Magnetic bead purification as a rapid and efficient method for
enhanced antibody specificity for plant sample immunoblotting
and immunolocalization
Ian J. Quitadamo
a,1, Todd A. Kostman
1 b, Margaret E. Schelling
a,
Vincent R. Franceschi
b,*
aSchool of Molecular Biosciences,Washington State Uni
6ersity,Pullman,WA99164-4234,USA
bSchool of Biological Sciences,Washington State Uni
6ersity,Pullman,WA99164-4236,USA
Received 1 October 1999; received in revised form 7 October 1999; accepted 7 October 1999
Abstract
Molecular analysis of plant tissues with antibodies has traditionally been hindered by the presence of high non-specific binding by plant cell walls and other components along with significant contaminants within sera that retard identification of specific plant tissue targets. Methods which rely on immobile solid supports conjugated with high-affinity molecular entities, have been used to purify sera. Despite their wide use, traditional antibody purification methods can result in low yields or activity and can produce significant levels of secondary contaminants, resulting in high non-specific background and dilution of tissue-specific signals. Mobile support matrixes like magnetic beads conjugated with high-affinity antisera have recently become an efficient alternative method for isolating and identifying diverse molecular targets. In this study, rabbit anti-calreticulin (CRT) immunoglobulin G (IgG) was isolated from whole anti-CRT sera with magnetic beads and tested by Western blot and immunocytochemistry for CRT localization inPistia stratiotesplant tissues. IgG protein quantitation and purity was compared between purified and non-purified pre-immune and anti-CRT sera using spectrophotometric, reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and fluorescence staining followed by quantitative densitometry analysis. Anti-CRT IgG binding specificity after purification was determined by Western blot of total soluble protein extract. Purified and non-purified pre-immune and anti-CRT samples were subsequently utilized for CRT immunogold localization in Pistia tissue sections and visualized with confocal microscopy. The results demonstrate that magnetic bead purified anti-CRT IgG from whole serum shows enhanced specificity and reduced background. The ease of use and speed of this IgG purification technique should find widespread use in the plant biology field. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Antibody; Calreticulin; Immunocytochemistry; Magnetic bead; Microscopy; Purification
www.elsevier.com/locate/plantsci
1. Introduction
Calreticulin, a low-affinity, high-capacity cal-cium binding protein found in the endoplasmic reticulum (ER) of most eukaryotic cells [1 – 3], has been isolated from several plant species including barley, Euglena, Gingko and pea [4 – 7]. The exact role(s) of calreticulin (CRT) in plant systems is
still in question and is the focus of much investiga-tion [8]. CRT is hypothesized to be an important component of the calcium buffering system found in the ER, but has also been reported to serve as an ER chaperone and signaling molecule, and to mediate protein – protein interactions [1].
We are particularly interested in the role of high capacity calcium binding proteins in calcium ox-alate formation in plants [9], which is a calcium buffering process unique to plants [10,11]. Im-munological techniques are critical to our analysis of the role of such proteins in the specialized cells that produce calcium oxalate.
* Corresponding author. Tel.:+1-509-335-3025; fax:+ 1-509-335-3517.
E-mail address:[email protected] (V.R. Franceschi) 1Co-contributors.
However, protein localization in plant tissue presents unique problems. Plant cells contain a multitude of components including pigments (phe-nolics), polysaccharides and glycoproteins that non-specifically bind antibody serum protein com-ponents like immunoglobulin G (IgG) and serum albumin [12]. In particular, cell walls are a persis-tent source of antibody non-specific binding due the presence of complex polysaccharides [13] and lignins. The reactivity of plant cell wall and other components can make target protein localization by immuncytochemical techniques difficult. To al-leviate antibody non-specific binding, plant scien-tists employ a variety of measures. Common approaches include increasing blocking agent and/
or detergent concentrations as well as buffer strin-gency by increasing salt levels. However, these approaches may also decrease antibody specific binding to target proteins. Thus, a delicate balance must be struck between eliminating antibody non-specific binding and promoting antibody non-specific target recognition.
