Ž .
Aquaculture 193 2001 65–80
www.elsevier.nlrlocateraqua-online
Losses of lipid, protein and n
y
3 fatty acids in
enriched Artemia franciscana starved at different
temperatures
J.O. Evjemo
a,), T.L. Danielsen
c, Y. Olsen
ba
Department of Botany, Faculty of Chemistry and Biology, Brattøra Research Centre, Norwegian UniÕersity
( )
of Science and Technology NTNU , Gryta 2, N-7491 Trondheim, Norway
b
Trondhjem Biological Station, Norwegian UniÕersity of Science and Technology,
N-7491 Trondheim, Norway
c ( ´ )
TiMar Culturas Em Agua , Lda Apartado 49, Fuzeta 8700, Olhao, Portugal˜ Received 10 February 2000; received in revised form 28 June 2000; accepted 28 June 2000
Abstract
The loss rates of protein and lipids were determined for enriched Artemia franciscana starved
Ž y1.
at different temperatures after enrichment. Following 12-h enrichment with DHA Selco 0.2 g l
Ž
at 288C, the nauplii were temperature acclimated and transferred to starving condition 0–96 h;
.
58C to 308C .
y1 Ž . Ž
The total lipid content during enrichment increased from 145 mg g dry weight DW newly
. y1
hatched nauplii to 222 mg g DW after 12 h. The DHArEPA ratio reached an optimum after
Ž .
12 h 1.85 , where DHA and EPA constituted 12% and 6.7% of total fatty acids, respectively. When the nauplii were transferred to starving conditions the contents of all lipid components became reduced. The specific loss rate was found to be exponential for all components and significantly higher for DHA than for EPA and the sum of the other ny3 fatty acids. A.
Ž . y1Ž .
franciscana starved at the highest temperature 308C showed a loss rate of 92% day of DHA .
y1Ž . Ž .
Nauplii starved at 128C had a loss rate of 51% day of DHA . At the same temperature 128C the corresponding loss rate of EPA, the sum of the other ny3 fatty acids and for the total lipid content was 15%, 30% and 11% dayy1, respectively.
Ž
The protein content was relatively stable in the nauplii kept at the lowest temperatures 58C
.
and 88C , but as temperature increased, the loss rate of protein gradually increased reaching a loss rate of 28% dayy1in nauplii starved at 268C. The survival of the nauplii was )67% throughout
)Corresponding author. Tel.:q47-73-55-03-48; fax:q47-73-59-63-11.
Ž .
E-mail address: [email protected] J.O. Evjemo .
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved. Ž .
Ž .
the starvation period 96 h , but at temperatures below 88C and above 198C, the survival was
-13% after 96 h.
Results show that the when Artemia nauplii are starved the lipid and protein content decreases as a function of temperature. This might affect the nutritional value of A. franciscana quite strongly if the nauplii reside in the fish tanks before being eaten by the larvae, or stored at ambient temperature before they are transferred to the fish tanks.q2001 Elsevier Science B.V. All rights reserved.
Keywords: Artemia; Starvation; Protein content; Lipid content; Fatty acid; EPA; DHA
1. Introduction
Several studies have shown that marine fish tissues are rich in ny3 HUFA, and that
long chain polyunsaturated ny3 fatty acids are essential components in the diet of
Ž
marine fish Yone and Fuji, 1975; Watanabe, 1982; Koven et al., 1990; Rainuzzo et al.,
. Ž .
1992 , in particular docosahexaenoic acid DHA; 22:6 ny3 and eicosapentaenoic acid
ŽEPA; 20:5ny3. ŽKanazawa et al., 1979; Leger et al., 1981; Watanabe, 1988; Navarro
´
.et al., 1993 . Phospholipids from vertebrate neural tissue have a high content of DHA
ŽSargent et al., 1993 , and it has been shown that deficiency during critical stages of the. Ž
embryonic development may lead to neurological abnormalities Neuringer et al., 1988;
.
Bell and Tocher, 1989; Bazan and Scott, 1990 . Malpigmentation observed in halibut, turbot and red sea bream larvae has been suggested to be a consequence of suboptimal
Ž
DHA content or DHArEPA ratio in the diet Watanabe, 1993; Reitan et al., 1994; Næss
.
et al., 1995 .
The overall nutritional quality of the live food organisms depend on the content and
Ž
nutritional balance of carbohydrates, protein and lipids Leger et al., 1986; Lubzens et
´
.al., 1989 . The content of essential amino acids and protein in the live food must meet
Ž
the requirements for growth and cell maintenance of the fish larvae Fauconneau, 1985;
.
