www.elsevier.nlrlocateraqua-online
Optimization of tetraploid induction in Pacific
oysters, Crassostrea gigas, using first polar body as
a natural indicator
Benoit Eudeline
a, Standish K. Allen Jr.
b,), Ximing Guo
ca
Laboratoire de Biologie et Biotechnologies Marine, UniÕersite de Caen, Esplanade de la Paix,
14032 Caen Cedex, France
b
Virginia Institute of Marine Science, Aquaculture Genetics and Breeding Technology Center, College of William and Mary, Gloucester Point, VA 23062-1346, USA
c
Haskin Shellfish Research Laboratory, Rutgers UniÕersity, 6959 Miller AÕenue, Port Norris, NJ 08349, USA
Received 15 April 1999; received in revised form 20 December 1999; accepted 22 December 1999
Abstract
Tetraploid Crassostrea gigas were first successfully produced in 1993 by inhibiting the first
Ž
polar body of eggs from triploids that had been fertilized with sperm from diploids Guo and
.
Allen method . However, attempts to repeatedly produce high yields of tetraploids were inconsis-tent. Because of these uncertainties, we examined some of the fundamental aspects of tetraploid production in an attempt to optimize tetraploid induction using the Guo and Allen method.
Ž .
Varying the duration of the treatment to inhibit polar body 1 PB 1 of triploid eggs had clear
Ž
effects on ploidy of progeny. Short treatments 15–35 min after fertilization — about half the
. Ž
period of meiosis 1 in triploid eggs yielded tetraploid and heptaploid cells. Long treatments 7–43
.
min — about three quarters of the period of meiosis I in triploid eggs yielded only heptaploid cells among the embryos. Tetraploid induction was most consistent when treatments were accomplished on eggs from individual triploid females rather than pooled from a number of females, and when treatments were metered according to biological landmarks. That is, eggs from
Ž .
individual triploids were fertilized and 0.5 mgrl cytochalasin B CB added after 10 min. A subsample of the fertilized eggs was kept aside untreated. When 50% of the untreated eggs
Ž .
showed PB 1 extrusion as judged by microscopic examination of dividing, untreated eggs , the CB treatment was discontinued. In eight treatments based on these ‘‘biological criteria,’’
)Corresponding author.
Ž . Ž .
E-mail addresses: [email protected] B. Eudeline , [email protected] S.K. Allen , [email protected]
ŽX. Guo .. 1
Current address: Whiskey Creek Oyster Farm, 3395 Bayshore Road, Tillamook, OR 97141, USA 0044-8486r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
proportions of tetraploids ranged from 13% to 92% after 8 days for an average of 55%, and seven of eight replicates went through metamorphosis and settlement. At settlement, the percentage of tetraploids ranged from 7% to 96%, averaging 45%. Average survival in all the replicates at 8 days was 4.4%, which is acceptable considering tetraploid progeny are destined for use as brood stock.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Triploid; Tetraploid; Crassostrea gigas; Cytochalasin B; Meiosis; Flow cytometry; Oyster;
Shellfish; Breeding
1. Introduction
In the last 10 years, triploids have become an important part of Pacific oyster,
Crassostrea gigas, aquaculture. For example, about one third of the hatchery produced
Ž .
seed on the US West Coast were triploid Chew, 1994 and there is considerable interest in Australia, China and Europe. Triploids are interesting principally due to their reduced
Ž
gonadal development which prevents deterioration of meat quality Downing and Allen,
. Ž .
1986 . Consequently, triploids can be marketed year-round Allen, 1988 . Reduced gonadal development may also favor more rapid growth rates, although probably only in
Ž .
favorable conditions Davis, 1989 . Given their very low fecundity, triploids have also been used in some cases for population control and biological containment of non-native
Ž .
species Allen, 1993; Allen and Wattendorf, 1987; Guo and Allen, 1994a .
Currently, in commercial hatcheries, triploid oysters are produced by inhibiting polar
Ž . Ž . Ž .
body 2 PB 2 with cytochalasin B CB Allen et al., 1989 , or more recently with
Ž . Ž .
