Disease: Therapeutic Implications
Agneta Nordberg
The neuronal nicotinic acetylcholine receptors (nAChRs) in the brain are important for functional processes, including cognitive and memory functions. The nAChRs acting as neuromodulators in communicative processes regulated by different neurotransmitters show a relatively high abundance in the human cortex, with a laminar distribution of the nAChRs of superhigh, high, and low affinity in the human cortex. The regional pattern of messenger RNA (mRNA) for various nAChR subtypes does not strictly follow the regional distribution of nAChR ligand-binding sites in the human brain. Consistent losses of nAChRs have been measured in vitro in autopsy brain tissue of Alzheimer’s disease patients (AD), as well as in vivo by positron emission tomography (PET). Measure-ment of the protein content of nAChRs showed reduced levels of thea4,a3, anda7 nAChR subtypes. The finding that thea4 anda3 mRNA levels were not changed in AD brains suggests that the losses in high-affinity nicotinic-binding sites cannot be attributed to alterations at the transcriptional level of thea4 anda3 genes and that the causes have to be searched for at the translational and/or posttranslational level. The increased mRNA level of the a7 nAChR subtyep in the hippocampus indicates that subunit-specific changes in gene expression of the a7 nAChR might be associated with AD. The PET studies reveal deficits in nAChRs as an early phenomena in AD, stressing the importance of nAChRs as a potential target for drug intervention. PET ligands measuring the a4 nAChRs are under development. Studies of the influence of b-amyloid on nAChRs in brain autopsy tissue from pa-tients with the amyloid precursor protein 670/671 muta-tion have shown that there is no direct relamuta-tionship between nAChR deficits and pathology. Treatment with cholinergic drugs in AD patients indicate improvement of the nAChRs in the brain, as visualized by PET. Further studies on neuroprotective mechanisms mediated via nAChR subtypes are exciting new avenues. Biol Psychi-atry 2001;49:200 –210 © 2001 Society of Biological Psychiatry
Key Words: AD, human brain, nicotinic receptor sub-types, PET ligands, nicotinic agonists, APP 670/671, cholinesterase inhibitors
Introduction
T
he neuronal nicotinic acetylcholine receptors (nAChRs) are transmitter-gated ion channels that belong to a superfamily of ion channels of homologous receptors including g-aminobutyric acid, glycine, and serotonin 3 (5-HT3) (Karlin and Akabas 1995; Patersonand Nordberg 2000; Sargent 1993, 2000). The nAChRs are obvious candidates for transducing cell surface inter-actions, not only for acetylcholine but also for other neurotransmitters (Kaiser et al 2000; Wonnacott 1997). Experimental data suggest that the nAChRs might act as neuromodulators in communicative processes in the brain (Kaiser et al 2000; Lindstro¨m 1997; Wonnacott 1997). It is therefore of great importance to define by which mecha-nisms the nAChRs exert their action in the brain. The nAChRs are found to be involved in a complex range of central nervous system disorders including Alzheimer’s disease (AD), Parkinson’s disease, schizophrenia, Tourette’s syndrome, anxiety, depression, and epilepsy (Newhouse and Kelton 2000; Newhouse et al 1997; Paterson and Nordberg 2000). The exact role of nAChRs and their full potential as a therapeutic target in these diseases have yet to be clarified. Interestingly enough, a considerable body of evidence exists to suggest that the nAChRs are involved in cognitive and memory functions (Levin 2000; Newhouse and Kelton 2000; Newhouse et al 1997; Sahakian and Coull 1994).
Alzheimer’s disease is the most common form of dementia and one of the most devastating diseases of the middle aged and elderly. Although the last decade has witnessed a steadily increasing effort directed at discovery of the etiology and neuropathologic and neurochemical mechanisms involved in the disease, there is still no cure (Braak and Braak 1998; Cohen-Mansfield 2000; Fratigli-oni et al 1999; Hardy 1997; Master and Beyreuther 1998; St George-Hyslop 2000,). However, extensive research activities have stimulated the development of new
From the Department of NEUROTEC, Division of Molecular Neuropharmacology, Karolinska Institute, Huddinge University Hospital, Huddinge, Sweden. Address reprint requests to Agneta Nordberg, M.D., Ph.D., Department of
NEU-ROTEC, Division of Molecular Neuropharmacology, Huddinge Hospital B84, S-141 86 Huddinge, Sweden.
Received June 1, 2000; revised November 13, 2000; accepted December 8, 2000.
© 2001 Society of Biological Psychiatry 0006-3223/01/$20.00
treatment strategies in AD, and several drugs that improve cholinergic transmitter activity have reached clinical use (for a review, see Nordberg and Svensson 1998). The role of nAChRs in AD is discussed below, with the focus mainly on new therapeutic implications.
nAChRs in the Human Cortex
The nAChRs are distributed in many regions of the human brain. So far the nAChR subunitsa3,a4,a5,a7,b2,b3, and b4 have been cloned (Anand and Lindstrom 1990; Chini et al 1994; Fornasari et al 1990; Matter and Ballivet 2000; Raimondi et al 1991; Willoughby et al 1993). The distribution of the nAChRs and their transcripts have been mapped in vitro in the human brain, using autoradiography (Adem et al 1988, 1989; Court and Perry 1994; Sihver et al 1998b), in situ hybridization (Rubboli et al 1994; Wever et al 1994), and reverse transcription polymerase chain reaction (Hellstro¨m-Lindahl et al 1998). In vitro receptor binding studies in human autopsy brain tissue suggest that nAChRs are heterogeneous and can be rationalized to three different nAChR sites (superhigh, high, and low affinity) (Marutle et al 1998; Nordberg et al 1988; Warp-man and Nordberg 1995). The distribution of high-affinity nAChRs was studied with [3H]nicotine and [3 H]epibati-dine as radioactive receptor ligands (Marutle et al 1998; Sihver et al 1998b). Two high-affinity nAChR sites were identified in the human cortex with [3H]epibatidine, most likely representing the a3 and a4b2 nAChR subtypes. Differences in the regional binding between [3H]nicotine and [3H]epibatidine were observed in the human brain, with a proportionally higher level of [3H]epibatidine binding sites in the thalamus and cerebellum. The differ-ence possibly reflects a selectivity for different nAChR subtypes between the nAChR ligands, nicotine, and epi-batidine—that is, a greater selectivity for [3H]epibatidine for the a3 nAChRs in the human brain (Marutle et al 1998).