Cowey, 1994; Oie et al., 1997 . The fish larvae grow rapidly during early stages, and the demand for protein is believed to be relatively high in small marine larvae of fish and
Ž .
shellfish Dabrowski, 1984; Cowey, 1994 .
DHA is present only in trace amounts in newly hatched Artemia-nauplii, whereas EPA may be present in higher amounts, depending on Artemia species and cyst batch
ŽLeger et al., 1986; Lavens et al., 1989 . Earlier studies have established enrichment
´
.methods for enhancement of the ny3 HUFA content in Artemia by feeding the nauplii
Ž .
with emulsified lipids Watanabe et al., 1978; Watanabe, 1982; Leger et al., 1986 ,
´
Ž
which affect the lipid level and fatty acid profile of the nauplii quite strongly Leger et
´
.al., 1986; Evjemo and Olsen, 1997; Evjemo et al., 1997 . These techniques for
enrichment of ny3 HUFA in Artemia are well developed, but the stability of the
( )
J.O. EÕjemo et al.rAquaculture 193 2001 65–80 67
content in Artemia post enrichment may reduce their overall nutritional value. This may have consequences in the rearing of both warm and cold water species. Relatively few
Ž .
studies have been carried out in this field. Evjemo et al. 1997 have measured levels of DHA in different Artemia species following enrichment and subsequent starvation,
Ž .
while Estevez et al. 1998 have measured the effect of starvation on the fatty acid profiles of Artemia enriched with arachidonic acid-rich and eicosapentaenoic acid-rich diets.
The aim of the present study was to examine the quantitative changes in the protein, lipid and fatty acid content of enriched Artemia franciscana, and thereafter maintained under starving conditions at different temperatures. The results of this study may improve hatchery practice and develop feeding routines leading to a more efficient utilisation of Artemia as a live food organism for marine fish larvae.
2. Materials and methods
2.1. Culture conditions
Ž .
Cysts from A. franciscana Great Salt Lake, UT, USA, INVE Aquaculture, Belgium were decapsulated and hatched following a modified procedure of Sorgeloos et al.
Ž1986 . The nauplii were enriched in 600-l conical tanks for 12–30 h at a density of.
y1 w x y1
140–160 ind ml . The cultures were aerated with airstones to attain O2 )3.5 mg l
Ž
at a temperature of 288C and a salinity of 34‰. The nauplii were fed DHA Selco INVE
. Ž .
Aquaculture, Belgium at incubation time 0 and again 13 h later, both times at 0.2 g ly1.
After enrichment dead animals were removed and the nauplii were rinsed in seawater.
Ž
The cultures were then acclimated to nine different temperatures for 45–60 min 5, 8,
.
10, 12, 15, 19, 22, 26 and 308C . The cultures were thereafter starved in black
Ž . Žw x y1.
cylindrical tanks 100 l under continuous illumination and aeration O2 )3.5 mg l
for 96 h. Initial animal density was 37–100 ind mly1 and percent survival was
Ž
determined daily in all cultures by counting individuals ns10, each replicate with
. Ž y1 .
40–100 animals . The total lipid and fatty acid contents mg g DW were determined
both during enrichment and during the starvation period. Samples for fatty acid analyses were taken from newly hatched nauplii, every 6 h during enrichment and every 24 h
during the starvation period. The samples were immediately frozen and stored aty808C
under N2 atmosphere. Samples for protein analysis were taken from newly hatched
nauplii, after enrichment and every 12 h during the starvation period.
2.2. Analytical methods
Ž .
The lipid analysis was performed according to Rainuzzo et al. 1992 using a
Ž .
modified method of Bligh and Dyer 1959 for lipid extraction and a method described
Ž .
by Metcalfe et al. 1966 for methylation. The FAMEs were determined quantitatively
Ž .
The protein content was based on the sum of all hydrolysed amino acids in the dry
Ž .
weight sample % protein of dry weight, DW . Amino acids were quantified by reversed
Ž .
phase high-pressure liquid chromatography HPLC as described by Oie and Olsen
Ž . Ž y1 .
1997 . The amino acids mg g DW were estimated based on three replicate
Ž .
measurements and the protein content calculated from Eq. 1 :
Protein mg g
Ž
y1DW.
ssum of amino acids mg gŽ
y1DW.
r0.9Ž .
1Ž Ž ..
The sum of free amino acids was divided by 0.9 Eq. 1 because the HPLC analysis
did not include all amino acids i.e. proline, cysteine, tryptophan, serine, methionine and threonine which are partly destroyed during acidic hydrolysis. These amino acids have
Ž
been estimated to constitute approximately 9.5% of the total amino acids Watanabe et
.
al., 1983 .
Ž .
Specific loss rates SLR of the total lipid and protein content, total fatty acids, DHA,
Ž .
