Thyroid Function and Response to 48-Hour Sleep
Deprivation in Treatment-Resistant Depressed Patients
Michela M. David, James A. Owen, Gebrehiwot Abraham, Nicholas J. Delva,
Stephen E. Southmayd, Eric Wooltorton, and J. Stuart Lawson
Background: Clinical depression is associated with
ab-normalities of the hypothalamic–pituitary–thyroid axis. Changes in thyroid function during sleep deprivation may be related to its antidepressant effects.
Methods: Levels of thyroid-stimulating hormone,
tri-iodothyronine, tri-iodothyronine uptake, thyroxine, and free thyroxine were measured before, during, and after a 48-hour sleep deprivation in nine treatment-resistant de-pressed patients. Clinical state was assessed every 4 hours. A retrospective study of 26 similar patients was added for cross-validation.
Results: Significant increases in thyroid-stimulating
hor-mone and tri-iodothyronine during sleep deprivation were not correlated with clinical improvement. Sleep depriva-tion responders had lower tri-iodothyronine uptake levels than nonresponders in both the prospective (p,.02) and the retrospective (p ,.03) samples.
Conclusions: The lower tri-iodothyronine uptake values
in responders may identify a subgroup of depressed patients who respond to sleep deprivation by virtue of some abnormality of the hypothalamic–pituitary–thyroid axis that is temporarily corrected by sleep deprivation.
Biol Psychiatry 2000;48:323–326 © 2000 Society of
Bio-logical Psychiatry
Key Words: Sleep deprivation, thyroid, thyroid-stimulat-ing hormone, tri-iodothyronine uptake, treatment-resistant depression, affective disorders
Introduction
A
disruption of the normal homeostasis of the hypo-thalamic–pituitary–thyroid axis may be of etiologic significance in depressive illness (Stein and Avni 1988). Total sleep deprivation (SD), which temporarily amelio-rates depressive symptoms in over 60% of patients (Wuand Bunney 1990), is a heuristic method by which this hypothesis may be tested.
Although differences in thyroid function between SD responders and nonresponders have not been demonstrated consistently, some studies have found thyroid function to be predictive of SD response. An increase in thyroid-stimulating hormone (TSH) during SD has been reliably demonstrated (Baumgartner et al 1990a, 1990b; Kaschka et al 1989; Kasper et al 1988; Parekh et al 1998), although a causal link between TSH and clinical response has not been firmly established. The finding by Southmayd et al (1992) that thyroxine (T4) can reliably prolong the clinical improvement induced by SD strongly suggests that changes in thyroid function are etiologically relevant to this antidepressant effect.
Our study examined thyroid function before, during, and after SD. It differs from previous studies by incorpo-rating an extended (48-hour) SD period, 4-hour assess-ments of clinical state, and measureassess-ments of tri-iodothy-ronine uptake (T3U). A retrospective study of thyroid function before SD in an independent patient sample is also described, for cross-validation.
Methods and Materials
Prospective Study
Nine treatment-resistant inpatients (seven female, two male; mean age 41 years) with unipolar major depression (DSM-IV; American Psychiatric Association 1994) underwent a 48-hour SD. At baseline, the 17-item Hamilton Rating Scale for Depres-sion (Hamilton 1967) mean score was 24.8. Two patients had been medication free for 3 weeks or more; seven were on stable psychotropic medication regimes for at least 2 weeks, with no new antidepressants having been introduced for 6 weeks. The women were between days 2 and 7 of their menstrual cycles; none were taking hormones.
During the 3-day baseline subjects were awake between 6:00
AM and 10:00 PM. They then remained awake for 48 hours. Subjects slept between 6:00AMand 12:00PMon day 5, returning to the baseline schedule for 2 “recovery” days. Brief structured videotaped interviews were conducted at 4-hour intervals during wakefulness to assess clinical state. These were rated on a visual analogue scale (Aitken 1969), independently and in random
From the Department of Psychiatry, Queen’s University and Kingston Psychiatric Hospital (MMD, JAO, GA, NJD, SES, JSL), and School of Medicine, Queen’s University (EW), Kingston, Canada.