The laminar distribution of nAChRs in human cortex was studied in autopsy brain tissue, using autoradiographi-cal analysis and the nAChR ligands [3H]nicotine, [3 H]epi-batidine, and [3H]cytisine (Sihver et al 1998b). A general high binding was observed in layers 1, 11, and V, with particularly high levels in the layer of primary sensory motor cortex and the inferior frontal sulcus (Sihver et al 1998b). All three ligands appeared to bind to a common high-affinity binding site, which most likely represents binding to the nAChRa4 subunits. A significantly greater binding for [3H]nicotine and [3H]epibatidine than for [3H]cytisine is observed in areas of the primary motor cortex, layer 111b of the occipital cortex, and layer V of the superior temporal sulcus, most likely representing binding of an additional nAChR site. High levels of
[3H]nicotine binding have been observed in layers 1 and V1 of the primary cortex, deeper layer V of the primary cortex, layer 111 of the superior temporal sulcus, and layer V1 of the parietal cortex. This suggests the presence of an additional third nAChR site in the human cortex (Sihver et al 1998b) that may be the a7 nAChR subtype.
The distribution of messenger RNA (mRNA) for differ-ent nAChR subunits has been examined in differdiffer-ent cortical layers (Schro¨der et al 1995). Thea4 mRNA seems to be abundant in all layers except 1 and IV of the frontal cortex (Schro¨der et al 1995). The a3 mRNA has been found to be most prominently expressed in the pyramidal neuron layers 111–V1, moderately expressed in layer 11, and minimally expressed in layer IV of the human cortex (Schro¨der et al 1995; Wever et al 1994). A high expression ofa7 mRNA was observed in layers 11 and 111, moderate in layers V and V1, and low in layers I and IV of the human cortex (Schro¨der et al 1995). A significant decrease in [3H]epibatidine binding has been observed with aging in the human cortical brain regions and cerebellum (Ma-rutle et al 1998). The levels ofa4 anda7 nAChR mRNA showed a decrease with aging, whereas the levels ofa3 mRNA were unchanged in the elderly brain relative to the fetal brain (Hellstro¨m-Lindahl et al 1998). Interestingly, the observed regional pattern of expression of nAChR subunit mRNA in the human brain does not directly correspond to the regional pattern of nAChR binding sites revealed by ligand-binding studies.
nAChR Changes in AD
A consistent, significant loss of nAChRs has been ob-served in cortical autopsy brain tissue from AD patients relative to age-matched healthy subjects (Nordberg and Winblad 1986). More recently, we have have found that the nAChR deficits in AD brains probably represent an early phenomenon in the course of the disease, which can be detected in vivo by positron emission tomography (PET) (Nordberg et al 1990, 1995, 1997). The cortical nAChR deficits significantly correlate with cognitive im-pairment in AD patients (Nordberg, in press; Nordberg et al 1995, 1997).
the vesamicol binding sites may be more preserved in the existing presynaptic terminals of AD cortical tissue, thereby expressing a compensatory capacity to maintain cholinergic activity. In addition, the observation suggests that nAChRs are present on noncholinergic nerve termi-nals to a significant extent. Basal forebrain lesions in rats, with the selective cholinergic immunotoxin 192Ig saporin and ibotenic acid, also show the rich presence of nAChRs on noncholinergic nerve terminals (Bednar et al 1998), supporting the assumption of nAChR as a neuromodulator also in noncholinergic nerve terminals (Kaiser et al 2000). b-Amyloid (Ab) is also an important factor, which may initiate and promote AD (Selkoe 1999). Recently, the possible role of Ab as a neuromodulator in the brain has stressed the possible regulatory mechanisms between Ab and cholinergic neurotransmission and nAChRs in the
was approximately 15 years younger than the sporadic patients. Both positive and negative correlations were observed between the number of nAChR binding sites and the number of neuronal plaques and neurofibrillary tan-gles, respectively, in the temporal cortex and parietal cortex of mutation carriers. This finding suggests that these processes may be closely related but not directly dependent on each other (Figure 2). Different brain re-gions may show different kinetics for the development of neurofibrillary tangles and neuritic plaques. We have recently characterized the nAChRs in APP 670/671 trans-genic mice and found compensatory mechanisms for some of the nAChR subtypes, which correlates to behavior and pathology in the transgenic mice (Paterson et al, unpub-lished data).
A decrease in the protein levels of the a3 and a4 nAChR subunits was recently measured in the temporal cortex and of thea3,a4, anda7 nAChR subtypes in the hippocampi of AD brains relative to age-matched control subjects (Guan et al 2000b). A decrease in protein levels of
the a4 nAChR but not of the a3 and a7 nAChRs was reported by Martin-Ruiz et al (1999). The explanation for the differences in results obtained by Martin-Ruiz et al (1999) and Guan et al (2000b) is unknown. Lee et al (2000) recently also reported a significant decrease in the a7 nAChR protein level of the AD hippocampus. Inter-estingly, a reduction in the protein level ofa7 has also been measured in the frontal cortex of patients with schizophrenia (Guan et al 1999), whereas no decrease was measured in the a4 nAChR protein level (Guan et al 1999). The deficits in nAChRs seen in AD brains may be related to alterations of the nAChR synthesis on different levels such as transcription, translation and postranslation modifications, and receptor turnover.
Examination of the regional expression of mRNA of the nAChRa4 and a3 subunits has shown no difference in autopsy AD brain tissue in any region analyzed (Hell-stro¨m-Lindahl et al 1999; Terzano et al 1998), whereas the level of the a7 mRNA was significantly higher in the hippocampus (Hellstro¨m-Lindahl et al 1999). The studies suggest that the nAChR deficits in AD brains mainly reflect posttranscriptional events (Hellstro¨m-Lindahl et al 1999; Schro¨der and Wever 1998). The mechanisms for changes in protein levels of nAChRs in AD are unclear. Possible factors such as amyloid peptide accumulation, hyperphosphorylation of tau protein, oxidative stress, and modification of cell membrane during the development of AD may be related to decreased protein levels of nAChRs (Farooqui et al 1995; Smith et al 1996). Interestingly enough, lipid peroxidation has been shown to decrease the number of nAChRs in PC12 cells (Guan et al 2000a).