EPA and the content of other ny3 fatty acids were calculated from Eq. 2 :
SLRsln Q
Ž
tyQ0.
rtŽ .
2where Q and Q corresponds to protein, lipid, or fatty acid content at time 0 and time t,0 t
respectively.
y1 Ž .
Loss rate as % day was calculated from Eq. 3 :
%LRs
Ž
eSLRy1.
P100Ž .
33. Statistics
All experimental data were tested for statistical significance using Student’s t-test
Žtwo-way . The general linear model procedure GLM , SPSS Base 8.0 ANCOVA ,. Ž . Ž .
was used to compare and determine differences in the regression analysis. The variation of the data is shown as SD.
4. Results
4.1. Enrichment of A. franciscana
Ž . Ž .
The total lipid content in the newly hatched nauplii time 0 was mean"SD
y1 Ž . Ž .
145"4.6 mg g dry weight DW and the fatty acid content FA constituted 68% of
Ž y1 .
the total lipids 99 mg g DW . Total ny3 fatty acids minus DHA and EPA, hereafter
named other ny3 fatty acids, constituted 34% of the total fatty acids. DHA was not
found in the newly hatched nauplii whereas the content of EPA was 3.9 mg gy1 DW
ŽFig. 1A and B ..
The content of DHA increased gradually during enrichment and levelled off after 24
Ž . Ž y1 .
( )
J.O. EÕjemo et al.rAquaculture 193 2001 65–80 69
Ž y1 . Ž .
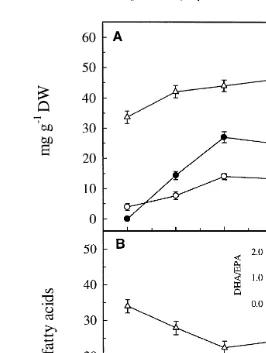
Fig. 1. Absolute mg g DW and relative % content of DHA, EPA and other ny3 fatty acids in newly
Ž . Ž . Ž .
hatched nauplii of A. franciscana time 0 and during enrichment 6–30 h with DHA Selco. Inset in B shows the DHArEPA ratio during enrichment. Each data point represents the mean of three replicates. Error bars indicate SD.
reflected in the nauplii. The ratio DHArEPA reached a maximum after 6–12 h of
Ž .
enrichment 1.85; inset of Fig. 1B , whereafter it decreased slightly toward the end of
Ž .
the enrichment 1.67 after 30 h . A. franciscana were enriched for 12 h before being transferred to starving conditions.
Ž . Ž .
The protein content of newly hatched nauplii time 0 was mean"SD 380"11.2
y1 Ž . y1 Ž .
mg g DW 38% of DW and decreased to 306"8.9 mg g DW 31% of DW after
Ž .
12 h of enrichment. The dominant amino acids were glutamine 14% , aspartic acid
Ž9% , alanine 8.3% , lysine 11.5% , leucine 8% and arginine 8.2% . The amount of. Ž . Ž . Ž . Ž .
free amino acids increased from 0.024 to 0.043mg indy1
4.2. StarÕation of A. franciscana at different temperatures
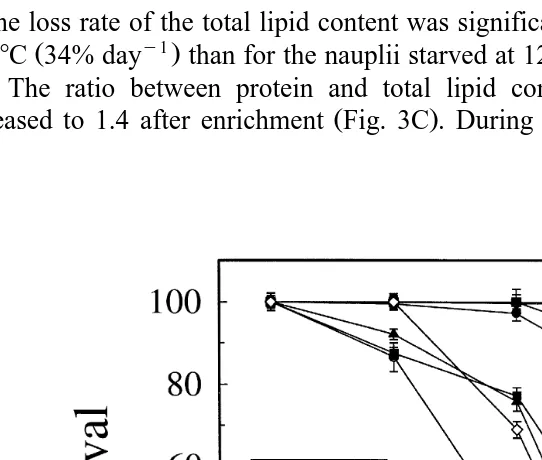
The survival of A. franciscana during starvation for 96 h was)67% at temperatures
Ž .
between 88C and 198C Fig. 2 . The animals starved at 128C showed the highest survival
Ž93% . At temperatures below 8. 8C and above 198C, survival was in the range of 0–12%
after 96 h. The nauplii starved at 58C and temperatures G198C was, as a consequence
of the high mortality, sampled for protein, lipid and fatty acid analysis once or twice during the starvation period.