6-dimethylaminopurine 6-DMAP Desrosiers et al., 1993 . However, there are major limitations to the use of these chemicals. First, these techniques always produce less than 100% triploidy because of inherent variation among dividing eggs. This leads to larval populations containing both diploids and triploids. Batches of triploids in lower proportions than 80% are a problem for hatchery management and a waste of production space and money. Secondly, CB is toxic, and has come under the scrutiny of the United States Food and Drug Administration because of its potential danger to hatchery personnel. Health and safety concerns currently restrict its use in oyster hatcheries. Finally, blocking PB 2 may negatively affect the survival and growth of induced
Ž .
triploids, probably due to the treatment itself Downing and Allen, 1987 or to genetic
Ž .
considerations, such as inbreeding Chourrout et al., 1986; Guo et al., 1990 . All these problems are eliminated with the development of tetraploids. After more than a decade
Ž .
of attempts, Guo and Allen 1994b finally succeeded in producing tetraploid Pacific oysters using eggs from triploids in which they blocked the first polar body with CB
Ž .
treatment applied from 5 to 20 min post fertilization PF . Since then, they have shown
Ž .
the general utility of tetraploids for making 100%-triploid populations Guo et al., 1996 .
Ž .
While Guo and Allen 1994b reported a successful spawn for tetraploids from work in 1993, there were in fact a considerable number of other tetraploid attempts that were less successful, including a major effort in 1996 to produce tetraploids in a number of
Ž .
commercial hatcheries Allen, unpublished data . Treatments were based on the timing
Ž .
uncertainties, we examined some of the fundamental aspects of tetraploid production,
Ž .
including dynamics of development in triploid eggs Eudeline et al., 2000 . Here we report the results of our investigations into improving production of tetraploids in C.
gigas.
2. Materials and methods
2.1. Brood stock and gametes
Triploid Pacific oysters used in this study were 2 years old, and produced by blocking the release of PB 2 with CB. Ploidy was confirmed in all individuals by flow cytometry prior to spawning. Diploid Pacific oysters used in this study were 2 years old and came
Ž .
from a randomly mated population reared in Willapa Bay Washington State . Both
Ž .
diploid and triploid brood stock were kept in a warm seawater pond about 238C before being transferred in 10,000-l conditioning tanks for about 1.5 – 2 months before spawning.
2.2. Preparation of gametes
Certified triploids were randomly selected, opened, and sexed under the microscope. Males and females were separated to prevent any accidental fertilization. All surfaces in contact with broodstock were cleaned with diluted bleach. Gametes were obtained by strip-spawning.
Ž
Eggs from each individual female were stripped without adding water i.e., without .
aid of a spray bottle ; seawater was added only when all females from an experiment
Ž .
had been stripped. This ‘‘dry stripping’’ Allen and Bushek, 1992 ensured that all eggs were exposed to seawater simultaneously, which is important to get a synchronous
Ž .
development and a consistent duration of hydration Eudeline et al., 2000 . Eggs were separated from cellular debris by passing them through an 80-mm Nytex screen. Eggs
Ž . were caught on a 25-mm screen, then resuspended in 1-mm filtered seawater FSW at 258C and a salinity of 30 – 32 ppt. Eggs were counted using appropriate dilutions in a Sedgwick–Rafter chamber. After counting, the eggs — arrested at prophase of meiosis 1 — were held in seawater at 258C and checked microscopically until germinal vesicle
Ž .
breakdown GVBD was observed, a sign that meiosis was ready to resume. At this stage, we assumed that the eggs were blocked in metaphase of meiosis 1. Eggs remained in seawater at least 45 min, but no longer than 60 min during counting.
A pool of sperm obtained by stripping three different diploid males was suspended in 1mm FSW, sieved through a 25 mum screen, and used to fertilize the eggs.
2.3. Fertilization and treatment
the eggs fertilized. For each spawning, a diploid female was used as control, and the egg density adjusted to that of the triploid egg density. Depending on the experiment, the egg density ranged from 1 to 5 million eggs by liter. Fertilization occurred at 258C in 1 mm FSW, and aliquots of the sperm suspension were added to obtain about 10 to 20 spermatozoa per egg. CB treatment consisted of adding 0.5 mg CB dissolved in 1 ml
Ž .
dimethyl sulfoxide DMSO per liter of seawater. At the end of the treatment, eggs were drained on a 25-mm Nytex screen, rinsed with 1 mm FSW, then soaked in 0.05%
Ž .
DMSO vrv dissolved in 1-mm FSW for 20 min. After this rinse, eggs were recounted and poured into culture tanks.