Visualization of nAChRs in AD by PET
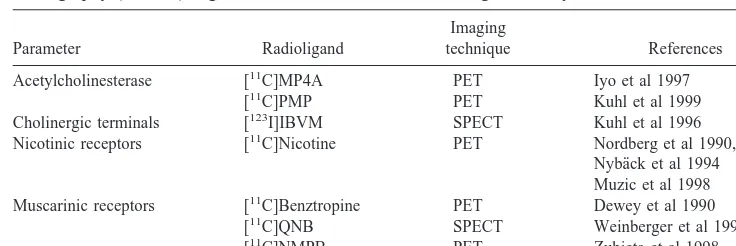
Significant progress has been made during recent years in developing and applying functional brain imaging tech-niques to allow for early diagnosis of AD and evaluation of drug treatment efficacy. Positron emission tomography might be a suitable method for functional studies of pathologic changes in the brain, not only revealing dys-functional changes early in the course of the disease but also providing a deep insight into the functional mecha-nisms of new potential drug treatment strategies. A limited number of PET ligands are so far available for mapping the cholinergic system in the human brain (Table 1).
[11C]Physostigmine, [11C]MPA4A, and [11C]PMP have been used in PET studies to measure acetylcholinesterase in brains of healthy volunteers (Kuhl et al 1999; Namba et al 1999; Pappata et al 1996). Positron emission tomogra-phy studies have revealed a reduced cortical acetylcho-linesteserase activity in AD patients (Iyo et al 1997; Kuhl et al 1999). A progressive loss of cortical acetylcholines-terase activity has been observed in AD patients with Figure 2. (A) Correlation between neuronal plaques and [3
H]nic-otine binding in the temporal cortex and parietal cortex of four patients (P1–P4) carrying the Swedish amyloid precursor protein (APP) 670/671 mutation. (B) Correlation between neurofibrillary tangles and [3
cognitive decline (Shinotoh et al 2000). The presynaptic vesicular acetylcholine transporter vesamicol ([123 I]iodo-benzovesamicol) has been used in vivo as a marker of presynaptic cholinergic activity in single photon emission computed tomography (SPECT) studies (Kuhl et al 1996). Greater reduction in [123I]iodobenzovesamicol binding was observed throughout the cerebral cortex in AD pa-tients with early onset of the disease (Kuhl et al 1996). The loss in cortical acetylcholinesterase activity was less pro-nounced in mildly demented AD patients relative to autopsy material and did not strictly correlate with cere-bral glucose metabolism impairment (Kuhl et al 1999).
The development of methods for the synthesis of radiolabeled nicotinic ligands with short half-life [11C] has enabled the study of the binding of [11C]nicotine in both normal and AD brains by PET (Nordberg et al 1990, 1995). The use of [11C]nicotine in PET studies was reviewed by Mazie´re and Delforge (1995), who identified some problems with the tracer due to high nonspecific binding, rapid metabolism, and rapid washout from the brain. [11C]Nicotine has drawbacks such as rapid dissoci-ation from the receptor ligand and strong dependence of accumulation on cerebral blood flow. Different kinetic modeling approaches have been developed to improve the analysis of the PET studies, and the dual tracer kinetic rate constant (k2*) model for [
11
C]nicotine compensates for some of the problems (Lundqvist et al 1998). The mea-surement of [11C]nicotine in the human brain in vivo by PET (k2*) agrees with the distribution of nAChRs
ob-served by in vitro radioligand binding in human brain tissue autopsy (Nordberg 2000b). A significant decrease in [11C]nicotine binding (increase in k2* value) was
mea-sured in the temporal cortex, frontal cortex, and hippocam-pus (Nordberg et al 1995, 1997). Selective cortical deficits in [11C]nicotine binding are often observed by PET early in the course of the AD disease (Figure 3A). A significant correlation can be observed between cognitive function and nicotinic receptor binding (k2*) (Figure 3A). The
findings might be promising in that PET imaging com-bined with genetic testing (e.g., apolipoprotein E typing) could be used to detect subjects with a greater risk of developing AD.
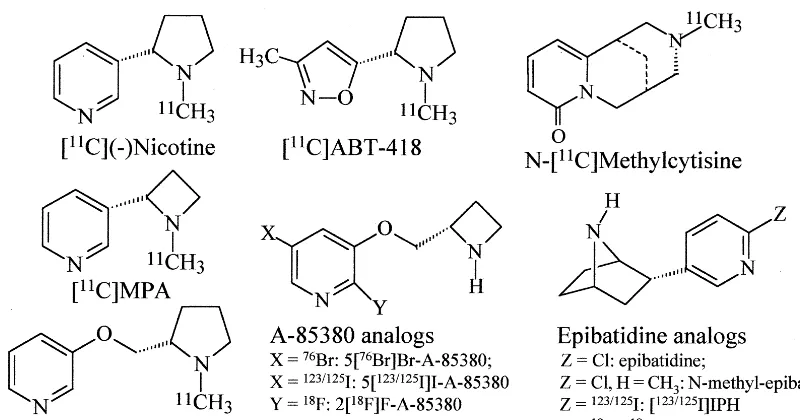
The past several years have seen an expanded effort to develop PET probes for noninvasive study of nAChRs. This has also led to the search for new nAChR PET ligands. A ligand with a selectivity for thea4b2 nAChRs would be particularly preferable because the a4b2 has been recognized as the predominant subtype that is defi-cient in AD (for a review, see Sihver et al 2000). Several PET ligands have been synthesized, including [11 C]ABT-418, [11C]MPA, and [76Br]BAP (A-85380) (Sihver et al 1998a, 1999a, 1999b) (Figure 4). [11C]MPA and [76Br]BAP (A-85380) showed a more distinct binding pattern in the rat brain relative to [11C]nicotine and [11C]ABT, as revealed by autoradiography (Figure 3B). Administration of the PET ligands to monkeys also re-vealed a slower binding dissociation, and higher specific binding for [11C]MPA and [76Br]A-85380 than for [11C]nicotine and [11C]nicotine (Sihver et al 1999a, 1999b). The very high affinity for [76Br]A-85380, the high specific in vivo uptake seen in monkeys (Figure 3C), and thereby the possible a4b2 nAChR selectivity increase interest in using the ligand in human PET studies. The application of A-85830 analog with an [11C] label is ongoing. Positron emission tomography studies using 2-[18F]A-85380 and SPECT studies with [123I]5-A-85380 have recently been performed in monkeys (Fujita et al 2000; Sihver et al 2000). Both PET and SPECT studies reveal a high uptake of the radioligand to areas rich in a2b2 nAChR such as the thalamus (Figure 3C). Recently, several epibatidine analogs such as norchloro[18F]fluoroepibatidine (Ding et al 2000) have been developed, but it is still uncertain whether the epibatidine analogs can be applied in humans due to risk of toxicity.
