uriel Halbreich
Background: Platelet
a
2A-adrenoceptors (
a
2AAR) and
imidazoline binding sites (subtype I
1) have been proposed
as peripheral markers of brain stem receptors that
medi-ate sympathetic outflow and are reported to be elevmedi-ated in
major depression.
Methods: In our study, p[
125I]-iodoclonidine was used to
assess platelet
a
2AAR and I
1binding sites in healthy
postmenopausal women (n
5
34) compared with healthy
women of reproductive age (n
5
26). Receptor
determi-nations were repeated in 19 postmenopausal women
following 59 – 60 days of estrogen replacement therapy
(ERT; 0.1 mg estradiol transdermal patches).
Results: I
1binding sites were twofold higher in platelets
of postmenopausal women compared with women of
re-production age but were down-regulated (normalized)
after 59 – 60 days of ERT. All other binding parameters,
including platelet
a
2AAR density, were not different
be-tween groups nor were they changed after ERT. Platelet I
1densities after 59 – 60 days of ERT were positively
corre-lated with plasma luteinizing hormone concentrations.
Conclusions: It is suggested that increased imidazoline
binding sites might be associated with mood and
behav-ioral changes in postmenopausal women. Biol Psychiatry
2000;48:932–939 © 2000 Society of Biological Psychiatry
Key Words: Imidazoline receptors,
a
2adrenoceptors,
monoamine oxidase, estrogen, luteinizing hormone,
meno-pause, depression
Introduction
P
ostmenopausal women are at high risk for osteoporosis
(American Medical Association 1984) and
cardiovas-cular disease (Stampfer et al 1991). Accordingly, estrogen
replacement therapy (ERT) has been indicated for the
prevention of osteoporosis and cardiovascular disease in
postmenopausal women. Menopausal status may also
influence cognitive problems (Yaffe et al 1998) and
vulnerability to depression (Halbreich 1997; Halbreich
and Lumley 1993). Some studies have suggested that ERT
might improve cognition (Haskell et al 1997; Sherwin,
1988) and stabilize mood (Ditkoff et al 1991; Klaiber et al
1997) in postmenopausal women.
Clonidine is a centrally active, partial agonist for
a
2-adrenoceptors (
a
2AR) that elicits a number of
cardio-vascular (Parsons and Morledge 1970), hormonal (Siever
and Uhde 1984), and cognitive effects (Ammassari-Teule
et al 1991). Clonidine’s agonistic activities at
a
2AR can be
discriminated from its nonadrenergic activities by
induc-tion of a G-protein “coupled” state of
a
2AR, yet not all of
clonidine’s effects are mediated through adrenoceptors
(Piletz et al 1994). Specifically, clonidine has nanomolar
affinity for nonadrenergic I
1-imidazoline binding sites (I
1sites: Ernsberger et al 1995a).
The molecular nature and function of I
1sites has
remained a subject of debate (Ernsberger and Haxhiu
1997) even while attempts to clone I
1sites are being
reported (Ivanov et al 1998b). In radioligand binding
assays, I
1sites can be distinguished from three subtypes of
a
2AR (
a
2A,
a
2B, and
a
2CAR) by phenylephrine and
guanidine compounds, which, in contrast to all
a
2AR
subtypes, lack high affinities for I
1sites (Ernsberger et al
1995a). Prolonged hypotension is induced by
microinjec-tion of imidazoline compounds, such as clonidine, into the
rostroventral lateral medulla (RVLM) region of the brain
stem (Bousquet et al 1989), the potency of which
corre-lates with affinities of these agents at I
1sites but not at any
a
2AR subtypes (Ernsberger et al 1990). Based on
phar-macologic studies, I
1sites in the RVLM were proposed to
reside on imidazoline receptors that regulate sympathetic
outflow (Bousquet et al 1989; Ernsberger et al 1990).
More recently, another subtype of I-sites (I
2sites) has been
identified as a modulatory domain within monoamine
oxidase enzymes (Raddatz et al 1997; Tesson et al 1995).
The latter finding has raised the possibility that
imidazo-From the Department of Psychiatry and Human Behavior, and Departments of Pharmacology and Physiology, University of Mississippi Medical Center, Jackson (JEP) and Biobehavioral Program, Departments of Psychiatry and Gynecology and Obstetrics, State University of New York, Buffalo (UH). Address reprint requests to Uriel Halbreich, M.D., Professor of Psychiatry,
Research Professor of Gyn/OB, Director of Biobehavioral Research, SUNY Clinical Center, Biobehavioral Program, Room BB 170, 462 Grider Street, Buffalo NY 14215.
Received September 23, 1999; revised February 14, 2000; accepted February 15, 2000.
lines might also mediate sympathetic outflow via the
metabolism of catecholamines in the brainstem.
There is also evidence that platelet I
1and
a
2AAR
clonidine-binding sites may be codysregulated in
depres-sion. This is based on reports (Piletz et al 1990, 1996a)
that I
1and
a
2AAR clonidine-binding sites are elevated in
depressed patients compared with healthy control subjects,
and that both sites are down-regulated following certain
types of antidepressant treatments (Piletz et al 1991,
1996b). Furthermore, platelet
a
2AAR and I
1sites appear to
possess identical binding properties as their counterparts
in the brain (Piletz and Sletten 1993). Elevations in brain
I
1and
a
2AAR clonidine-binding sites have also been
suggested (Callado et al 1998; Garcia-Sevilla et al 1996,
1999) in depressed suicide victims compared with
sudden-death matched control subjects. Thus, high densities of I
1and
a
2AAR sites may be associated with depressed mood
(Garcia-Sevilla et al 1999).