In the first experiment, we attempted to produce tetraploid larvae based on techniques
Ž .
described by Guo and Allen 1994b , without looking at the time of meiotic events. For
Ž .
these trials, pair matings a single male and female were used. After hydration, eggs
Ž .
from triploids were treated with CB 0.5 mgrl from 5 to 20 min PF. Four trials were attempted, using four different females.
In the second experiment, we varied the duration of CB treatment to try to increase the yields of tetraploids. These experiments were accomplished on five triploid females Žreplicate spawns , with eggs split into two batches for either a ‘‘short’’ or ‘‘long’’ CB.
Ž .
treatment. Based on some work on timing of meiosis first polar body extrusion in
Ž .
triploid eggs Eudeline et al., 2000 , we decided to use two treatments: short — from 15 to 35 min PF, corresponding approximately to half of the period required for the
Ž .
expulsion of 50% first polar bodies PB 1 in triploid eggs; and long — from 7 to 43 min PF, a duration estimated to include about three quarters of that period.
In our last experiment, we adapted the treatment to the timing of meiosis of
Ž .
individual females ns8 . That is, for the eggs of each female, CB treatment was Ž
started when first signs of polar body extrusion were observed or at 12 min, if none .
were seen before and proceeded until 50% PB 1 were evident or until 37 min PF, whichever came first. In order to observe 50% PB 1 extrusion, it was necessary to take a subsample of fertilized eggs prior to the addition of CB and hold them at the same water temperature as the experimental group, observing meiotic events over time by repeated sampling. One trial of tetraploid induction was also attempted by mixing eggs from seven different females whose fecundity ranged from 1.3 to 9.2 million eggs, the total number of treated eggs being 24.7 million. Treatments for this batch were based on the biological criteria described above.
2.4. LarÕal culture
Ž .
All batches of larvae were cultured in 200-l tanks American Plastics at a density of
Ž .
less than 10rml according to standard practices e.g., Breese and Malouf, 1975 . Rearing temperature ranged from 238C to 258C and salinity from 30 to 32 ppt. Survival
Ž .
of approximately 700 – 800mm. At this time, they were transferred to upwelling system and the densities adjusted according to need as spat grew.
2.5. Flow cytometry
2.5.1. LarÕae
Approximately 10,000 48-h-old larvae were sampled from each culture for flow Ž .
cytometric FCM analysis to determine the percentage of tetraploids produced. In preparation for flow cytometry, larvae were concentrated into a 1 ml suspension, then pelleted by centrifugation at 1500=g for 10 s in a microcentrifuge. Supernatant
Ž
seawater was withdrawn and 0.5 ml of DAPIrdetergentrDMSO solution 146 mM NaCl, 10 mM Tris, adjust to pH 7.4, 2 mM CaCl , 22 mM MgCl , 0.05g BSA, 0.1%2 2
.
Triton X, 10 mgrml DAPI and 10% DMSO was added to the tube. Larvae were resuspended by vortex. Larval suspensions were frozen aty808C, and, after at least 1 h, larvae were thawed and disaggregated by repeated aspiration with a 1-ml syringe fitted with a 26G needle. Cell suspensions were passed through a 25-mm screen immediately before the assay. FCM analyses were accomplished on a Partec CA-II flow cytometer. Analysis of larval samples yielded data from a population of larvae, about 10,000 prepared by disaggregation. The proportion of cells in each ploidy class was calculated relative to the proportion of observations in all ploidy classes, after curve fitting with
Ž . Ž .
Modfit Verity Software House, Topsham, ME, USA Allen and Bushek, 1992 .
2.5.2. Spat
When the spat reached about 1000 – 2000mm, about 30 individual spat from each experiment were examined by flow cytometry to assess ploidy. Spat were placed into separate tubes and crushed gently. About 0.5 ml of DAPI solution was added to each tube, and the contents vortexed and screened on a 25-mm Nytex screen before FCM analysis.
3. Results
3.1. Treatment from 5 to 20 min PF
Ž .
Treatment according to strict criteria of timing described by Guo and Allen 1994b
Ž .