Positron emission tomography studies not only allow
Table 1. Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) Ligands for Visualization of Cholinergic Activity in the Human Brain
Parameter Radioligand
Imaging
technique References Acetylcholinesterase [11C]MP4A PET Iyo et al 1997
[11C]PMP PET Kuhl et al 1999
Cholinergic terminals [123I]IBVM SPECT Kuhl et al 1996
Nicotinic receptors [11C]Nicotine PET Nordberg et al 1990, 1995
Nyba¨ck et al 1994 Muzic et al 1998 Muscarinic receptors [11C]Benztropine PET Dewey et al 1990
[11C]QNB SPECT Weinberger et al 1991
measurement of nAChRs in steady-state situation in AD, but also allow measurement of nAChRs during functional activation studies. We have recently performed studies
where functional activation patterns in the brain during an episodic memory task in healthy subjects as well as in AD patients have been measured by cerebral blood flow
Figure 3. (A) (Top) Horizontal positron emission tomography images of the uptake and distribution of [11
C]nicotine at the level of the basal ganglia (left) and the frontal association cortex–parietal cortex (right) in the brain of an Alzheimer’s disease patient with mild dementia. The figure represents a summation picture of the brain uptake of [11
C] radioactivity after an intravenous injection of a tracer dose of [11
C]nicotine. The color scale indicates radioactivity expressed in nCi/cm3
/body weight. Red, high uptake; yellow, medium uptake; green, low uptake. The patient shows left-side cortical deficits in [11
C]nicotine. (Bottom) Correlation between cognitive function (Mini-Mental State Examination [MMSE] score) and nicotinic receptor binding (k2* rate constant) in the temporal cortex of Alzheimer’s patients. p,.001. (B) Binding of
different nicotinic acetylcholine receptor ligands in the rat brain as visualized by in vitro autoradiography of coronal (left) and sagittal (middle) rat brain sections. (Right) The nonspecific binding obtained in the presence of 100mmol/L (2)nicotine is shown in the sagittal sections. The binding density increases in the sequence black, yellow, red, white. ctx, cortex; hip, hippocampus; th, thalamus; cbl, cerebellum; str, striatum; MPA, (R,S)-1-methyl-2-(3-pyridyl)azetidine; BAP, bromo-3-[[2(5)-azetidinyl]methoxy] pyridine. Data are from Sihver et al (1998a, 1999a).
(C) (a) Positron emission tomography image of [76
Br]Br-A-85380 in a rhesus monkey brain (transverse section). The total uptake was determined at a baseline scan (left), and a second scan was performed 3.5 hours later after injection of 0.5 mg/kg cytisine to block the uptake of [76
Br]Br-A-85380 (right). The ratios of total to block were 2.5 in the thalamus and 1.2 in the cortex. (b) Distribution of [76
changes with PET (Ba¨ckman et al 1997, 1999). In some ongoing studies we are now measuring the changes in nAChRs in different brain regions before and during task performances such as attentional tests. This type of study will provide further insight into the regional network in the brain, where nAChRs play an important role, and how drug treatment can improve brain function.
nAChRs: Target for AD Treatment?
Transmitter replacement therapy is the mainstay treatment for AD. Cholinergic therapy is based on the assumption that low levels of acetylcholine are responsible for the cognitive decline associated with AD. Cholinesterase in-hibitors including tacrine, donepezil, rivastigmine, and galantamine have in clinical studies shown palliative effects on symptoms and some trend to slow disease progression (Giacobini 2000; Nordberg and Svensson 1998; Van den Berg et al 2000). It is likely that the therapeutic benefit of cholinesterase inhibitors occurs at least in part through activation of the nAChRs, by the direct action of increased levels and/or through a direct activation of the allosteric site on the nAChR (Maelicke et al 1995, 2000).
Positron emission tomography and SPECT studies per-formed in AD patients under treatment with cholinergic drug therapy have shown an improvement in the cerebral blood flow and glucose metabolism (Nordberg 1999). In addition, PET studies also have revealed an improvement in nAChRs in AD patients during long-term treatment with cholinesterase inhibitors such as tacrine and NXX-066 (Nordberg 2000; Nordberg et al 1992, 1998). Since an
enhanced activity of acetylcholinesterase has been mea-sured in cerebrospinal fluid following long-term treatment with tacrine (Nordberg et al 1999), possibly as a result of an increased acetylcholinesterase gene expression, it might be an advantage to use drugs interacting with nAChRs. Nerve growth factor intraventricularly administered to AD patients for 3 months resulted in an increased [11 C]nico-tine binding (Eriksdotter-Jo¨nhagen et al 1998), whereas treatment with the 5-HT3blocker ondansetron showed a
cholinesterase inhibitors produce similar improvement in cognitive function in AD patients (Nordberg et al 1998). Although epidemiologic data are somewhat conflicting about the possibility that smoking can protect against AD (Doll et al 2000; Van Duijn et al 1995), experimental data suggest that a neuroprotective effect against Ab toxicity might be obtained via the nAChR (e.g., the a7 subtype) (Kihara et al 1997; Svensson and Nordberg 1998; Zamani et al 1997). Estrogen, which in epidemiologic studies has been shown to reduce the risk of AD (Henderson 1997), has in experimental studies in PC 12 cells shown neuro-protective effects against Ab toxicity that are at least partly mediated by thea7 subtype nAChR (Svensson and Nordberg 1998). It is reasonable to assume that to obtain significant neuroprotective effects the drug probably must be given during a very early stage of AD, probably a presymptomatic level. There are tremendous possibilities for the development of nAChR agonists as potential therapeutic agents in AD.