Address reprint requests to Michela M. David, Ph.D., Kingston Psychiatric Hospital, Mood Disorders Service, Postal Bag 603, Kingston Ontario K7L 4X3, Canada.
Received September 23, 1999; revised April 3, 2000; accepted April 4, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
order, by two clinicians blinded to time and date. Sleep depri-vation response was defined as an improvement of least 2 standard deviations from the baseline mean, or clinical state reaching the normal range during SD (Southmayd et al 1990). Subjects whose moods reached the normal range at any point during baseline were excluded.
Blood samples were drawn at 9:00AMand 9:00PMfrom day 2 through day 6 (except 9:00AMon day 5, when subjects slept). Tests for TSH, T3, T4, free T4 (fT4), and T3U were performed by a commercial medical laboratory on batched samples using automated instruments.
Changes in thyroid function were assessed using a multivariate analysis of variance, with group (responders and nonresponders) as the between-subjects variable and time (9:00AMand 9:00PM) and phase (baseline, SD, and recovery) as within-subjects vari-ables. The linear component of the phase effect contrasted recovery with baseline; the quadratic component contrasted SD with the average of baseline and recovery.
A correlational analysis compared the quadratic component of the phase effect for the thyroid measures with the corresponding effect for clinical state.
Retrospective Study
Twenty-six subjects were selected who 1) had completed a 40-hour SD with clinical ratings, 2) had major depression or bipolar mood disorder and were depressed at baseline, 3) had records of thyroid function before SD, and 4) had normal TSH and were not taking thyroid supplements.
Of the responders (11 female, four male; mean age 58.3 years), 14 were unipolar and one bipolar, four were medica-tion free, and 11 were taking psychotropic medicamedica-tions. Of the nonresponders (six female, five male; mean age 50.3 years), eight were unipolar and three bipolar, two patients were medication free, and nine were taking psychotropic medica-tions. Independent sample t tests evaluated group differences for TSH, T4, and T3U.
Results
Prospective Study
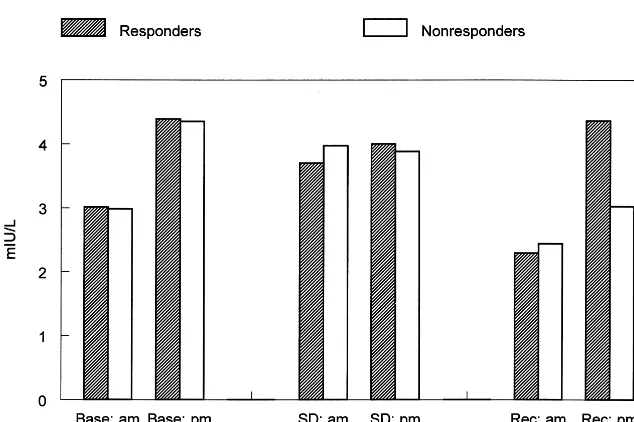
Sleep deprivation induced changes in TSH, as indicated by the phase effect [F(2,14) 5 8.239, p , .004]. The quadratic component of the phase effect [F(1,7)57.890,
p , .03] reflects a rise during SD (Figure 1). The significant quadratic component of the phase by time interaction [F(1,7)55.620, p,.05] reflects the fact that the rise in TSH during SD was only evident at 9:00 AM,
counteracting an overall time of day effect whereby TSH was generally higher at 9:00 PM than at 9:00 AM [F(1,7)
513.136, p, .008]. The linear component of the phase effect [F(1,7)5 8.575, p , .03] indicates that recovery levels of TSH dropped below baseline.
Tri-iodothyronine was lower at 9:00PMthan at 9:00AM
[F(1,7)55.590, p5.05] and rose significantly with SD, as indicated by the quadratic component of the phase effect [F(1,7)56.590, p,.04].