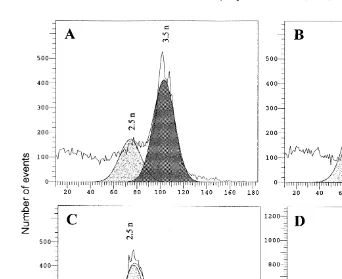
were relatively unsuccessful Fig. 1A–D according to the ploidy determined 48 h PF. Generally, these profiles show significant numbers of aneuploid cells with indistinct ploidy peaks. Although not well separated, these peaks seem organized around two
Ž .
values relative to a diploid standard peak values62 : about 75–76 for the first peak Žabout 2.40–2.45n and 104–108 for the second 3.30–3.48 n . These values are shown. Ž . in Table 1. None of the replicates presented major peaks located at the tetraploid level Žexpected mean relative DNA contents124 , indicating very few, if any, tetraploids. It.
Ž .
is interesting to note that for the first replicate Fig. 1A the second peak is dominant
Ž .
Fig. 1. Flow cyometric ploidy profiles of 48-h-old larvae obtained from triploid eggs=diploid sperm treated
Ž .
with CB 0.5 mgrl from 5 to 20 min after fertilization. Ploidy levels have been calculated relative to a diploid standard.
3.2. Short and long treatments
The results of our experiments with short and long treatments were highly repro-ducible. Fig. 2 shows the ploidy profiles obtained from the larvae at 48 h PF in three of
Table 1
Ž .
Mean relative DNA content of 48-h old larvae from unmodified treatments from Guo and Allen, 1994b to induce tetraploidy in C. gigas. The mean and coefficient of variation are derived from a curve fitting program. Ploidy is calculated relative to the diploid standard
Replicate Peak 1 Peak 2
Mean CV Ploidy Mean CV Ploidy
1 76 15.8 2.45 104 10.4 3.35
2 76 16.7 2.45 107 11.9 3.45
3 75 19.6 2.42 102 14.7 3.30
4 75 11.3 2.40 108 7.8 3.48
Ž .
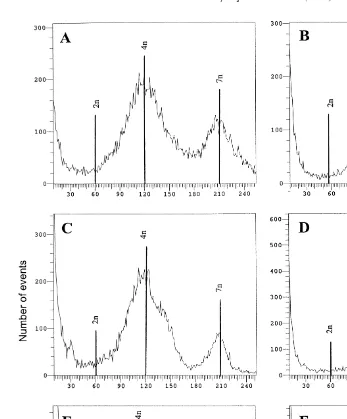
Fig. 2. Flow cytometric ploidy profiles of 48-h-old larvae resulting from short 15–35 min PF — left column
Ž . Ž .
and long 7–43 min PF — right column CB treatments 0.5 mgrl of triploid eggs fertilized with diploid sperm. Ploidy levels have been calculated relative to a diploid standard.
Table 2
Ž . Ž .
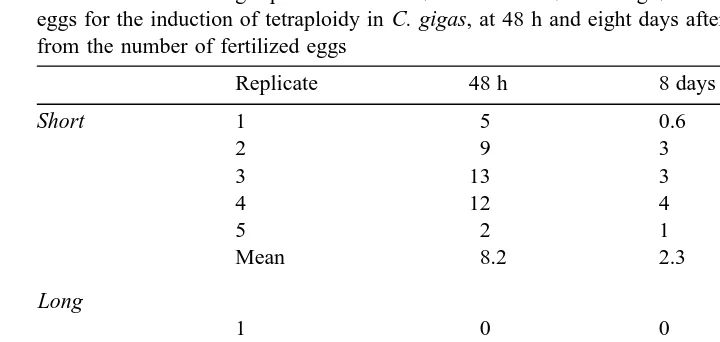
Percent survival among replicates of short 15–35 min PF and long 7–42 min PF CB treatments of triploid eggs for the induction of tetraploidy in C. gigas, at 48 h and eight days after treatment. Values are calculated from the number of fertilized eggs
Replicate 48 h 8 days
Short 1 5 0.6
2 9 3
3 13 3
4 12 4
5 2 1
Mean 8.2 2.3
Long
1 0 0
2 1.6 0
3 1.5 0
4 0.6 0
5 0 0
Mean 0.7 0.0
2 n std 68 14
Ž .
Short treatment Fig. 2A,C, and E yielded ploidy profiles with a major tetraploid Ž . peak at about 120, and a secondary peak at 210, corresponding to heptaploid cells 7n . Replicate 3 has an additional peak with a mean relative DNA content of 74, correspond-ing to 2.5n larvae.