This study was supported by grants from the Swedish Medical Research Council (Project No. 05817), Loo and Hans Osterman’s foundation, Stohne’s foundation, the Swedish Alzheimer foundation, Foundation for Old Servants, and Swedish Match.
Aspects of this work were presented at the symposium “Nicotine Mechanisms in Alzheimer’s Disease,” March 16 –18, 2000, Fajardo, Puerto Rico. The conference was supported by the Society of Biological Psychiatry through an unrestricted educational grant provided by Janssen Pharmaceutica LP.
References
Adem A, Nordberg A, Singh-Jossan S, Sara V, Gillberg PG (1989): Quantitative autoradiography of nicotinic receptors in large cryosections of human brain hemispheres. Neurosci Lett 101:247–252.
Adem A, Singh-Jossan S, dA´ rgy R, Brandt I, Winblad B, Nordberg A (1988): Distribution of nicotinic receptors in human thalamus as visualized by [3
H]nicotine and [3
H] acetylcholine receptor autoradiography. J Neural Transm 73:77– 83.
Anand R, Lindstrom J (1990): Nucleotide sequence of the human nicotinic acetylcholine receptorb2-subunit gene. Nucl Acid
Res 18:4272.
Auld DS, Kar S, Quirion R (1998): Beta-amyloid peptides as direct cholinergic neuromodulators. a missing link? Trends
Neurosci 21:43– 49.
Ba¨ckman L, Almkvist O, Andersson J, Nordberg A, Winblad B, Reineck R, Långsstro¨m B (1997): Brain activation of youn and old adults during implicit and explicit retrieval. J Cogn
Neurosci 9:378 –391.
Ba¨ckman L, Andersson JLR, Nyberg L, Winblad B, Nordberg A, Almkvist O (1999): Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology 52:1861–1870.
Bednar I, Zhang X, Dastranj-Sedghi R, Nordberg A (1998):
Differential changes of nicotinic receptors in the rat brain following ibotenic acid and 192-IgG saporin lesions of the nucleus basalis magnocellularis. Int Dev Neurosci 16:661– 668.
Braak H, Braak E (1998): Evolution of neuronal changes in the course of Alzheimer’s disease. J Neural Transm 53(suppl): 127–140.
Chini B, Raimond E, Elgoyhen AB, Moralli D, Balzaretti M, Heinemann S (1994): Molecular cloning and chromosomal localization of the human alpha 7-nicotinic receptor subunit gene (CHRNA7). Genomics 19:379 –381.
Cohen-Mansfield J (2000): Heterogenity in dementia: Challenge and opportunities. Alzheimer Dis Assoc Disord 14:60 – 63. Court JA, Perry E (1994): CNS nicotinic receptors—possible
therapeutic target in neurodegenerative disorders. CNS Drugs 2:216 –233.
Dewey SL, Volkow ND, Logan J, MacGregor RR, Fowler JS, Schlyer DJ, et al (1990): Age-related decrease in muscarinic cholinergic receptor binding in human brain measured with positron emission tomography (PET). J Neurosci Res 27: 569 –575.
Ding YS, Logan J, Bermel R, Garza V, Rice O, Fowler JS, et al (2000): Dopamine receptor-mediated regulation of striatal cholinergic activity: Positron emission tomography studies with norchloro[18F]fluoroepibatidine. J Neurochem 74:1514 – 1521.
Doll R, Peto R, Boreham J, Sutherland I (2000): Smoking and dementia in male British doctors prospective study. BMJ 320:1097–1102
Eriksdotter-Jo¨nhagen M, Nordberg A, Amberla K, Ba¨ckman L, Ebendal T, Meyerson B, et al (1998): Intracerebroventricular infusion of nerve growth factor in three patients with Alzhei-mer’s disease. Dement Geriatr Cogn Disord 9:246 –257. Farooqui AA, Wells K, Horrocks LA (1995): Breakdown of
membrane phospholipids in Alzheimer disease. Involvement of excitatory amino acid receptors. Mol Chem Neuropathol 25:155–173.
Fornasari D, Chini B, Tarroni P, Clementi F (1990): Molecular cloning of human nicotinic a3 subunit. Neurosci Lett 111: 351–356.
Fratiglioni L, DeRonchi D, Aguero-Torres H (1999): Worldwide prevalence and incidence of dementia. Drugs Aging 15:365– 375.
Fujita M, Tamagnan G, Zoghbi SS, Al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB (2000): Measurement ofa4b2 nicotinic acetylcholine receptors with [123I]5-I-A-85380 SPECT.
J Nucl Med 41:1552–1560.
Giacobini E (2000): Cholinesterase inhibitor therapy stabilizes symptoms of Alzheimer’s disease. Alzheimer Dis Assoc
Disord 14(suppl 1): S3–S10.
Guan ZZ, Zhang X, Blennow K, Nordberg A (1999): Decreased protein level of nicotinic receptor a7 subunit in the frontal cortex from schizophrenic brain. Neuroreport 10:1779 –1782. Guan ZZ, Zhang X, Nordberg A (2000a): Influence of lipid peroxidaion on the nicotinic acetylcholine receptors in PC12 cells. Neurosci Lett 286:163–166.
Guan ZZ, Zhang X, Ravid R, Nordberg A (2000b): Decreased protein levels of nicotinic receptor subunits in the hippocam-pus and temporal cortex of patients with Alzheimer’s disease.
Hardy J (1997): Amyloid, the presenilins and Alzheimer’s disease. Trends Neurosci 20:154 –159.
Hellstro¨m-Lindahl E, Gorbounova O, Seiger Å, Mousavi M, Nordberg A (1998): Regional distribution of nicotinic recep-tors during prenatal development of human brain and spinal cord. Dev Brain Res 108:147–160.