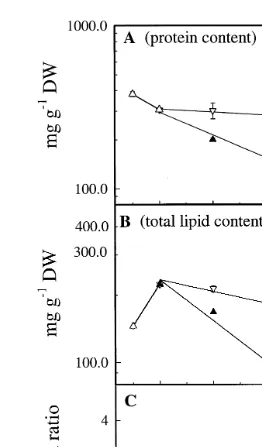
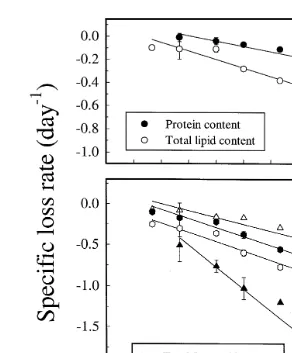
When A. franciscana were transferred to starving conditions, we found an
exponen-Ž .
tial loss with time of both protein and lipid content Fig. 3 . After enrichment, the nauplii contained 306 mg protein gy1 DW. During starvation at 12 and 268C the specific
Ž y1 Ž .. Ž .
loss rate day ; Eq. 2 was y0.04 andy0.32, respectively Table 1 , corresponding
Ž y1 Ž .. y1 Ž .
to a loss rate %, day ; Eq. 3 of 4% and 28% day , respectively Fig. 3A . The
results did not reveal any specific pattern of selective catabolism of amino acids. Essential amino acids constituted 47% of the total amino acids and the balance between essential and non-essential amino acids remained constant.
The loss rate of the total lipid content was significantly higher for the nauplii starved
Ž y1. Ž y1. Ž . Ž
at 268C 34% day than for the nauplii starved at 128C 11%, day P-0.01 Fig.
.
3B . The ratio between protein and total lipid content was 2.6 after hatching and
Ž .
decreased to 1.4 after enrichment Fig. 3C . During starvation the ratio increased as a
Ž .
( )
J.O. EÕjemo et al.rAquaculture 193 2001 65–80 71
Ž . Ž . Ž y1 . Ž .
Fig. 3. Total lipid A and protein content B mg g DW and proteinrlipid ratio C in newly hatched A.
Ž . Ž .
franciscana H on the x-axis , following enrichment H –0 on the x-axis , and during the starvation period
Ž0–96 h at 12. 8C and 268C. Legend refers to both figures. Error bars indicate SD from three replicates.
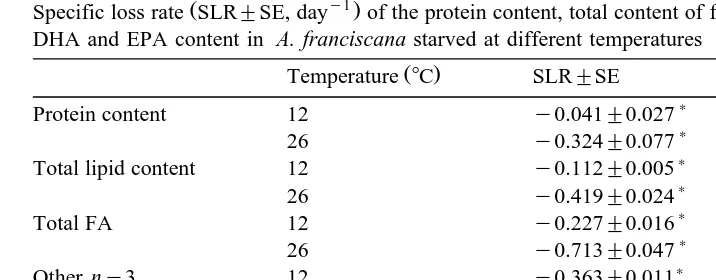
Table 1
Ž y1.
Specific loss rate SLR"SE, day of the protein content, total content of fatty acids, other ny3 fatty acids, DHA and EPA content in A. franciscana starved at different temperatures
2
Ž .
Temperature 8C SLR"SE r n
)
Protein content 12 y0.041"0.027 0.99 5
)
26 y0.324"0.077 0.92 3
)
Total lipid content 12 y0.112"0.005 0.98 5
)
Specific loss rates significantly higher than 0 P-0.05 .
The total content of fatty acids, other ny3 fatty acids, DHA and EPA decreased as a
function of the temperature, and there was a significant difference in loss rate of these
Ž . Ž .
components in nauplii starved at 128C and 268C P-0.001 Fig. 4A–D . Of the initial
fatty acid content 20% was lost per day in the nauplii starved at 128C which increased to
51% in the nauplii starved at 268C. Also the loss rate of the other ny3 fatty acids
increased more than twofold, from 30% per day in the nauplii starved at 128C, to 61%
per day in the nauplii starved at 268C.
The loss rate of DHA was higher than the loss rate of EPA and other ny3 fatty
acids. During the first day of starvation at 128C the DHA content of A. franciscana
y1 Ž . y1
decreased from 25.7 mg g DW after enrichment to 12.3 mg g DW, corresponding
y1 Ž . Ž .
to a loss rate of 51% day Fig. 4D . At the higher temperature 268C the reduction of
y1 Ž .
DHA in the nauplii was 86% day . DHA decreased much faster than EPA Fig. 5A ,
which seemed to accumulate relative to other ny3 fatty acids. Nauplii starved at 12
Ž .
and 268C increased in fact their relative content of EPA % of total fatty acids towards
Ž . Ž y1 .
the end of the starvation period Fig. 5B . The absolute content of EPA mg g DW in
y1 Ž .
nauplii starved at 12 and 268C was reduced by 15 and 45% day Fig. 4C . Table 1
Ž y1.
summarises the estimated specific loss rates SLR, day of the various lipid
compo-nents and protein content during the starvation period at 128C and 268C.