Ž .
The ploidy profiles obtained from long treatments Fig. 2B,D, and F had their Ž .
principal peak at 210 7n with virtually no tetraploid cells. For all the treatments, the
Ž .
percentage of tetraploids after 48 h e.g., eight days of larval rearing was difficult to estimate accurately because of their high variance due to aneuploids. However, we can reasonably deduce that after the aneuploids and heptaploids died, larvae from short treatments will yield higher levels of viable tetraploids than will long treatments.
Larval survival at 48 h and eight days PF are presented for all five replicates in Table 2. Larval survival at 48 h in short treatments ranged from 2% to 13% averaging 8.2%. After 8 days, this mean was 2.3%. For long treatments, larval survival was very low, ranging from 0% to 1.6% at 48 h PF, with no larvae surviving to day 8. For the diploid standard, larval survival was 68% at 48 h and 14% at day 8.
3.3. Adapting the treatment to meiosis timing
Fig. 3. Flow cyometric ploidy profiles of 48-h-old larvae obtained from eggs of an individual triploid
Ž .
female=diploid male treated with CB 0.5 mgrl based on the meiotic landmark, 50% PB one. Ploidy levels have been calculated relative to a diploid standard.
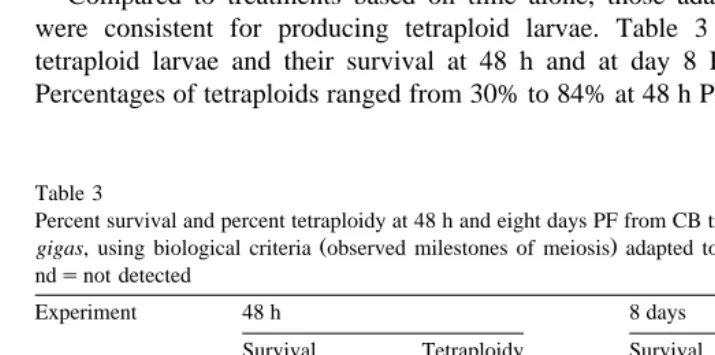
Compared to treatments based on time alone, those adapted to individual females were consistent for producing tetraploid larvae. Table 3 shows the percentage of tetraploid larvae and their survival at 48 h and at day 8 PF for all eight replicates. Percentages of tetraploids ranged from 30% to 84% at 48 h PF with a mean of 58% and,
Table 3
Percent survival and percent tetraploidy at 48 h and eight days PF from CB treatments of eggs from triploid C.
Ž .
gigas, using biological criteria observed milestones of meiosis adapted to treatment of individual females.
ndsnot detected
Experiment 48 h 8 days
Survival Tetraploidy Survival Tetraploidy
IndiÕidual females
X4N14 01 26 84 07 92
02 18 33 10 14
03 10 70 06 90
X4N15 01 04 nd 02 13
02 13 30 02 36
03 22 nd 04 63
04 15 nd 02 77
P4N21 07 73 02 nd
Mean 14.4 58.0 4.4 55.0
2 n std. 65 – 37 –
Pooled eggs, multiple females
after eight days, when most of the aneuploid larvae have died, tetraploids ranged from 13% to 92%, with a mean of 55.0%. Survival at 48 h ranged from 4% to 26%, averaging 14.4%. After eight days, survival ranged from 2% to 10%, averaging 4.4%. In the diploid standard, survival was 65% at 48 h and 37% at day 8.
Treatments of pooled eggs from different females produced larvae with 11%
te-Ž .
traploids and 15% survival after 48 h Table 3 . At day 8, survival decreased to 0.2% and the percentage of tetraploids remained 12%. Treatment was started at 12 min PF and stopped after 37 min, the maximum allowable time under our default parameters. According to our observations, 50% PB 1 was not reached in this pooled batch of triploid eggs.
4. Discussion
Our first trials to produce tetraploids confirmed the fact already shown by Guo and
Ž .
Allen 1994b that the inhibition of the first polar body in eggs from triploids leads to the formation of a majority of aneuploids in the first 48 h PF. However, these authors showed that their treatment led to two principal populations of larvae with ploidies between 3–4 n and 4–5n, followed by a shift in the ploidy of larvae when aneuploids died. In their paper, the majority of the larvae surviving at 7 days were tetraploids. In our case, where we treated eggs based on timing alone, it seems clear that the percentage of tetraploids would have been very low even following the mortality of aneuploids, because major tetraploid peaks were absent at 48 h. It is interesting to note that some
Ž .