An alternative to these approaches is antibody purification, which reduces serum secondary con-taminants. Traditional methods like affinity chro-matography are often employed for antibody purification; however these methods may produce low yields of active antibody [14] that often still contains significant amounts of secondary contam-inants [15,16]. An alternative method for enhanc-ing antibody purification and specificity in plant biology use involves magnetic bead purified anti-body [16]. Previous studies using magnetic bead purified antibodies have shown enhanced antigenic specificity and immunologic activity in mam-malian tissues [16]. As compared to other antibody purification methods like traditional affinity chro-matography, magnetic bead purification methods can produce antibodies of superior purity and activity [16]. Magnetic bead antibody purification is also a very rapid technique, but to our knowl-edge it has not been applied to plant biology work.
For our research purposes, we have generated polyclonal antibody in rabbits to plant CRT. The purpose of this study was to analyze the effective-ness of magnetic bead imunoaffinity purification
for enhancing CRT immunolocalization in Pistia
stratiotes plant tissues. Sheep anti-rabbit IgG-con-jugated paramagnetic beads were utilized for a quantitative IgG purification scheme from whole
pre-immune and anti-CRT sera. Following mag-netic bead purification of preimmune and anti-CRT IgG, antibody purity and immunologic activity were assessed using sodium dodecyl
sul-fate-polyacrylamide gel electrophoresis
(SDS-PAGE) and Sypro Red fluorescence staining in addition to Western blot analysis. Non-purified and purified pre-immune and anti-CRT IgG were subsequently used for CRT localization in Pistia plant tissue sections. We show that magnetic bead IgG purification is a quick and effective method for antibody preparation to be used on plant samples.
2. Materials and methods
2.1. Total soluble protein extraction
P. stratiotes (water lettuce) shoot tips were har-vested, weighed, and frozen in liquid nitrogen. Pistia tissues where then ground to a fine pow-der with mortar and pestle, after which two vol-umes of extraction buffer (25 mM Tricine, 10 mM
b-mercaptoethanol, 1% w/v polyvinylpolypyrro-lidone (PVPP), 1 mM EGTA, pH 7.5) were added per gram of tissue. One micromolar leupeptin, 1
mM pepstatin A, and 10 mg/100 ml phenylmethyl-sulfonyl fluoride (PMSF) proteinase inhibitors were added to the extraction buffer just prior to use. Resultant extract was centrifuged at 17 000×
g for 10 min at 4°C, the supernatant collected and Pistia total soluble protein concentration deter-mined using the Pierce Coomassie method (Pierce, Rockford, IL).
2.2. Magnetic bead IgG purification
Magnetic bead antibody purification was per-formed as previously published [16] with some modifications. Briefly, two 500-ml polystyrene-coated paramagnetic iron bead slurry aliquots pre-conjugated with sheep anti-rabbit IgG antibody (M-280 Dynabeads; Dynal, Lake Success, NY)
were washed with 2×500 ml cold
pre-immune or anti-CRT serum diluted in 250 ml cold PBS-T for 1 h at room temperature under constant bidirectional mixing (Dynal Sample Mixer 947.01; Dynal, Lake Success, NY). Bead
pellets were then washed with 3×500 ml cold
PBS-T and collected as described above. Pre-im-mune or anti-CRT rabbit IgG was eluted from magnetic beads with 500ml 0.5 M acetic acid, pH 4.5 for 15 min at room temperature under con-stant bidirectional mixing. Beads were again col-lected with a magnetic particle collector, then supernatant removed from the immobilized bead pellet and immediately neutralized with 500ml 1 M Tris – HCl, pH 8.5. Eluant protein concentration was determined by dividing A280readings collected
from a Shimadzu UV-240 UV-Visible Recording Spectrophotometer (Shimadzu, Kyoto, Japan) by a protein extinction coefficient of 1.4. Neutralized eluant was subsequently centrifuge-concentrated via a 30-kDa-cutoff Centricon spin concentrator (Millipore, Bedford, MA). Final pre-immune and anti-CRT concentrates were stored at 4°C.
2.3. IgG purity and densitometry analysis
Pre-immune and anti-CRT IgG content in crude whole sera was determined using 1:1 and 1:2 whole serum diluted in 10 mM Tris – HCl, 150 mM NaCl, pH 7.2 (TBS), separated on a 12% acry-lamide SDS-PAGE gel under reducing conditions, and visualized using Coomassie staining. Pre-im-mune and anti-CRT IgG were compared to a non-specific IgG standard. Percent IgG was visu-ally determined and multiplied by the dilution factor to estimate average percent IgG.