Alpha
2AR in uteri and brain have been reported to be
influenced by gonadal hormones and to regularly fluctuate
along the menstrual cycle (Bottari et al 1983; Orensanz et
al 1982; Roberts et al 1979). On the other hand, we have
found irregular fluctuations of platelet
a
2AAR and I
1sites
during the menstrual cycle in correlation with changes in
plasma epinephrine and norepinephrine levels (Piletz et al.
1998). Women with dysphoric premenstrual symptoms
also exhibited higher [
3H]-para-aminoclonidine binding to
platelet I
1and
a
2AAR sites than control subjects, which
was especially pronounced during the symptomatic late
luteal phase of the disorder (Halbreich et al 1993).
To our knowledge, platelet
a
2AAR and I
1sites have not
previously been studied in postmenopausal women, and
the effects of ERT on these sites have not been reported.
Considering the possible role of these systems in the
modulation of blood pressure and mood, their influence by
menopause and ERT is of interest from both a clinical and
a heuristic perspective.
Methods and Materials
Subjects
Thirty-four postmenopausal women (aged 52.663.4 SD; range 45–59 years) and 26 women of reproductive age (aged 36.16
6.5, range 22– 45 years) participated in the baseline study. Of the postmenopausal women, 19 were retested for radioligand binding on days 59 – 60 of ERT (aged 52.263.4 years). Six postmeno-pausal women completed ERT but were unable to be rescheduled for blood drawing until 2–3 days after estrogen (E2)
discontin-uation (their posttreatment data will not be included here). Nine postmenopausal women discontinued ERT prematurely or were lost to follow-up.
All women of reproductive age as well as postmenopausal women were recruited, evaluated, and treated, and their samples
were drawn and processed at the same site (Biobehavioral Program, State University of New York at Buffalo).
Eligibility of subjects was determined according to several criteria. Postmenopausal women had not experienced menstrual cycles for at least 2 years. They were at least 1 year after cessation of menopausal symptoms (including hot flashes, night sweats, and irritation by vaginal dryness) but were not older than 60 years of age. Luteinizing hormone (LH) and follicle-stimu-lating hormone (FSH) concentrations were within range for menopause, and no plasma progesterone was detected on at least two occasions before the study began. These postmenopausal women had no medical contraindications to ERT. The subjects weighed within 20% of ideal body weight, were physically healthy (including normal blood pressure and pulse), and met research diagnostic criteria (Spitzer et al 1978) for “not currently mentally ill” for at least 2 years before the study. Ten of the enrolled women had lifetime histories of major depressive disorder (MDD) and were therefore treated as a subgroup. Women were excluded if they had any active physical illnesses or other disorders necessitating chemical or somatic treatments or had any Axis I diagnosis of the DSM-III-R (APA 1987) at the time of testing. Postmenopausal subjects were compared with women of reproductive status who had regular menstrual cycles and otherwise met the same inclusion and exclusion criteria. Women of reproductive age were also administered the Premen-strual Assessment Form (Halbreich et al 1982) and daily rating forms (Endicott et al 1986) to exclude subjects with dysphoric symptoms during their midfollicular phases or those with pre-menstrual syndrome. This study was approved by an institutional review committee. All subjects signed consent forms before the study.
slowly through an 18-gauge needle into a heparinized syringe (19 units/mL blood). An additional 5 mL of blood was collected to obtain plasma (stored at270°C) for hormone determinations. The laboratory personnel were unaware of the subject’s age, diagnosis, or treatment (single blind).
Platelet Preparations
The preparation of intact platelets began according to a slight modification of the method of Corash (1980). Platelets were then washed four times in sterile Ca11/Mg11-free Hank’s balanced
salt solution, pelleted, resuspended in the same solution with 0.1 mmol/L phenyl methylsulfonylflouride, a protease inhibitor, and snap frozen at280°C. After thawing, sonication, and centrifu-gation at 4°C (Piletz et al 1990), the platelet particulates were resuspended in ice-cold reaction buffer (designated washed lysates; formula of reaction buffer given below). Washed lysates were then layered over a discontinuous 14.5% and 34% sucrose gradient and centrifuged at 105,000 g for 90 min at 4°C using a Beckman (Allendale, NJ) SW-28 rotor in a Sorvall OTD60B ultracentrifuge (Dupont, Wilmington, DE). The interface layer, containing the plasma membranes, was diluted in 40 mL of ice-cold water, pelleted, and resuspended in reaction buffer (Piletz et al 1990). The concentration of protein was determined using a Lowry-Biuret reagent kit from Sigma Chemical Co. (St. Louis). Samples were stored at280°C until use.