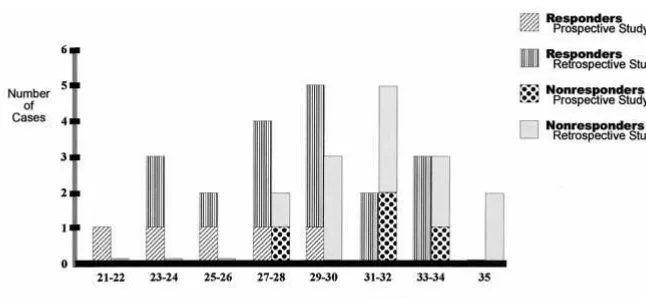
Baseline T3U levels were significantly lower for the responders [n55; F(1,7)59.9662, p,.02] and remained stable. The frequency distribution of T3U was bimodal.
Given the small sample size, we evaluated the possibility of type II errors using power analyses. We placed an upper bound with 80% confidence on any critical effect where we were unable to reject the null hypothesis with 95% confi-dence. The results suggest it is unlikely that we have missed effects of clinically or theoretically important size.
The correlations between clinical state and various measures of thyroid function were not significant.
Retrospective Study
Results replicated those of the prospective study, with a significant group difference for T3U (t52.15, p5 .03) Figure 1. Mean levels of thyroid-stimulat-ing hormone for the responder and nonre-sponder groups at 9:00 AMand 9:00 PM
during the baseline (Base), sleep depriva-tion (SD), and recovery (Rec) phases. SD (p 5.03) and recovery (p 5.03) levels were significantly different from baseline;
AMand PM levels were significantly dif-ferent (p5.008; see Results).
324 BIOL PSYCHIATRY M.M. David et al
but not for T4 or TSH. Responders had lower T3U levels than nonresponders, with a bimodal frequency distribution similar to that of the prospective study (Figure 2).
Discussion
The rise in T3 during SD is consistent with other studies (Baumgartner et al 1990a, 1990b; Kaschka et al 1989; Parekh et al 1998); however, we did not observe previ-ously documented increases in T4 and fT4 (Baumgartner et al 1990b; Kaschka et al 1989; Parekh et al 1998).
As reported by others (e.g., Baumgartner et al 1990a), TSH was generally higher at 9:00PMthan at 9:00AM. Our
study also replicates the reliable finding of an increase in TSH during SD. The rise in TSH at 9:00AM during SD
counteracted its diurnal rhythm, which supports the obser-vation by Parker et al (1987) that the normal inhibition of TSH release occurring near sleep onset is temporarily bypassed by SD.
Whereas some studies found that the SD-related rise in TSH correlated with clinical improvement (Baumgartner et al 1990a; Parekh et al 1998), others, including ours, did not (Baumgartner et al 1990b; Kaschka et al 1989; Kasper et al 1988). This inconsistency may be related to method-ological or sample differences. Furthermore, the potential connection between TSH and antidepressant response may not be a direct one. Elevated TSH may be a response to changes in those systems that may mediate the antidepres-sant response at a higher level. Despite the consistent effect of SD on TSH, the relevance and proximity of this rise in TSH to the antidepressant action remain unclear.
Differences in thyroid function between SD responders and nonresponders have not been demonstrated reliably. For example, Baumgartner et al (1990a, 1990b) reported higher T4 levels in responders, whereas others (e.g., Kaschka et al 1989), including ourselves, did not. This inconsistency between studies makes interpretation difficult.
To our knowledge, our study is the first to examine
changes in T3U during SD. The robust group difference in T3U observed in the prospective study, and substantiated by the retrospective analysis of a larger independent sample, is noteworthy. It strongly suggests that T3U is a predictor of SD response.
Lower T3U, seen in the responders, reflects an increase in the number of unoccupied plasma T4-binding protein sites. This can be caused by an increased concentration of thyroid hormone– binding protein, although no causal factors (e.g., pregnancy, exogenous estrogens) applied in the present study. A more likely explanation is hypothy-roidism, which some researchers (e.g., Hickie et al 1996), but not all (Joffe 1999), have found to occur more commonly in treatment-resistant depressed patients than in their non–treatment-resistant counterparts. If the lower T3U values in responders reflect subclinical hypothyroid-ism, the condition may be temporarily alleviated by SD, which increases T3 availability. This fits with the obser-vation that exogenous T4 can extend the beneficial effects of SD (Southmayd et al 1992). Shelton et al (1992) found that, in comparison to nonresponders, SD responders had a more robust response to thyrotropin-releasing hormone stimulation, which can be a manifestation of subclinical hypothyroidism. Further study is needed to test this hypothyroidism hypothesis and to determine whether the lower T3U values in SD responders are state or trait specific.