The Laemmli method [17] was used with the following modifications. Two 0.75-mm, 10% SDS-PAGE gels were run at 150 V (62 mA) for 90 min
under reducing conditions, then fluorescence
stained with Sypro Red (FMC, Rockland, ME) as follows. SDS-PAGE gels were briefly rinsed with double distilled water (ddH2O), then incubated
with 1:5000 Sypro Red in 7.5% v/v acetic acid in a polypropylene container for 1 h at room tempera-ture under constant mixing. Gels were rinsed with 1×20 ml additional 7.5% acetic acid and stored in ddH2O prior to signal detection.
Sypro Red-stained SDS-PAGE gels were excited using a Molecular Dynamics Storm 860 laser scan-ner (version 4.1; Molecular Dynamics, Sunnydale, CA) set to 530 nm connected to a Power
Macin-tosh G3 300 MHz running Mac OS 8.1.
Fluores-cent signals were visualized with Molecular
Dynamics ImageQuant (version 1.2; Molecular Dynamics, Sunnydale, CA) software and exported as tagged image format (TIF) files. TIFs were then imported into and quantitatively analyzed using BioRad Quantity One densitometry software (ver-sion 4.0.3; BioRad, Hercules, CA). Absolute pro-tein amounts for magnetic bead purified pre-im-mune and anti-CRT gels were determined by comparison to a six-point BSA standard calibra-tion curve using extrapolacalibra-tion and linear regres-sion.
2.4. Western blot analysis
Total soluble proteins were separated on a 10% SDS-PAGE gel, stained with Sypro-Red and imaged as described above, then transferred to Immun-Blot polyvinylidene difluoride (PVDF) membrane (BioRad; Hercules, CA) at 200 mA constant current overnight. Following transfer, the membrane was rinsed briefly with TBS, then non-specific protein binding sites blocked in 1% Western Blocking Solution (WBS; Boehringer-Mannheim; Indianapolis IN) in TBS for 1 h at room temperature. Membrane was then incubated with a 1:200 dilution of either magnetic bead purified anti-CRT IgG or anti-CRT whole serum in 0.5% WBS in TBS for 2 h at room temperature. Incubated membranes were subsequently washed with 2×100 ml TBS containing 1% v/v Tween-20
(TBS-T) and 2×100 ml 0.5% WBS in TBS for 10
2.5. CRT immunolocalization
Pistia shoot tips were dissected into 2 mm2
sections and fixed in 4.0% paraformaldehyde in 50 mM [Piperazine,N, N-bis-(2-ethane-sulfonic acid), 1.5 sodium salt] (Pipes), pH 7.2 overnight at 4°C. Fixed tissue was dehydrated through a 30, 50, 70, 80, 95 and 100% increasing ethanol series, infiltrated with LR White acrylic resin (EM Sci-ences; Ft. Washington, PA) and cured overnight at
60°C. One micrometer Pistia shoot tip sections
were mounted on silane-coated slides (Digene Di-agnostics; Beltsville, MD), then non-specific bind-ing sites blocked for 1 h in 10 mM Tris – HCl, 150
mM NaCl, 0.3% v/v Tween-20, 1% w/v bovine
serum albumin (BSA), pH 7.2 (TBS-T/BSA). Sec-tions were incubated in 1:100 whole or magnetic bead purified pre-immune or anti-CRT sera di-luted in TBS-T/BSA for 4 h at room temperature. Shoot tip sections were subsequently washed 4×
15 min in TBS-T/BSA, then incubated with 1:100 protein A-gold diluted in TBS-T/BSA for 1 h at room temperature. Sections were sequentially
washed 2×15 min in TBS-T/BSA, TBS-T, and
ddH2O, then silver-enhanced and visualized on a
BioRad MRC1000 laser scanning confocal micro-scope (BioRad; Hercules, CA). Reflected and transmitted tissue images were obtained then merged to generate the final Pistia shoot tip sec-tion images.