Binding Assays
Radioligand binding with p[125
I]-iodoclonidine ([125
I]PIC; New England Nuclear, Boston) was according to our previous proce-dure (Ernsberger et al 1995b; Piletz et al 1996b). Specifica2AAR
binding was determined from “total binding” minus “nonadren-ergic binding,” as displaced in the presence of 10 mmol/L norepinephrine. With another aliquot, I1 binding sites were
measured under a mask of 10mmol/L NE (Piletz et al 1996b). Specific I1 binding was then determined from “nonadrenergic
binding” minus “total nonspecific binding,” by displacement in the presence of additional 100 mmol/L moxonidine (kindly contributed by Dr. Siegfried Schafer, Solvay Pharma, Hanover, Germany). In most cases, the binding assays entailed seven concentrations of radioligand per site (Piletz et al 1996b); however, in some cases, a low protein yield permitted only 4 – 6 concentrations of radioligand per site. All measurements were made in hextuplicate (i.e., three total and three nonspecific values for each concentration). Reactions were initiated by adding 0.015– 0.03 mg plasma membrane proteins (up to 0.25 mL) to the radioligand and incubating at 21°C. The reaction buffer was 5 mmol/L HEPES, 0.5 mmol/L MgCl2, 0.5 mmol/L EGTA
(ad-justed to pH 7.4 with 0.1 mmol/L NaOH); with 0.1 mmol/L ascorbic acid added on the day of the binding assay. Trapped plasma membranes were washed three times with ice-cold wash buffer on thick glass fiber filters (Schleicher & Schuell #32, Keene, NH), and radioactivity was counted for 10 min per sample in a Cobra Gamma Counter (Packard Instruments, Meriden, CT) at 80% efficiency. Individual Bmaxand KDvalues
were quantified using LIGAND (McPherson 1985). Data were fit to both one-site and two-site models, but a one-site model was always preferred by an F test.
Hormonal concentrations were determined by radioimmuno-assays (RIA) with commercially available kits. 17-Beta estradiol (E2) was assayed (Diagnostic Products, Los Angeles) with 100ml
aliquots of plasma that were incubated at room temperature for 3 hours in an antibody-coated tube with125
I-free estradiol. Bound estradiol was counted after decantation of the supernatant. The intra- and interassay coefficients of variation (CV) ranged from 4.0% to 8.1%; the sensitivity limit was 8 pg/mL. Plasma progesterone determinations (Diagnostic Products) followed pro-cedures similar to those used for estradiol. The intra- and inter-assay CV ranged from 6% to 10%; the sensitivity limit was 0.05 ng/ml. Plasma testosterone was assayed by a solid phase RIA (Diagnostic Products) in an aliquot of 50mL. Interassay CV ranged from 9%–13%, and intraassay CV ranged from 4% to 6%. The sensitivity limit for the testosterone assay was 0.04 ng/mL. Plasma FSH and LH were also determined with RIA reagents purchased from Diagnostic Products in 200-mL aliquots. The interassay CV ranged from 4% to 6% (FSH) and 2% to 7% (LH), and the intraassay CV ranged from 2% to 4% (FSH) and 1% to 2% (LH). The sensitivity limit for FSH was 0.06 mIU/mL and for LH was 0.1 mIU/mL.
Statistical Analyses
Subject groups were compared with each other using nonpaired Student’s t tests. Pre- to posttreatment values were compared with paired t tests. Pearson’s regression tests were performed for associations of radioligand binding values with hormone values. All p values are two tailed. The data are expressed as means6
standard deviations.
Results
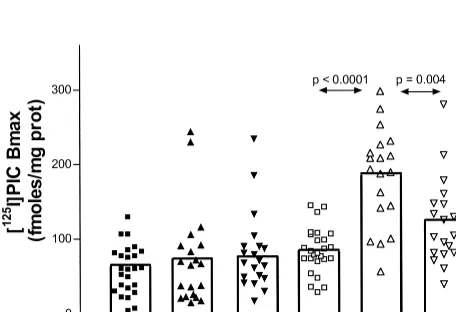
As shown in Table 1 and Figure 1, platelet I
1density was
significantly higher (pre-ERT) in postmenopausal women
(n
5
34) compared with women of reproductive status
(n
5
26; I
1B
maxt
5
5.4, p
,
.0001). None of the
other platelet radioligand binding parameters were
signif-icantly different between postmenopausal women and
women of reproductive status (
a
2AAR B
maxt
5
0.38 p
5
.7;
a
2AAR K
Dt
5
0.68, p
5
0.5; I
1site K
Dt
5
1.17, p
5
.25). There was no correlation between the
ages of postmenopausal women and any K
Dor B
maxparameters (pretreatment rs:
5
.04 to .28, posttreatment
rs
5 2
.02 to
2
.24).
Before ERT, postmenopausal women had plasma E
2concentrations of 7.4
6
6.8 pg/mL, progesterone
concen-trations of 0.18
6
0.13 ng/mL, LH concentrations of
81.4
6
10.0 mIU/mL, FSH concentrations of 102.6
6
52.1
mIU/mL, and testosterone concentrations of 0.24
6
0.12
pg/mL. After 59 – 60 days of estrogen treatment, plasma
E
2concentrations were 63.6
6
39.7 pg/mL (p
,
.0001
vs. baseline), plasma LH concentrations were 9.3
6
5.7
mIU/mL (p
,
.0001 vs. baseline), plasma FSH
concen-trations were 39.2
6
11.3 mIU/mL (p
,
.0001 vs.
baseline), and plasma testosterone concentrations were
0.31
6
0.40 pg/mL (ns). Progesterone concentrations after
ERT were below the sensitivity of the RIA for most
women. Baseline and posttreatment concentrations of E
2or other hormones did not correlate with each other (r
5
.03, p
5
.86 for E
2). Plasma E
2concentrations after 30
days of ERT (61.6
6
36.0 pg/mL) did not differ from E
2concentrations after 59 – 60 days of ERT (t
5
0.42, p
5
.63).