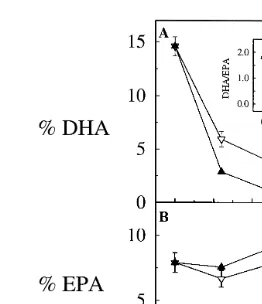
After enrichment, the DHArEPA ratio in the nauplii was 1.85. During the first 24 h
the value decreased to 0.89 at 128C and 0.38 at 268C. Thereafter the DHArEPA ratio
decreased steadily, reaching 0.21 after 96 h in the animals starved at 128C and 0.13 after
Ž .
48 h in the animals starved at 268C inset in Fig. 5A .
Ž y1.
The estimated specific loss rates SLR, day of the protein and lipid content, total
and other ny3 fatty acids, EPA and DHA for the range of temperatures examined are
shown as a function of temperature in Fig. 6. All components showed a linear relationship between the specific loss rates and the temperature. DHA and the content of
( )
J.O. EÕjemo et al.rAquaculture 193 2001 65–80 73
Ž . Ž . Ž . Ž . Ž y1 .
Fig. 4. Total content of fatty acid A , other ny3 fatty acids B , EPA C and DHA D mg g DW in
Ž . Ž .
newly hatched A. franciscana H on the x-axis , following enrichment H –0 on the x-axis , and during the
Ž .
starvation period 0–96 h at 128C and 268C. Legend refers to both figures. Error bars indicate SD from three replicates.
Ž .
only slightly reduced. The regression coefficients A and B are given in Table 2 and the
Ž .
equation SLRsAtqB is valid for temperatures in the range of 58C to 308C. The
results indicated that A. franciscana nauplii catabolise fatty acids rather than proteins during starvation.
5. Discussion
Ž .
Fig. 5. Relative contents of DHA and EPA % of total fatty acids in A. franciscana during starvation at 128C
Ž .
and 268C. Inset in B shows the DHArEPA ratio in nauplii starved at the same temperatures. Error bars indicate SD from three to five replicates.
become reduced at a variable rate during starvation, and that the loss rates of some
Ž . Ž .
essential components may be high, especially at high temperatures G158C Fig. 6 .
The loss rates of total lipid, other ny3 fatty acids, DHA and EPA in starved A.
Ž .
franciscana were positively correlated to temperature Figs. 3, 6 and Table 1 . The
content of DHA in starving nauplii decreased more rapidly than the content of EPA, and the loss rate of DHA showed much stronger response to increasing temperature
Ž . Ž .
compared to that of EPA P-0.001 Figs. 4, 5 and 6; Tables 1 and 2 . The results
showed that it is difficult to maintain high levels of DHA in A. franciscana at
Ž y1.
temperatures G208C reduction of DHA )80% day , indicating that
DHA-enrich-ment will have little effect unless the nauplii are not consumed by the fish larvae shortly
Ž .
after enrichment. Similar results were reported by Estevez et al. 1998 showing that
Ž .
both EPA and arachidonic acid AA were catabolized during starvation, although in a lower rate than DHA.
( )
J.O. EÕjemo et al.rAquaculture 193 2001 65–80 75
Fig. 6. Specific loss rate of protein and total lipid content, other ny3 fatty acids, EPA and DHA in A.
Ž .
franciscana plotted as a function of the temperature 8C during starvation. Error bars indicate SD from three replicates
ŽDHA content 19% of total fatty acids; Reitan et al., 1993 has very little effect on the.
Ž .
DHA content of Artemia nauplii Olsen et al., 1997 . It is therefore important to establish feeding strategies of the fish and crustacean larvae, which minimise the retention time of Artemia in the fish tanks. If the Artemia is eaten by the larvae relatively fast after transfer, the DHA level in the nauplii might still satisfy the needs of the larvae.
Another strategy for maintaining a high DHA level in the nauplii after enrichment is commercialise Artemia-species or strains which have the ability to retain high levels of DHA after enrichment. It has been demonstrated that one specific strain of Artemia
sinica, a Chinese species, exhibits relatively stable DHA content during starving
Ž .
Table 2
Ž . Ž y1.
Coefficients "SE for calculation of the specific loss rate SLR, day of the protein and total lipid content, total fatty acid content, other ny3 fatty acids, EPA and DHA in A. franciscana starved at different
Ž Ž ..
temperatures SLRsAtqB; t is the temperature 8C
2
A B r
Protein content y0.015"0.002 0.144"0.044 0.96
)
Total lipid content y0.021"0.004 0.002"0.082 0.88 )
Total fatty acid content y0.037"0.003 0.154"0.055 0.95 )
Other ny3 y0.038"0.003 0.057"0.065 0.97
)
EPA y0.028"0.005 0.171"0.091 0.88
)
DHA y0.089"0.007 0.298"0.145 0.97
) Ž .