Hellstro¨m-Lindahl E, Mousavi M, Zhang X, Ravid R, Nordberg A (1999): Regional distribution of nicotinic receptor subunit mRNA in human brain: Comparison between Alzheimer and normal brain. Mol Brain Res 66:94 –103.
Henderson VW (1997): Estrogen replacement therapy for the prevention and treatment of Alzheimer’s disease. CNS Drugs 8:343–351.
Iyo M, Namba H, Fukushi K, Shinotoh H, Nagatsuka S, Suhara T, et al (1997): Measurement of acetylcholinesterase by positron emission tomography in the brain of healthy controls and patients with Alzheimer’s disease. Lancet 349:1805– 1809.
Jones GMM, Sahakian BJ, Levy R, Warburton DM, Gray JA (1992): Effects of acute subcutaneous nicotine on attention, information processing, and short-term memory in Alzhei-mer’s disease. Psychopharmacology (Berl) 108:485– 494. Kaiser S, Soliakov L, Wonnacott S (2000): Presynaptic neuronal
nicotinic receptors: Pharmacology, heterogeneity, and cellu-lar mechanisms. In: Clementi F, Fornasari D, Gotti C, editors.
Neuronal Nicotinic Receptors. Experimental Pharmacology,
Vol 14. Berlin: Springer, 193–211.
Karlin A, Akabas MH (1995): Towards a structural basis for the function of nicotinic acetylcholine receptors and their cous-ins. Neurons 15:1231–1244.
Kihara T, Shimohama S, Sawada H, Kimura J, Kume T, Kochiyama H, et al (1997): Nicotinic receptor stimulation protect neurons against beta-amyloid toxicity. Ann Neurol 42:159 –163.
Kuhl DE, Koeppe RA, Minoshima S, Snyder SE, Ficaro EP, Foster NL, et al (1999): In vivo mapping of cerebral acetyl-cholinesterase activity in aging and Alzheimer’s disease.
Neurology 52:691– 699.
Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, et al (1996): In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease.
Ann Neurol 40:399 – 410.
Lee DH, Da´ndrea MR, Plata Salaman CR, Wang HY (2000): Decreaseda7 nicotinic acetylcholine receptor protein levels in sporadic Alzheimers disease hippocampus. Alzheimer Rep 3:217–220.
Levin ED (2000): The role of nicotinic acetylcholine receptors in cognitive function. In: Clementi F, Fornasari D, Gotti C, editors. Neuronal Nicotinic Receptors. Experimental
Phar-macology, Vol 14. Berlin: Springer, 587– 602.
Lindstro¨m J (1997): Nicotinic acetylcholine receptors in health and disease. Mol Neurobiol 15:193–222.
Lundqvist H, Nordberg A, Hartvig P, Långstro¨m B (1998): (S)(2)[11
C] nicotine binding assessed by PET a dual tracer model evaluated in the rhesus monkey brain. Alzheimer Dis
Assoc Disord 12:238 –246.
Maelicke A, Schrattenholz A, Samochocki M, Radina M, Albu-querque EX (2000): Allostrically potentiating ligands of nicotinic receptors as a treatment strategy for Alzheimer’s disease. Behav Brain Res 113:199 –206.
Maelicke A, Schrattenholz A, Storch A, Schro¨der B, Gutbrod O, Methfesel C, et al (1995): Noncompetitive agonism at nico-tine acetylcholine receptors: Functional significance for CNS signal transduction. J Recept Signal Transduct Res 15:333– 353.
Martin-Ruiz CM, Court JA, Molnar E, Lee M, Gotti C, Ma-malaki A, et al (1999): Alpha 4 but not alpha 3 and alpha 7 nicotinic acetylcholine receptor subunits are lost in the temporal cortex in Alzheimer’s disease. J Neurochem 73: 1635–1640.
Marutle A, Warpman U, Bognanovic N, Lannfelt L, Nordberg A (1999): Neuronal nicotinic receptor deficits in Alzheimer patients with the swedish amyloid precursor 670/671 muta-tion. J Neurochem 72:1161–1169.
Marutle A, Warpman U, Bogdanovic N, Nordberg A (1998): Regional distribution of subtypes of nicotinic receptors in human brain and effect of aging studied by6 [3H]epibati-dine. Brain Res 801:143–149.
Master CL, Beyreuther K (1998): Alzheimer disease. BMJ 316:446 – 448.
Matter JM, Ballivet M (2000): Gene structures and transcrip-tional regulation of the neuronal nicotinic acetylcholine re-ceptors. In: Clementi F, Fornasari D, Gotti C, editors.
Neuronal Nicotinic Receptors. Experimental Pharmacology,
Vol 14. Berlin: Springer, 33–55.
Mazie´re M, Delforge J (1995): PET imaging of (11
C) nicotine: Historical aspects. In: Domino EF, editor. Brain Imaging of
Nicotine and Tobacco Smoking. Ann Arbor, MI: NPP Books,
13–28.
Mullan J, Crawford F, Axelman K, Houlden H, Lillius L, Winblad B, et al (1992): A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of
b-amyloid. Nat Genet 1:345–347.
Muzic RF Jr, Berridge MS, Friedland RP, Zhu N, Nelson AD (1998): PET quantification of specific binding of carbon-11-nicotine in human brain. J Nucl Med 39:2048 –2054. Namba H, Masaoimi I, Fukushi K, Shinotoh H, Nagatsuka S,
Suhara T, et al (1999): Human cerebral acetylcholinesterase activity measured with positron emission tomography proce-dure, normal values and effect of age. Eur J Nucl Med 25:135–143.
Newhouse PA, Kelton M (2000): Clinical aspects of nicotinic agents: Therapeutic application in central nervous system disorders. In: Clementi F, Fornasari D, Gotti C, editors.
Neuronal Nicotinic Receptors. Experimental Pharmacology,
Vol 14. Berlin: Springer, 779 – 812.
Newhouse PA, Potter A, Levin ED (1997): Nicotinic system involment in Alzheimer’s disease and Parkinson’s diseases. Implication for therapeutics. Drugs Aging 11:206 –228. Nordberg A (1999): PET studies and cholinergic therapy in
Alzheimer’s disease. Rev Neurol (Paris) 155(suppl 4):53– 63. Nordberg A (2000): The effect of cholinesterase inhibitors studied with brain imaging. In: Giacobini E, editor.