Correlations between Binding Sites and Hormones
At baseline (pre-ERT), none of the platelet receptor
parameters were significantly correlated with any of the
plasma
hormone
concentrations
in
postmenopausal
women (r values ranged from .1 to .45, ns).
Because ERT induced a down-regulation of platelet I
1sites (Table 1 and Figure 1), it was anticipated that E
2concentrations at post-ERT might be negatively correlated
with I
1B
maxvalues. In fact, the opposite was observed. A
positive correlation was found between posttreatment E
2concentrations and I
1B
maxvalues at the p
5
.04
significance level (r
5
.49, n
5
18); however,
pre-to-post treatment I
1B
maxchange scores (the
magni-tude of decrease) were positively correlated with
posttreat-ment E
2concentrations (r
5
.46, p
5
.04).
It was also anticipated that plasma LH concentrations,
FSH concentrations, or both might be positively correlated
with platelet I
1B
maxvalues at posttreatment. This was
because all three of these measures appeared to decrease
following ERT. In accord with this prediction, plasma
concentrations of LH after 59 – 60 days of ERT were
highly positively correlated with I
1B
maxvalues (r
5
.73,
p
5
.001, n
5
17) (Figure 2). Furthermore, the
pre-to-post treatment I
1B
maxchange scores were
nega-tively correlated with posttreatment LH concentrations
(r
5 2
.48, p
5
.04). Thus, higher E
2and lower LH
concentrations after ERT were associated with higher
Figure 1. Effect of menopause and estrogen therapy (ERT) on plateleta2-adrenergic and imidazoline-1 sites. [
125
I]PIC, p[125
I]-iodoclonidine; prot, protein.
Figure 2. Platelet imidazoline binding site and plasma luteiniz-ing hormone followluteiniz-ing estrogen replacement therapy in post-menopausal women. prot, protein.
Table 1. Platelets Imidazoline anda2AR Binding
Subjects
Baseline Post-ERT
I1Bmax I1KD a2Bmax a2KD I1Bmax I1KD a2Bmax a2KD
All postmenopausal women (n5 34)
177.6689.3a 4.2
61.2 64.6659.8 0.6660.53 — — — —
Subjects who completed 60 days of E2(n5 19)
188.3663.3b 4.1
61.2 76.6664.0 0.7260.59 122.7658.1 4.461.7 80.7653.7 0.6260.37
Reproductive age women (n5 26) 84.6630.5 4.661.6 60.0631.9 0.5560.61 — — — —
Values represent mean6SD. E2, 17-beta estradiol.ap,.0001 vs. women of reproductive age.
change scores (i.e., more down-regulation) of I
1B
maxvalues. No other significant correlation was observed
between any of the hormone concentrations and either I
1or
a
2AAR parameters.
In general, there was no association between blood
pressure or pulse rate with any of the platelet I
1or
a
2AAR
parameters (rs: .028 –.184). A negative correlation (r
5
2
.4040, p
5
.037) was observed between platelet
a
2AAR affinity (K
D) and pulse rate at baseline, however.
Subgroup with Lifetime History of MDD
Postmenopausal women with lifetime histories of MDD
(n
5
10) tended to have higher I
1B
maxvalues than those
who were never mentally ill (n
5
24), but this did not
reach statistical significance (221
6
132 vs. 159
6
69
fmol/mg protein, t
5
1.62, p
5
.12). Similarly, I
1K
Dvalues did not differ (4.1
6
1.0 vs. 4.2
6
1.2, t
5
.3, p
5
.77), nor did
a
2AAR K
Dvalues differ (0.42
6
0.40 versus
0.74
6
0.63, t
5
1.39, p
5
.18) in regard to lifetime
histories of MDD. Postmenopausal women with lifetime
histories of MDD differed in their
a
2AAR B
maxvalues at
baseline, however, which were high (73.8
6
61.2 vs.
30.1
6
16.6 fmol/mg protein, t
5
2.83, p
5
.01). The
two groups (with or without lifetime MDD) did not
significantly differ in baseline hormonal concentrations.
Following 59 – 60 days of treatment with Estraderm, the
women with past MDD (n
5
7) did not differ from those
without lifetime histories of MDD on any of the I
1parameters; however, after E
2treatment, the LH
concen-trations of women with histories of MDD decreased to
lower concentrations than did the LH of women who were
never mentally ill (3.9
6
2.4 vs. 11.1
6
5.6 ug/L, t
5
3.23, p
5
.008).
Discussion
We report two main findings. The first is a higher
radioligand binding density of I
1sites in platelets of
postmenopausal women compared with women of
repro-ductive age (Table 1 and Figure 1). The second is
down-regulation of platelet I
1binding sites after 59 – 60
days of ERT (Table 1 and Figure 1). These findings are
distinguished from
a
2AAR sites on platelets, which were
not different in postmenopausal women compared with
women of reproductive age and were not affected by
ERT.