Regression coefficients significantly higher than 0 P-0.05 .
The DHA Selco used for nauplii enrichment contains no protein and it was therefore not surprising to find that the protein content became reduced during enrichment. This loss of protein is probably related to a yolk protein which is used as an energy reserve just after hatching, and losses of 20% have been reported when the nauplii are grown
Ž .
from Instar I to Instar II Benijts et al., 1976 . The protein content of A. franciscana
Ž .
was relatively stable during starvation at the lowest temperatures 58C and 88C , but as for the lipid, the loss rate of protein increased as the temperature increased. In the
nauplii starved at 268C the protein level decreased by 28% dayy1. It has been
Ž . Ž
Fig. 7. Relative contents of DHA % of total fatty acids in A. franciscana, B. plicatilis data from Olsen et
. Ž .
( )
J.O. EÕjemo et al.rAquaculture 193 2001 65–80 77
Ž .
demonstrated that starving zooplankton 108C increase nitrogen excretion after 4 days
ŽIkeda, 1977 , indicating that lipids are being used as an energy source during the first.
days of a starvation period whereas the metabolic degradation of proteins may be increased in later stages.
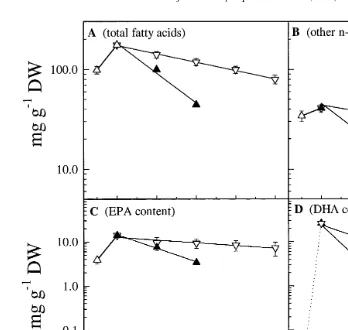
Compared to other organisms that are used as live food, the high loss rate of DHA is
Ž .
only found in Artemia Fig. 7 . The loss rate of lipid and other ny3 fatty acids also
Ž .
increases with temperature in the rotifer Brachionus plicatilis Olsen et al., 1993 , but the loss rates are considerable lower than in A. franciscana. On the other hand, copepods that are normally included in the natural diet of several marine fish larvae, tend to retain the DHA content during starvation. In the copepods examined the relative
Ž . Ž .
content of DHA % of total fatty acids increase during starvation Fig. 7 , indicating that fatty acids other than DHA are used as the major metabolic energy sources
ŽEvjemo, unpublished results ..
The proteinrlipid ratio of enriched A. franciscana was 1.4, increasing to 1.8 when
Ž .
the nauplii were starved for 24 h 268C, Fig. 3C . In other live food organisms, the
proteinrlipid ratio is considerably higher. As for cultivated rotifers the ratio is in the
Ž
range of 3 and 4 decreasing to 2–2.4 when the animals are starved 24 h Oie and Olsen,
.
1997; and calculations based on Olsen et al., 1993 and Makridis and Olsen, 1999 . In copepods the proteinrlipid ratio is between 4.8 and 5.8 which decreases to 3.7–4.5 after
Ž .
a starvation period of 3 days Evjemo, unpublished results . These results might indicate that rotifers use a high proportion of the protein during starvation whereas A.
francis-cana tend to metabolise a higher proportion of lipid. These comparisons show that
among the live food organisms that have been examined so far A. franciscana and
Ž .
several other Artemia species and strains Triantaphyllidis et al., 1995 show a high loss rate of lipid and particularly DHA after enrichment. It has been suggested that DHA is
Ž .
retroconverted into EPA Barclay and Zeller, 1996; Furuita et al., 1996 and Navarro et
Ž .
al. 1999 have shown that Artemia nauplii are capable of retroconverting DHA into EPA both during enrichment and subsequent starvation. The relatively stable content of
Ž .
EPA % of total fatty acids shown in Fig. 5B and the low loss rate of EPA during
Ž .
starvation Table 1, Figs. 4 and 6 might also indicate and support the findings that DHA is retroconverted into EPA.
The differences between A. franciscana and the other live food organisms could be related to the stage of the animals. In this study, only Artemia nauplii were analysed whereas the rotifers and the copepods were adult or at least pre-adult stages. A possibility to explain some of the differences between these organisms could be that A.
franciscana metabolise other lipids, and in particular, not so high amounts of DHA
when it reaches pre-adult or adult stage.
6. Conclusion
The loss rates of the lipid and protein content in A. franciscana were quantified in order to describe the biochemical changes that take place in the nauplii after enrichment.
larvae and in this period the protein, lipid and fatty acid content decreases. The loss rates of all these components increased with increasing temperature. The total lipid content decreases at a higher rate than the protein content. Highest loss rate was found for DHA whereas the loss rate for EPA was considerably lower.