2.5n aneuploids were still alive at day 8, and also at day 18 data not shown . Some FCM analyses on 1-year-old spat revealed that 2.5n were still alive, confirming the
Ž .
viability of aneuploids Guo and Gaffney, 1993; Guo and Allen, 1994b .
The presence of a 2.5n peak in our first inductions is interesting, since theoretically, a triploid egg fertilized by a spermatozoa from a diploid should lead to 2.5n embryos after
Ž
expulsion of both polar bodies previously confirmed by Akashige, 1990 and Guo and .
Allen 1994c . Our data show that for at least three of the four tetraploid induction trials using time alone to indicate treatment, CB treatment failed to retain the first polar body as designed. These results led us to conclude that CB treatment missed the critical windows of first polar body extrusion and was probably applied too early, or for too
Ž .
short duration, even though it apparently worked for Guo and Allen 1994b . It should be noted, however, that Guo and Allen did their work under different conditions of temperature and salinity. They used temperatures of up to 288C versus 258C in our work. Guo and Allen were also working with salinities of about 20–22 ppt whereas our experiments were accomplished at 30–32 ppt. Temperature and salinity have clear
Ž .
effects on meiotic and early mitotic events Lu, 1986 , and the times suggested by Guo and Allen were probably appropriate to their conditions, their brood stock, or both.
In the second experiment, short treatments were effective in producing tetraploid larvae as indicated by the major tetraploid DNA peak. Tetraploids are obtained when the first polar body from the triploid eggs is retained in all or most of the eggs, and
Ž .
chromosomes segregate following a united bipolar segregation Guo and Allen, 1992 . In diploid=diploid crosses, blocking PB1 with CB led to united bipolar segregation in
Ž .
from triploids, 30 dyads would be aligned on a single metaphase plate, 30 chromosomes would be released as PB 2 and 30 would remain in the egg and unite to the 10 chromosomes from the spermatozoa, forming a tetraploid embryo. Although the fre-quency of united bipolar segregation in triploid eggs is probably low, it would be the only segregation pattern leading to a tetraploid and euploid condition. Other segregations
Ž .
would lead to aneuploids Guo et al., 1992; Guo and Allen, 1994b , most of which are unviable within the first days, leading to a high mortality in the first week. Thus, for the
Ž .
short treatments, the average survival after 8 days 2.3% were similar to the results
Ž .
obtained by Guo and Allen 1994b . Long treatments are clearly inappropriate, since they produce principally heptaploid larvae, and no survival after day 8.
Treatments that commence too late or too early and for too short a duration will miss the first polar body in many eggs and yield larvae with 2.5n peaks. Also, treatments that last too long will retain both the first and second polar body, leaving the egg in an
Ž . Ž .
hexaploid state 6 n and, with the addition of sperm, produce heptaploid larvae 7n . These experiments, together with those on the timing of meiotic events in triploid
Ž .
eggs Eudeline et al., 2000 , allowed us to determine more precisely the optimal duration of CB treatment and the critical window of PB1 extrusion. However, given the wide variation among females in timing of meiosis, we decided to develop a technique that relied on biological criteria of individual females than on general rules applicable to all females. Results obtained on the eight replicates of tetraploid induction — based on biological criteria — produced tetraploids consistently, although variation among spawns was still high. Proportions of tetraploids ranged from 13% to 92% after eight days for an average of 55%, and seven of the eight replicates went through metamorphosis and settlement. At settlement, the percentage of tetraploids ranged from 7% to 96% for an average of 45%. The average survival on all the replicates at eight days was 4.4%, which is acceptable if we keep in mind that these tetraploid progeny are destined for use as brood stock and not to be marketed directly. Production of tetraploids from single females means that brood stocks will have to be carefully pedigreed since each batch will be no more than a half-sib family. Serious inbreeding could occur from repeated use of the same tetraploid brood stock, especially if tetraploids were, in turn, produced from 4 n=4 n matings of this same family. But the half-sib family structure of tetraploid brood stocks also provides an opportunity for carefully tracking performance of various family ‘‘lines’’ over time and making significant improvements.