We suggest that the high density of platelet I
1binding
sites observed in postmenopausal women might relate to
altered hormonal status, rather than to age per se. This is
for the following reasons: 1) In our study, there were no
within-group correlations between ages and I
1or
a
2AAR
binding parameters. 2) In our five previous studies
(Hal-breich et al 1993; Piletz et al 1990, 1996a, 1996b, 1998),
with more than 90 subjects of varying diagnoses (male and
female subjects, ages 20 – 65), no statistically significant
associations have been found between ages and densities
of platelet I
1or
a
2AAR sites. 3) Even considering only
those reports (Piletz et al 1996a; 1996b, 1998) that used an
identical binding assay and where a sizeable number of
healthy female control subjects were available (n
5
44),
we could find no association between female ages (23– 63
years) and densities of platelet I
1or
a
2AAR sites. To our
knowledge, no other reports exist on aging effects on these
platelet sites. Because no previous reports have even
hinted at an effect of age per se on these sites, the best
explanation for the present data seems to be the
postmeno-pausal status of women and its related hormonal state.
I
1and
a
2AR sites are known to be differentially
distributed in the brain (DeVos et al 1994; Kamisaki et al
1990). I
1sites are almost completely absent in the brain
stem nucleus locus coeruleus, which contains a high
density of
a
2AAR (Ernsberger et al 1994). The frontal
cortex is another region of high density of
a
2AAR, but
where I
1sites are of relatively low density (DeVos et al
1994; Kamisaki et al 1990). On the other hand, I
1sites
have been extensively studied in the RVLM brainstem
nucleus (Bricca et al 1989; Ernsberger et al 1990), where
they are associated with the hypotensive action of
cen-trally applied imidazoline compounds. I
1sites in the
RVLM might serve a physiologic role in the tonic and
reflex control of the sympathetic nervous system
(Erns-berger and Haxhiu 1997). An endogenous clonidine
dis-placement substance has also been characterized (Atlas
1991) and identified to contain agmatine, a decarboxylated
arginine metabolite (Li et al 1994). Thus, agmatine and the
imidazoline binding sites (I
1and I
2) might compose a
neurotransmitter-receptor system. Although agmatine is
now realized to be only one of several molecules that
constitute clonidine displacement substance (Piletz et al
1995; Sun et al 1995), agmatine fulfills several criteria for
a neurotransmitter (Reis and Regunathan 1999).
Al-though we did not measure platelet I
2sites in our study, it
is conceivable that the platelet I
1site is physically related
to I
2sites.
Our finding of no effect of ERT on platelet
a
2AAR sites
in postmenopausal women is in accord with a previous
study by Best and coworkers (Best et al 1992) in which
yohimbine binding to platelet
a
2AAR sites was unchanged
in postmenopausal women given an estradiol implant (100
mg) for 6 weeks. By contrast, another study reported
increased platelet
a
2AAR sites in human male volunteers
treated with E
2(U’Prichard and Snyder 1979), and three
earlier studies of female rabbits reported that estrogen
treatment decreases
a
2AAR sites in platelets (Elliott et al
1980; Mishra et al 1985; Roberts et al 1979). In our study,
and in the study by Best and colleagues (Best et al 1992),
estradiol was administered for a much longer time (42– 60
days) than in the human male study (4 days) or the rabbit
studies (6 –24 days). Therefore, the length of E
2treatment
and species and gender differences might be critical
factors.
We have also reported (Halbreich et al 1993) higher
platelet
a
2AAR and I
1sites in women with dysphoric
premenstrual syndrome compared with healthy control
women. In that study, elevations in binding were most
pronounced during the symptomatic late luteal phase of
the disorder when plasma norepinephrine and adrenaline
concentrations typically peak (Blum et al 1992). The
literature is uniformly negative for regular, cyclic
fluctu-ations of platelet
a
2AAR or I
1sites along the human
menstrual cycle (Jones et al 1983; Piletz et al 1998;
Stowell et al 1988; Sundaresan et al 1985; Theodorou et al
1987). In certain brain regions and in uteri, however,
a
2AAR sites may be influenced by gonadal hormones and
appear to fluctuate along the estrus cycle (Bottari et al
1983; Orensanz et al 1982).
One potential paradox was that post-ERT I
1B
maxvalues
were positively associated with plasma E
2concentrations.
This would not be expected if E
2directly down-regulated
I
1sites; however, plasma E
2concentrations were found to
be positively associated with the magnitude of change in I
1B
maxvalues, which is in line with an E
2down-regulation
effect. We have previously reported (Piletz et al 1998)
irregular fluctuations of platelet
a
2AAR and I
1sites over
two menstrual cycles in healthy women, which were not
correlated with phase of the cycle but were instead
correlated with plasma concentrations of adrenaline and
norepinephrine. We have also reported (Ivanov et al
1998a) that immunoreactive I-sites on human
megakaryo-blastoma cells are up-regulated after 6 hours of exposure
to norepinephrine in vitro. Similar treatments with
estro-gen in vitro were without effect (unpublished
observa-tions). Thus, the mechanisms of the down-regulation of
platelet I
1sites in women after ERT (Table 1) is still
unclear. Future studies are needed to address whether
indirect changes in plasma catecholamines might underlie
platelet I
1changes.