Cholines-terases and Cholinesterase Inhibitors. London: Martin
Dun-itz, 237–247.
Nordberg A (2000): Noninvasive exploration of nicotinic acetyl-choline receptors in vivo. In: Clementi F, Fornasari D, Gotti C, editors. Handbook of Experimental Pharmacology, Vol 144. Berlin: Springer, pp 539 –561.
Heterogenous cholinergic nicotinic receptors in the CNS. In: Clementi F, Gotti C, Sher E, editors. Nicotinic Acetylcholine
Receptors in the Nervous System, NATO ASI Series H: Cell Biology, Vol H25. New York: Springer, 331–350.
Nordberg A, Almkvist O, Amberla K, Basun H, Corder B, Ebendal T, et al (1997): Responders and non-responders to tacrine, ondansetron and NGF treatment in Alzheimer pa-tients as evaluated by positron emission tomography and APOE genotype. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM, editors. Alzheimer’s Disease:
Biology, Diagnosis and Therapeutics. Chichester, UK: Wiley,
647– 653.
Nordberg A, Amberla K, Shigeta M, Lundqvist H, Viitanen M, Hellstro¨m-Lindahl E, et al (1998): Long-term tacrine treat-ment in three mild Alzheimer patients: Effects on nicotinic receptors, cerebral blood flow, glucose metabolism, EEG and cognitive abilities. Alzheimer Dis Assoc Disord 12: 228 –237.
Nordberg A, Hartvig P, Lilja A, Viitanen M, Amberla K, Lundqvist H, et al (1990): Decreased uptake and binding of
11
C-nicotine in brain of Alzheimer patients as visualized by positron emission tomography. J Neural Transm 2:215–224. Nordberg A, Hellstro¨m-Lindahl E, Almkvist O, Meurling L (1999): Activity of acetylcholinesterase in CSF increases in Alzheimer patients after treatment with tacrine. Alzheimer’s
Reports 2:347–352.
Nordberg A, Lilja A, Lundqvist H, Hartvig P, Amberla K, Viitanen M, et al (1992): Tacrine restores cholinergic nico-tinic receptors and glucose metabolism in Alzheimer patients as visualized by positron emission tomography. Neurobiol
Aging 13:747–758.
Nordberg A, Lundkvist H, Hartvig P, Andersson J, Johansson M, Hellstro¨m-Lindahl E, et al (1997): Imaging of nicotinic and muscarinic receptors in Alzheimer’s disease: Effect of tacrine treatment. Dement Geriatr Cogn Disord 8:78 – 84.
Nordberg A, Lundkvist H, Hartvig P, Lilja A, Långstro¨m B (1995): Kinetic analysis of regional (S)(–)11
C-nicotine bind-ing in normal and Alzheimer brains—in vivo assessment using positron emission tomography. Alzheimer Dis Assoc
Disord 9:21–27.
Nordberg A, Svensson AL (1998): Cholinesterase inhibitors in the treatment of Alzheimer’s disease: A comparison of tolerance and pharmacology. Drug Saf 19:465– 480. Nordberg A, Winblad B (1986): Reduced number of 3H-nicotine
and 3H-acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci Lett 72:115–119.
Nyba¨ck H, Halldin C, Åhlin A, Curvall M, Eriksson L (1994): PET studies of the uptake of (S) and (R)-[11
C] nicotine in the human brain: Difficulties in visulalizing specific receptor binding in vivo. Psychopharmacology (Berl) 115:31–36. Paterson D, Nordberg A (2000): Neuronal nicotinic receptors in
the human brain. Prog Neurobiol 61:75–111.
Pappata S, Tavitian B, Traykov L, Jobert A, Dalger A, Mangin JF, et al (1996): In vivo imaging of human cerebral acetyl-cholinesterase. J Neurochem 67:876 – 879.
Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA (1999): Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer’s disease.
Psychopharmacology 142:334 –342.
Raimondi E, Rubboli F, Moralli D, Chini B, Fornasari D, Tarroni
P, et al (1991): Chromosomal localization and physical linkage of the gene encoding the human a3,a5, and b4 neuronal nicotinic receptor subunits. Genomics 12:849 – 850. Rubboli F, Court J, Morris C, Chini B, Perry E, Clementi F (1994): Distribution of nicotinic receptors in human hip-pocampus and thalamus. Eur J Neurosci 6:1596 –1604. Rusted JM, Warburton DM (1992): Fascilitation of memory by
post-trial administration of nicotine evidence for an atten-tional explanation. Psychopharmacology (Berl) 108:452– 455. Sahakian BJ, Coull JT (1994): Nicotine and THA: Evidence for
improved attention in patients with dementia of the Alzhei-mer type. Drug Dev Res 31:80 – 88.
Sargent PB (1993): The diversity of neuronal nicotinic acetyl-choline receptors. Ann Rev Neurosci 16:403– 443.
Sargent PB (2000): The distribution of neuronal nicotinic ace-tylcholine receptors. In: Clementi F, Fornasari D, Gotti C, editors. Neuronal Nicotinic Receptors. Experimental
Phar-macology, Vol 14. Berlin: Springer, 163–192.
Schro¨der H, Van de Vos RA, Jansen EN, Birtsch C, Wever A, Lobron C, et al (1995): Gene expression of the nicotinic acetylcholine receptor a4 subunit in the frontal cortex of Parkinson’s disease patients. Neurosci Lett 187:173–176. Schro¨der H, Wever A (1998): Nicotinic acetycholine receptors in
Alzheimer’s disease. Alzheimer Dis Rev 3:20 –27.
Selkoe DJ (1999): Translation cell biology into therapeutic advances in Alzheimer’s disease. Science 399(suppl A):A23– A31.
Shinotoh H, Namba H, Fukushi K, Nagatsuka S, Tanaka N, Aotsuka A, et al (2000): Progressive loss of cortical acetyl-cholinesterase activity in association with cognitive decline in Alzheimer’s disease: A positron emission tomography study.
Ann Neurol 48:194 –200.