Financially supported by the Ontario Mental Health Foundation. The authors gratefully acknowledge the assistance of Deborah Collins, Judith Davidson, Salinda Horgan, Julia Kalotay, Paul Kasurak, and the MDU nursing staff.
References
Aitken RCB (1969): Measurement of feelings using visual analogue scales. Proc R Soc Med 62:989.
American Psychiatric Association (1994): Diagnostic and Sta-Figure 2. Frequency distribution of tri-io-dothyronine uptake (normal range 22–34%) in the prospective (n59) and retrospective (n526) studies. Differences between the responder and nonresponder groups were significant for both studies (t,.02 and t,
.03, respectively).
Thyroid Function in 48-Hour Sleep Deprivation BIOL PSYCHIATRY 325
tistical Manual of Mental Disorders, 4th ed. Washington, DC:
American Psychiatric Association.
Baumgartner A, Graf KJ, Kurten I, Meinhold H, Scholz P (1990a): Neuroendocrinological investigations during sleep deprivation in depression. I. Early morning levels of thyro-tropin, TH, cortisol, prolactin, LH, FSH, estradiol and testos-terone. Biol Psychiatry 28:556 –568.
Baumgartner A, Reimann D, Berger M (1990b): Neuroendocri-nological investigations during sleep deprivation in depres-sion. II. Longitudinal measurement of thyrotropin, TH, cor-tisol, prolactin, GH, and LH during sleep and sleep deprivation. Biol Psychiatry 28:569 –587.
Hamilton M (1967): Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278 –296. Hickie I, Bennett B, Mitchell P, Wilhelm K, Orlay W (1996):
Clinical and subclinical hypothyroidism in patients with chronic and treatment-resistant depression. Aust N Z J
Psy-chiatry 30:246 –252.
Joffe R (1999): Peripheral thyroid hormone levels in treatment resistant depression. Biol Psychiatry 45:1053–1055. Kaschka W, Marienhagen J, Bratenstein P (1989): Total sleep
deprivation and thyroid function in depression. Psychiatr Res 29:231–234.
Kasper S, Sack DA, Wehr TA, Kick H, Voll G, Vieira A (1988): Nocturnal TSH and prolactin secretion during sleep depriva-tion and predicdepriva-tion of antidepressant response in patients with major depression. Biol Psychiatry 24:631– 641.
Parekh PI, Ketter TA, Altshuler L, Frye M, Callahan A, Marangell L, et al (1998): Relationships between thyroid hormone and antidepressant responses to total sleep depri-vation in mood disorder patients. Biol Psychiatry 43:392– 394.
Parker DC, Rossman LG, Pekary AE, Hershman JM (1987): Effect of 64-hour sleep deprivation on the circadian wave-form of thyrotropin (TSH): Further evidence of sleep-related inhibition of TSH release. J Clin Endocrinol Metab 64:157– 161.
Shelton RC, Loosen PT, Orth DN (1992, May): Sleep depriva-tion hormonal response in depression. Paper presented at the 145th annual meeting of the American Psychiatric Associa-tion, Washington, DC.
Southmayd S, Kasurak P, MacDonald B, Waldron J (1992): Therapeutic sleep deprivation in a depressed patient: prolon-gation of response with concurrent thyroxine. Acta Psychiatr
Scand 86:84 – 85.
Southmayd SE, David MM, Cairns J, Delva NJ, Letemendia FJ, Waldron JJ (1990): Sleep deprivation in depression: Pattern of relapse and characteristics of preceding sleep. Biol
Psychi-atry 28:979 –988.
Stein D, Avni J (1988): Thyroid hormones in the treatment of affective disorders. Acta Psychiatr Scand 77:623– 636. Wu JC, Bunney WE (1990): The biological basis of an
antide-pressant response to sleep deprivation and relapse: Review and hypothesis. Am J Psychiatry 147:14 –21.
326 BIOL PSYCHIATRY M.M. David et al