Another interesting observation was that post-ERT I
1B
maxvalues were positively correlated with plasma LH
concentrations (Figure 2). Whether these are causally
linked (i.e., LH or the I
1site exerts a regulatory effect) or
whether LH and I
1sites are independently influenced by
E
2is open to speculation. The literature suggests no effect
of clonidine on LH release (Kaufman and Vermeulen
1989). Any effect of LH on I
1binding sites therefore is
unknown and will require additional studies (in the same
way that the possible effect of LH on mood and behavior
has not been sufficiently investigated).
It is also interesting to note that the abnormal I
1B
MAXvalues previously reported for depressed patients (Piletz et
al 1996b) are comparable in level to those of healthy
postmenopausal women (Table 1). Of course, exact
plate-let I
1B
MAXvalues are notoriously difficult to duplicate
across studies for technical reasons (Ernsberger et al
1995b). Even slight variations in platelet preparation may
be critical (Corash 1980). In fact, all the platelets in our
current study were prepared at a different site (Buffalo)
than in our previous studies with depressed patients
(Cleveland). Nevertheless, the B
MAXvalues in our
post-menopausal women (177.6
6
89.3 fmol/mg protein, n
5
34) are surprisingly close to what we previously reported
for a group of middle-aged male and female depressed
patients (161
6
66.3 fmol/mg, n
5
26) (Piletz et al
1996b). I
1densities in postmenopausal women after ERT
(122.7
6
58.1 fmol/mg, n
5
19) are also surprisingly
close to what we previously reported for healthy
middle-aged male and female subjects (126
6
63.6 fmol/mg, n
5
18) (Piletz et al 1996b). Furthermore, the few
postmeno-pausal women with lifetime histories of MDD had higher
B
MAXvalues for
a
2AAR (statistically significant) and I
1sites (trend) compared with women without lifetime
his-tories of MDD. If the platelet I
1site becomes validated as
a biological marker for depression (Piletz et al 1994), the
present findings in nondepressed postmenopausal women,
as well as the down-regulation by estrogen, will have to be
taken into account.
This work was supported in part by Grant Nos. RO1-MH-45901, RO1-MH-49248, and RO1-MH-57601. We greatly appreciate the clinical assistance provided by Judith Vedella, Ph.D., R.N., and Barbara Bernar-dis, B.S., R.N., the statistical analysis provided by Khalid Bibi, Ph.D., and the laboratory assistance of Jianhua Shen, M.D., Guojing Yuan, and Tammy Stefan.
References
American Psychiatric Association (1987): Diagnostic and
Sta-tistical Manual of Mental Disorders, 3rd ed rev. Washington,
DC: American Psychiatric Press.
Ammassari-Teule M, Maho C, Sara SJ (1991): Clonidine re-verses spatial learning deficits and reinstates 0 frequencies in rats with spatial fornix section. Behav Brain Res 45:1– 8. Atlas D (1991): Clonidine-displacing substance (CDS) and its
putative imidazoline receptor. New leads for further diver-gence ofa2-adrenergic receptor activity. Biochem Pharmacol
41:1541–1549.
Best NR, Rees MP, Barlow DH, Cowen PJ (1992): Effect of estradiol implant on noradrenergic function and mood in menopausal subjects. Psychoneuroendocrinology 17:87–93. Blum I, Nessiel L, David A, Graff E, Harsat A, Weissglas L, et
al (1992): Plasma neurotransmitter profile during different phases of the ovulatory cycle. J Clin Endocrinol Metab 75:924 –929.
Bottari SP, Vokaer A, Kaivez E, Lescrainier JP, Vauquelin GP (1983): Differential regulation ofa-adrenergic receptor sub-classes by gonadal steroids in human myometrium. J Clin
Endocrinol Metab 57:937–941.
Bousquet P, Feldman J, Tibirica E, Bricca G, Molines A, Dontenwill M, Belcourt A (1989): New concepts on the central regulation of blood pressure. Alpha2-adrenoceptors
and “imidazoline receptors. Am J Med 87:10S–13S. Bricca G, Dontenwill M, Molines A, Feldman J, Belcourt A,
Bousquet P (1989): The imidazoline preferring receptor: Binding studies in bovine, rat and human brainstem. Eur
J Pharmacol 162:1–9.
Callado LF, Meana JJ, Grijalba B, Pazos A, Sastre M, Garcia-Sevilla JA (1998): Selective increase of a2A-adrenoceptor
agonist binding sites in brains of depressed suicide victims.
J Neurochem 70:1114 –1123.
Chakravorty SG, Halbreich U (1997): The influence of estrogen on monoamine oxidase activity. Psychopharmacol Bull 33: 229 –233.
Corash L (1980): Platelet heterogeneity: Relevance to the use of platelets to study psychiatric disorders. Schizophr Bull 6:254 –258.
DeVos H, Bricca G, DeKeyser J, DeBacker J, Bousquet P, Vauquelin G (1994): Imidazoline receptors, non-adrenergic idazoxan binding sites, and a2-adrenoceptors in the human
central nervous system. Neuroscience 59:589 –598.