Sihver W, Fasth KJ, Horti AG, Koren AO, Bergstro¨m M, Lu L, et al (1999a): Synthesis and characterization of binding of 5[76Br]Bromo-3-[[2(S)-Azetidinyl]methoxy]pyridine, a novel nicotinic acetylcholine receptor ligand, in rat brain. J
Neuro-chem 73:1264 –1272.
Sihver W, Fasth KJ, O¨ gren M, Bivehed H, Bergstro¨m M, Nordberg A, et al (1998a): In vitro evaluation of11
C-labeled (S)-nicotine, (S)-3-methyl-5(1-methyl-2-pyrrolidinyl) isox-azole, and (R,S)-1-methyl-2-(3-pyridyl) azetidine as nicotinic receptor ligands for positron emission tomography studies.
J Neurochem 71:1750 –1760.
Sihver W, Fasth KJ, O¨ gren M, Lundqvist H, Bergstro¨m M, Watanange Y, et al (1999b): In vivo positron emission tomography studies on the novel nicotinic receptor agonist [11
C]MPA compared with [11
C]ABT-418 and (S)(2)[11
C]nico-tine in rhesus monkeys. Nucl Med Biol 26:633– 640. Sihver W, Gillberg PG, Nordberg A (1998b): Laminar
distribu-tion of nicotinic receptor subtypes in human cerebral cortex as determined [3
H](2)nicotine, [3
H]cytisine and [3
H]epibati-dine in vitro autoradiography. Neuroscience 85:1121–1133. Sihver W, Gillberg PG, Svensson AL, Nordberg A (1999c):
Autoradiographic comparison of [3
H](2)nicotine, [3
H]cy-tisine and [3
H]epibatidine binding in relation to vesicular acetylcholine transport sites in the temporal cortex in Alzhei-mer’s disease. Neuroscience 94:685– 696.
vivo imaging of cerebral nicotinic receptors. Behav Brain Res 113:143–158.
Smith MA, Sayre LM, Monnier VM, Perry G (1996): Oxidative posttranslational modification in Alzheimer disease. A possi-ble pathogenic role in the formation of senile plaques and neurofibrillary tangles. Mol Chem Neuropathol 28:41– 48. St George-Hyslop PH (2000): Molecular genetics of Alzheimer’s
disease. Biol Psychiatry 47:183–199.
Svensson AL, Nordberg A (1998): Tacrine and donepezil atten-uate the neurotoxic effect of A beta (25-35) in rat PC12 cells.
Neuroreport 9:1519 –1522.
Terzano S, Court JA, Fornasari D, Griffiths M, Spurden DP, Lloyd S, et al (1998): Expression of the a3 nicotinic receptor subunit mRNA in aging and Alzheimer’s disease. Mol Brain
Res 63:72–28.
Van den Berg CM, Kazmi Y, Jann MW (2000): Cholinesterase inhibitors for the treatment of Alzheimer’s disease in the elderly. Drugs Aging 16:123–138.
Van Duijn CM, Havekes LM, Van Broeckhoven C, de Knijff P, Hofman A (1995): Apolipoprotein E genotype and associa-tion between smoking and early onset Alzheimer’s disease.
BMJ 310:627– 631.
Warpman U, Nordberg A (1995): Epibatidine and ABT 418 reveal selective losses ofa4b2 nicotinic receptors in Alzhei-mer brains. Neuroreport 6:2419 –2423.
Weinberger DR, Jones D, Reba RC, Mann U, Coppola R, Gibson
R, et al (1991): A comparision of FDG PET and IQNB SPECT in normal subjects and in patients with dementia.
J Neuropsychiatry Clin Neurosci 4:239 –248.
Wever A, Jeske A, Lobron C, Birtsch C, Heinemann S, Maelicke A, et al (1994): Cellular distribution of nicotinic acetylcholine receptor subunits mRNAs in the human cerebral cortex as revealed by non-isotopic in situ hybridization. Mol Brain Res 25:122–128.
Willoughby JJ, Ninkina NN, Beech MM, Latchman DS, Wood JN (1993): Molecular cloning of a human neuronal nicotinic acetylcholine receptor b3-like subunit. Neurosci Lett 155: 136 –139.
Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, McFalls E, et al (1995): Nicotine patches in Alzheimer’s disease: Pilot study on learning, memory, and safety.
Phar-macol Biochem Behav 51:509 –514.
Wonnacott S (1997): Presynaptic nicotinic ACh receptors.
Trends Neurosci 20:92–98.
Zamani MR, Allen YS, Owen GP, Gray JA (1997): Nicotine modulates the neurotoxic effect of beta-amyloid protein (25-35) in hippocampal cultures. Neuroreport 8:513–517. Zubieta JK, Koeppe RA, Mulholland GK, Kuhl DE, Frey KA
![Figure 1. Laminar distribution of [3H]epibatidine through the entire cortical depth of the human temporal cortex from a control andan Alzheimer’s disease (AD) patient of young age (top) and from a control and an AD patient of a higher age (bottom)](https://thumb-ap.123doks.com/thumbv2/123dok/3142649.1383354/3.612.65.552.81.460/laminar-distribution-epibatidine-cortical-temporal-alzheimer-control-patient.webp)
![Figure 2. (A)parietal cortex of four patients (P1–P4) carrying the APPtangles and [ Correlation between neuronal plaques and [3H]nic-otine binding in the temporal cortex and parietal cortex of fourpatients (P1–P4) carrying the Swedish amyloid precursor pro](https://thumb-ap.123doks.com/thumbv2/123dok/3142649.1383354/4.612.64.298.80.389/parietal-patients-apptangles-correlation-neuronal-parietal-fourpatients-precursor.webp)
![Figure 3. (A)binding density increases in the sequence black, yellow, red, white. ctx, cortex; hip, hippocampus; th, thalamus; cbl, cerebellum; str, striatum;MPA, (R,S)-1-methyl-2-(3-pyridyl)azetidine; BAP, bromo-3-[[2(5)-azetidinyl]methoxy] pyridine](https://thumb-ap.123doks.com/thumbv2/123dok/3142649.1383354/6.612.63.536.78.510/increases-sequence-hippocampus-cerebellum-striatum-azetidine-azetidinyl-pyridine.webp)