The results show that the changes in protein, lipid and fatty acid content effect the nutritional value of A. franciscana quite strongly if the nauplii reside in the fish tanks a longer period of time before being eaten by the larvae.
Acknowledgements
Ž .
This study has been supported by the Research Council of Norway NFR and FINA Exploration Norway. We will thank Marte Schei and Merethe Selnes for skilled technical assistance during the experiments.
References
Barclay, W., Zeller, S., 1996. Nutritional enhancement of ny3 and ny6 fatty acids in rotifers and Artemia nauplii by feeding spray-dried Schizochytrium sp. J. World Aquacult. Soc. 27, 314–322.
Bazan, N.G., Scott, B.L., 1990. Dietary omega-3 fatty acids and accumulation of docosahexaeonic acid in rod photoreceptor cells of the retina and synapsis. Uppsala J. Med. Sci. Suppl. 48, 97–107.
Bell, M.V., Tocher, D.R., 1989. Molecular species composition of the major phospholipid in brain and retina
Ž .
from rainbow trout Salmo gairdneri . Biochem. J. 264, 909–915.
Benijts, F., Vanvoorden, E., Sorgeloos, P., 1976. Changes in the biochemical composition of the early stages of the brine shrimp, Artemia salina L. In: Proc. 10th Eur. Sym. on Mar. Biol. Persoone, G., Jaspers, E.
ŽEds. , Research in Mariculture at Laboratory- and Pilot Scale vol. 1. Universa Press, Wetteren, Belgium,.
620 pp.
Bligh, E.G., Dyer, W.J., 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917.
Cowey, C.B., 1994. Amino acid requirements in fish: a critical appraisal of present values. Aquaculture 125, 1–11.
Dabrowski, K., 1984. The feeding of fish larvae: present state of the art and perspectives. Reprod. Nutr. Dev. 24, 807–833.
Estevez, A., McEvoy, L.A., Bell, J.G., Sargent, J.R., 1998. Effects of temperature and starvation time on the pattern and rate of loss of essential fatty acids in Artemia nauplii previously enriched using arachidonic acid and eicosapentaenoic acid-rich emulsions. Aquaculture 165, 295–311.
Evjemo, J.O., Olsen, Y., 1997. Lipid and fatty acid content in cultivated live feed organisms compared to marine copepods. Hydrobiology 358, 159–162.
Evjemo, J.O., Coutteau, P., Olsen, Y., Sorgeloos, P., 1997. The stability of docosahexaenoic acid in two
Artemia species following enrichment and subsequent starvation. Aquaculture 155, 135–137.
Fauconneau, B., 1985. Protein synthesis and protein deposition in fish. In: Cowey, C.B., Mackie, A.M., Bell,
Ž .
J.G. Eds. , Nutrition and Feeding in Fish. Academic Press, London, pp. 17–43.
Furuita, H., Takeuchi, T., Toyota, M., Watanabe, T., 1996. EPA and DHA requirements in early juvenile red sea bream using HUFA enriched Artemia nauplii. Fish. Sci. 62, 246–251.
Ikeda, T., 1977. The effect of laboratory conditions on the extrapolation of experimental measurements to the ecology of marine zooplankton: IV. Changes in respiration and excretion rates of boreal zooplankton species maintained under fed and starved conditions. Mar. Biol. 41, 241–252.
( )
J.O. EÕjemo et al.rAquaculture 193 2001 65–80 79 animals and the capacity for bioconversion of linolenic acid to highly unsaturated fatty acids. Comp. Biochem. Physiol. 63, 295–298.
Ž .
Koven, W.M., Tandler, A., Kissil, G., Sklan, D., Friezlander, O., Harel, M., 1990. The effect of dietary ny3 polyunsaturated fatty acids on growth, survival and swim bladder development in Sparus aurata larvae. Aquaculture 91, 131–141.
Lavens, P., Leger, P., Sorgeloos, P., 1989. Manipulation of the fatty acid profile in Artemia offspring produced´ Ž .
in intensive culture systems. In: De Paw, N., Jaspers, E., Ackefors, H., Wilkins, N. Eds. , Aquaculture and Biotechnology in Progress. European Aquaculture Society, Bredne, pp. 731–739.
Leger, P., Fremont, L., Marion, D., Nassour, I., Desfrages, M.F., 1981. Essential fatty acids in trout serum´ lipoproteins, vitellogenin and egg lipids. Lipids 16, 593–600.