Ditkoff EC, Crary WG, Cristo M, Lobo RA (1991): Estrogen improves psychological function in asymptomatic postmeno-pausal women. Obstet Gynecol 78:991–995.
Elliott JM, Peters JR, Grahame-Smith DG (1980): Oestrogen and progesterone change the binding characteristics ofa -adren-ergic and serotonin receptors on rabbit platelets. Eur J
Phar-macol 66:21–30.
Endicott J, Cohen J, Nee J, Fleiss J, Sarantakos S (1981): Hamilton Depression Rating Scale. Extracted from regular and change versions of the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry 38:98 –103. Endicott J, Nee J, Cohen J, Halbreich U (1986): Premenstrual
changes: Patterns and correlates of daily ratings. J Affect
Disord 10:127–135.
Endicott J, Spitzer RL (1978): A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen
Psychiatry 35:837– 844.
Ernsberger P, Giuliano R, Willette RN, Reis DJ (1990): Role of imidazole receptors in the vasodepressor response to clonidine analogs in the rostral ventrolateral medulla. J
Phar-macol Exp Ther 253:408 – 418.
Ernsberger P, Graves ME, Graff LM, Zakieh N, Nguyen P, Collins LA, et al (1995a): I1-Imidazoline receptors:
Defini-tion, characterizaDefini-tion, distribution and transmembrane signal-ling. Ann N Y Acad Sci 763:22– 42.
Ernsberger P, Haxhiu MA (1997): The I1-imidazoline-binding
site is a functional receptor mediating vasodepression via the ventral medulla. Am J Physiol 42:R1572–R1579.
Ernsberger P, Haxhiu MA, Graff LM, Collins LA, Dreshaj I, Grove DL, et al (1994): A novel mechanism of action for hypertension control: moxonidine as a selective I-1 imidazo-line agonist. Cardiovasc Drugs Ther 8:27– 41.
Ernsberger P, Piletz JE, Graff LM, Graves ME (1995b): Opti-mization of radioligand binding assays for I-1 imidazoline sites. Ann N Y Acad Sci 763:163–168.
Garcia-Sevilla J, Escriba P, Guimon J (1999): Imidazoline receptors and human brain disorders. Ann N Y Acad Sci 881:392– 409.
Garcia-Sevilla JA, Escriba PV, Sastre M, Walzer C, Busquets X, Jaquet G, et al (1996): Immunodetection and quantitation of imidazoline receptor proteins in platelets of patients with major depression and in brains of suicide victims. Arch Gen
Psychiatry 53:803– 810.
Halbreich U (1997): Role of estrogen in postmenopausal depres-sion. Neurology 48:S16 –S19.
Halbreich U, Endicott J, Schacht S, Nee J (1982): The diversity of premenstrual changes as reflected in the Premenstrual Assessment Form. Acta Psychiatr Scand 65:46 – 65. Halbreich U, Lumley LA (1993): The multiple interactional
biological processes that might lead to depression and gender differences in its appearance. J Affect Disord 29:159 –73. Halbreich U, Piletz JE, Carson S, Halaris A, Rojansky N (1993):
Increased imidazoline anda2adrenergic binding in platelets
of women with dysphoric premenstrual syndromes. Biol
Psychiatry 34:676 – 686.
Haskell SG, Richardson ED, Horwitz RI (1997): The effect of estrogen replacement therapy on cognitive function in wom-en: A critical review of the literature. J Clin Epidemiol 50:1249 –1264.
Holsboer F, Benkert O, Demisch L (1983): Changes in MAO activity during estrogen treatment of females with endoge-nous depression. Mod Probl Pharmacopsychiatry 19:321– 326.
Ivanov T, Feng Y, Wang H, Regunathan S, Reis DJ, Chikkala DN, et al (1998a): Imidazoline receptor proteins are regulated in platelet-precursor MEG-01 cells by agonists and antago-nists. J Psychiatr Res 32:65–79.
Ivanov TR, Zhu H, Regunathan S, Reis DJ, Dontenwill M, Vonthron C, et al (1998b): Co-detection by two imidazoline receptor protein antisera of a novel 85 kilodalton protein.
Biochem Pharmacol 55:649 – 655.
Jones SB, Bylund DB, Rieser CA, Shekim W, Byer JA, Carr GW (1983): Alpha2adrenergic receptor binding in human
plate-lets: Alterations during the menstrual cycle. Clin Pharmacol
Ther 34:90 –96.
(1990): Binding of [3
H]p-aminoclonidine to two sites, a2
-adrenoceptors and imidazoline binding sites: Distribution of imidazoline binding sites in rat brain. Brain Res 514:15–21. Kaufman JM, Vermeulen A (1989): Lack of effect of the
a-adrenergic agonist clonidine on pulsatile luteinizing hor-mone secretion in a double blind study in men. J Clin
Endocrinol Metab 68:219 –222.
Klaiber EL, Broverman DM, Vogel W, Kobayashi Y, Moriarty D (1972): Effects of estrogen therapy on plasma MAO activity and EEG driving responses of depressed women. Am J
Psychiatry 128:1492–1498.
Klaiber EL, Broverman DM, Vogel W, Peterson LG, Snyder MB (1997): Relationships of serum estradiol levels, menopausal duration, and mood during hormonal replacement therapy.
Psychoneuroendocrinology 22:549 –58.
Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ (1994): Agmatine: An endogenous clonidine-displacing sub-stance in the brain. Science 263:966 –969.
McPherson CA (1985): Analysis of radioligand binding experi-ments. A collection of computer programs for the IBM PC.
J Pharmacol Methods 14:213–228.
Mishra N, Hamilton CA, Jones CR, Leslie C, Reid JL (1985): Alpha-adrenoceptor changes after oestrogen treatment in platelets and other tissues in female rabbits. Clin Sci 69:235– 238.
Orensanz LM, Guillamon A, Ambrosio E, Segovia S, Azuara MC (1982): Sex differences ina-adrenergic receptors in the rat brain. Neurosci Lett 30:275–278.
Parsons WB Jr, Morledge JH (1970): Antihypertensive effect of a new imidazoline compound (clonidine) and chlorthalidone, individually and in combination. Am J Cardiol 26:258 –261. Piletz J, Halaris A, Saran A, Marler M (1990): Elevated3
H-para-aminoclonidine binding to platelet purified plasma mem-branes from depressed patients. Neuropsychopharmacology 3:201–210.
Piletz JE, Andrew M, Zhu H, Feng YZ, Rains J, Halaris A (1998): Alpha2-adrenoceptors and I1-imidazoline binding
sites: Relationship with catecholamines in women of repro-ductive age. J Psychiatr Res 32:55– 64.
Piletz JE, Chikkala DN, Ernsberger P (1995): Comparison of the properties of agmatine and endogenous clonidine-displacing substance at imidazoline anda2adrenergic receptors. J
Phar-macol Exp Ther 272:581–587.
Piletz JE, Halaris A, Ernsberger PR (1994): Psychopharmacol-ogy of imidazoline anda2-adrenergic receptors: Implications
for depression. Crit Rev Neurobiol 9:29 – 66.
Piletz JE, Halaris A, Nelson J, Qu Y, Bari M (1996a): Platelet I1-imidazoline binding sites are elevated in depression but not
generalized anxiety disorder. J Psychiatr Res 30:147–168. Piletz JE, Halaris A, Saran A, Marler MR (1991): Desipramine
lowers 3
H-para-aminoclonidine binding in platelets of de-pressed patients. Arch Gen Psychiatry 48:813– 820. Piletz JE, Halaris AE, Chikkala D, Qu YS (1996b): Platelet I-1
imidazoline binding sites are decreased by two dissimilar
antidepressant agents in depressed patients. J Psychiatr Res 30:169 –184.
Piletz JE, Sletten K (1993): Nonadrenergic imidazoline binding sites on human platelets. J Pharmacol Exp Ther 267:1493– 1502.
Raddatz R, Lanier SM (1997): Relationship between imidazo-line/guanidinium receptive sites and monoamine oxidase A and B. Neurochem Int 30:109 –117.
Raddatz R, Parini A, Lanier SM (1997): Localization of the imidazoline binding domain on monoamine oxidase B. Mol
Pharmacol 52:549 –553.
Reis D, Regunathan S (1999): Agmatine: An endogenous ligand at imidazoline receptors is a novel neurotransmitter. Ann N Y
Acad Sci 881:65– 80.
Roberts JM, Goldfien RD, Tsuchiya AM, Goldfien A, Insel PA (1979): Estrogen treatment decreases a-adrenergic binding sites on rabbit pletelets. Endocrinology 104:722–728. Sherwin B (1988): Estrogen and/or androgen replacement
ther-apy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology 13:345–357.
Siever LJ, Uhde TW (1984): New studies and prespectives on the noradrenergic receptor system in depression: Effects of thea2
adrenergic agonist clonidine. Biol Psychiatry 19:131–156. Spitzer RL, Endicott J, Robbins E (1978): Research diagnostic
criteria: Rationale and reliability. Arch Gen Psychiatry 35: 773–782.
Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH (1991): Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med 325:756 –762. Stowell LI, McIntosh CJ, Cooke R, Ellis PM (1988):
Adreno-ceptor and imipramine reAdreno-ceptor binding during the menstrual cycle. Acta Psychiatr Scand 78:366 –368.
Sun M-K, Regunathan S, Reis DJ (1995): Cardiovascular re-sponses to agmatine, a clonidine-displacing substance, in anesthetized rat. Clin Exp Hypertens 17:115–128.
Sundaresan PR, Madan MK, Kelvie SL, Weintraub M (1985): Platelet a2 adrenoceptors and the menstrual cycle. Clin
Pharmacol Ther 37:337–342.
Tesson F, Limon-Boulez I, Urban P, Puype M, Vanderkerckhove J, Coupry I, et al (1995): Localization of I2-imidazoline
binding sites on monoamine oxidases. J Biol Chem 270: 9856 –9861.
Theodorou AE, Mistry H, Davies SL, Yamaguchi Y, Horton RW (1987): Plateleta2adrenoceptor binding and function during
the menstrual cycle. J Psychiatr Res 21:163–169.
U’Prichard DC, Snyder SH (1979): Distinct a-noradrenergic receptors differentiated by binding and physiological relation-ships. Life Sci 24:79 – 88.
Yaffe K, Grady D, Pressman A, Cummings S (1998): Serum estrogen levels, cognitive performance, and risk of cognitive decline in older community women [see comments]. J Am