Acknowledgements
This work was supported by a USDA SBIR awarded to Whiskey Creek Shellfish Farm. We are grateful to its owner, Lee Hanson, for supporting these experiments. We thank Sue Cudd and other hatchery staff for their logistical support, and Taylor United of Shelton, WA for additional assistance. This is contributiona 2276 of the Virginia
Institute of Marine Science.
References
Akashige, S., 1990. Growth and reproduction of triploid Japanese oyster in Hiroshima Bay. In: Hoshi, M.,
Ž .
Yamashita, O. Eds. , Advances in Invertebrate Reproduction 5 Elsevier, Amsterdam, pp. 461–468. Allen, S.K. Jr., 1988. Triploid oysters ensure year-round supply. Oceanus 31, 58–63.
Allen, S.K. Jr., 1993. Triploids for field tests? The good, the bad, and the ugly. J. Shellfish. Res. 12, 125,
Žabstract ..
Ž .
Allen, S.K. Jr., Bushek, D., 1992. Large scale production of triploid CrassostreaÕirginica Gmelin using ‘‘stripped’’ gametes. Aquaculture 103, 241–251.
Allen, S.K. Jr., Downing, S.L., Chew, K.K., 1989. Hatchery Manual for Producing Triploid Oysters. University of Washington Press, Seattle, WA, USA.
Allen, S.K. Jr., Wattendorf, R.J., 1987. Triploid grass carp: status and management implications. Fisheries 12, 20–24.
Breese, W.P., Malouf, R.E., 1975. Hatchery Manual for the Pacific Oyster. Oregon State University Sea Grant College Program, Corvallis, OR, USA, Publ. No. ORESU-H-75002.
Chew, K.K., 1994. Tetraploid Pacific oysters offer promise to future production of triploids. Aquacult. Mag. 20, 69–74.
Chourrout, D., Chevassus, B., Krieg, F., Happe, A., Burger, G., Renard, P., 1986. Production of second generation triploid and tetraploid rainbow trout by mating tetraploid males and diploid females — Potential of tetraploid fish. Theor. Appl. Genet. 7, 193–206.
Davis, J.P., 1989. Growth rate of sibling diploid and triploid oysters, Crassostrea gigas. J. Shellfish. Res. 8,
Ž .
319, abstract .
Desrosiers, R., Gerard, A., Peignon, J.M., Naciri, Y., Degresne, L., Morasse, J., Ledu, C., Phelipot, P., Guerrier, P., Dube, F., 1993. A novel method to produce triploids in bivalve molluscs by the use of´ 6-dimethylaminopurine. J. Exp. Mar. Biol. Ecol. 170, 29–43.
Downing, S.L., Allen, S.K. Jr., 1987. Induced triploidy in the Pacific oyster, Crassostrea gigas: optimal treatments with cytochalasin B depend on temperature. Aquaculture 61, 1–15.
Eudeline, B., Allen, S.K. Jr., Guo, X., 2000. Delayed meiosis and polar body release in eggs of triploid Pacific
Ž .
oysters, Crassostrea gigas, in relation to tetraploid production. J. Exp. Mar. Biol. Ecol., in press . Guo, X., Allen, S.K. Jr., 1994a. Reproductive potential and genetics of triploid Pacific oysters, Crassostrea
Ž .
gigas Thunberg . Biol. Bull. 187, 309–318.
Ž .
Guo, X., Allen, S.K. Jr., 1994b. Viable tetraploids in the Pacific oyster Crassostrea gigas Thunberg
produced by inhibiting polar body I in eggs from triploids. Mol. Mar. Biol. Biotechnol. 3, 42–50.
Ž .
Guo, X., DeBrosse, G.A., Allen, S.K. Jr., 1996. All-triploid Pacific oysters Crassostrea gigas Thunberg produced by mating tetraploids and diploids. Aquaculture 142, 149–161.
Guo, X., Hershberger, W.K., Cooper, K., Chew, K.K., 1992. Genetic consequences of blocking polar body I with cytochalasin B in fertilized eggs of the Pacific oyster, Crassostrea gigas: II. Segregation of chromosomes. Biol. Bull 183, 387–393.
Lu, J.-K., 1986. The combined effect of salinity and temperature on meiosis and early mitosis of the Pacific
Ž .