Leger, P., Bengtson, D.A., Simpson, K.L., Sorgeloos, P., 1986. The use and nutritional value of Artemia as a´ food source. Mar. Biol. Ann. Rev. 24, 521–623.
Lubzens, E., Tandler, A., Minkoff, G., 1989. Rotifers as a food in aquaculture. Hydrobiology 186r187, 387–400.
Makridis, P., Olsen, Y., 1999. Protein depletion of the rotifer starved Brachionus plicatilis during starvation. Aquaculture 174, 343–353.
Metcalfe, L.D., Schimtz, A.A., Pelka, J.R., 1966. Rapid preparation of fatty acids esters from lipids for gas chromatography. Anal. Chem. 38, 514–515.
Navarro, J.C., Batty, R.S., Bell, M.V., Sargent, J.R., 1993. Effects of two Artemia diets with different content
Ž .
of polyunsaturated fatty acids on the lipid composition of larvae of Atlantic herring Clupea harengus . J. Fish Biol. 43, 503–515.
Navarro, J.C., Henderson, R.J., McEvoy, L.A., Bell, M.V., Amat, F., 1999. Lipid conversions during enrichment of Artemia. Aquaculture 174, 155–166.
Neuringer, M., Anderson, G.J., Connor, W.E., 1988. The essentiality of ny3 fatty acids for the development and function of retina and brain. Ann. Rev. Nutr. 8, 517–541.
Ž .
Næss, T., Germain-Henry, M., Naas, K.E., 1995. First feeding of Atlantic halibut Hippoglossus hippoglossus using different combinations of Artemia and wild zooplankton. Aquaculture 130, 235–250.
Oie, G., Olsen, Y., 1997. Protein and lipid content of the rotifer Brachionus plicatilis during variable growth and feeding condition. Hydrobiology 358, 251–258.
Ž
Oie, G., Makridis, P., Reitan, K.I., Olsen, Y., 1997. Protein and carbon utilization of rotifers Brachionus
. Ž .
plicatilis in first feeding of turbot larve Scophthalmus maximus L. . Aquaculture 153, 103–122.
Olsen, Y., Reitan, K.I., Vadstein, O., 1993. Dependence of temperature on loss rates of rotifers, lipids, andv3 fatty acids in starved Brachionus plicatilis cultures. Hydrobiology 255r256, 13–20.
Olsen, A.I., Jensen, A., Evjemo, J.O., Olsen, Y., 1997. Effect of algal addition on the stability of fatty acids in enriched Artemia franciscana. Hydrobiology 358, 205–210.
Rainuzzo, J.R., Reitan, K.I., Jørgensen, L., 1992. Comparative study on the fatty acid and lipid composition of four marine fish larvae. Comp. Biochem. Physiol. 103B, 21–26.
Reitan, K.I., Rainuzzo, J.R., Øie, G., Olsen, Y., 1993. Nutritional effects of algal addition in first-feeding of
Ž .
turbot Scopthalmus maximus L. larvae. Aquaculture 118, 257–275.
Reitan, K.I., Rainuzzo, J.R., Olsen, Y., 1994. Influence of lipid composition of live feed on growth, survival and pigmentation of turbot larvae. Aquacult. Int. 2, 33–48.
Sargent, J.R., Bell, M.V., Tocher, D.R., 1993. Docosahexaenoic acid and the development of brain and retina
Ž .
in marin fish. In: Drevon, C.A., Baksaas, I., Krokan, H.E. Eds. , Omega-3 Fatty acids: Metabolism and Biological Effects. Birkhauser Verlag, Basel, Switzerland, pp. 139–149.¨
Sorgeloos, P., Lavens, P., Leger, P., Tackaert, W., Versichele, D., 1986. Manual for the Culture and use of´ Brine Shrimp Artemia in Aquaculture. Manual prepared for the Belgian Administration for Development Cooperation and the Food and Agriculture Organization of the United Nations. Artemia Reference Center. Faculty of Agriculture. State University of Ghent, Belgium, 319 pp.
Watanabe, T., 1982. Lipid nutrition in fish. Comp. Biochem. Physiol. 73B, 3–15.
Ž .
Watanabe, T., 1988. Nutrition and growth. In: Shepard, C.J., Bromage, N.R. Eds. , Intensive Fish Farming, Worcester. pp. 154–197.
Watanabe, T., 1993. Importance of docosahexaenoic acid in marine larval fish. J. World Aquacult. Soc. 24, 152–161.
salina, as live feed from view point of essential fatty acids for fish. Bull. Jpn. Soc. Sci. Fish. 44,
1115–1121.
Watanabe, T., Kitajima, C., Fujita, S., 1983. Nutritional values of live feed organisms in Japan for mass propagation of fish: a review. Aquaculture 34, 115–143.