3. Results
3.1. Serum and purified IgG protein determination
Whole anti-CRT serum protein concentration as determined by protein assays was 42 mg/ml. This value was used for total pre-immune serum protein concentration as well. Magnetic bead purified pre-immune and anti-CRT IgG protein A280 readings were 0.017 and 0.014, with
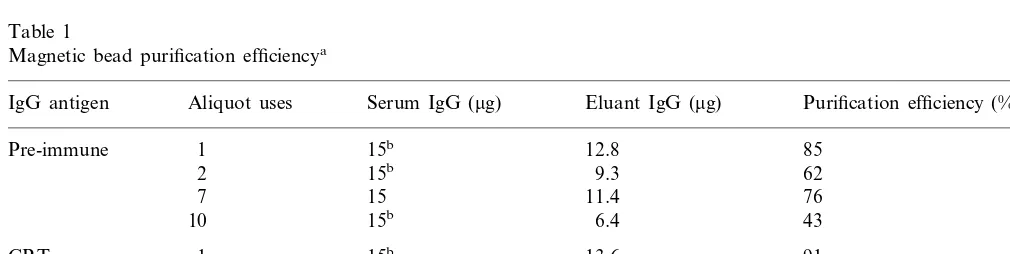
calcu-lated protein concentrations of 0.243 and 0.20 mg/ml, respectively. Absolute protein amounts and percent IgG yield via magnetic bead purifica-tion are depicted in Table 1.
3.2. BSA calibration cur6e
The BSA standard calibration curve was deter-mined by loading 0.01, 0.02, 0.04, 0.1, 0.2 and 1.0
mg known BSA quantities on a reducing 10%
acrylamide SDS-PAGE gel and subsequently staining with Sypro Red. Known BSA quantities produced 9.47, 20.3, 22.18, 36.45, 54.33, and 120.5 fluorescence signal densities, respectively. Plotted values produced a BSA linear regression curve correlation coefficient of 0.9928.
3.3. IgG analysis
Analysis of whole pre-immune and anti-CRT
Table 1
Magnetic bead purification efficiencya
Aliquot uses Serum IgG (mg) Eluant IgG (mg) Purification efficiency (%) IgG antigen
1 15b 12.8 85
Pre-immune
2 15b 9.3 62
15
7 11.4 76
10 15b 6.4 43
1
CRT 15b 13.6 91
2 15b 12.9 86
15 10.7
7 71
15b 9.3 62
10
aMagnetic bead uses refers to the number of times a single bead aliquot was used for purification. Pre-immune and anti-CRT
bead aliquots were used specifically for same antibody isolation as previous purifications. Percent yield values were rounded to the nearest whole percent.
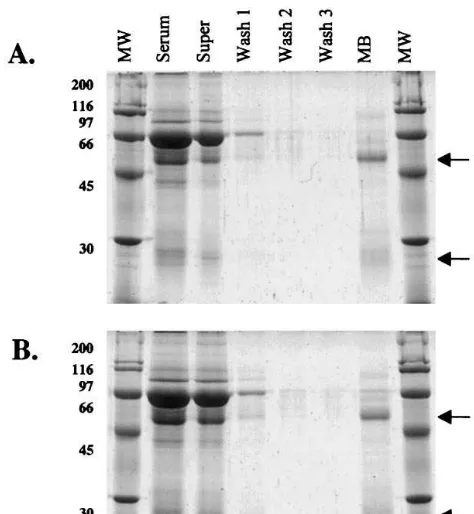
Fig. 1. SDS-PAGE of magnetic bead purified pre-immune (A) and anti-CRT (B) sera. Arrows indicate positions of IgG heavy chain (HC) and light chain (LC) proteins. Samples include molecular weight marker (MW), whole pre-immune (A) or anti-CRT (B) serum diluted 1:30 (Serum), supernatant collected following whole serum incubation with magnetic beads (Super), washes following serum incubation with mag-netic beads (Washes 1 – 3), and centrifugal-concentrated pre-immune or anti-CRT magnetic bead eluant product (MB).
Fig. 2. A. Western blot of Pistia total soluble protein from
−80°C stored stock, incubated with either whole anti-CRT serum (WS) or magnetic bead purified anti-CRT IgG (MB). Whole serum gives multiple bands while bead purified IgG detects CRT and a breakdown product of CRT (BDP). B. Western blot ofPistiafreshly prepared total soluble protein extract incubated with magnetic bead purified anti-CRT. Breakdown product is very minor in non-frozen samples. BDP, Break down product.
albumin, IgG heavy and light polypeptide chain proteins, respectively. Whole anti-CRT serum
di-luted 1:30 contained 3.04, 1.83 and 0.82 mg,
whereas supernatant collected following magnetic bead incubation with undiluted whole serum con-tained 2.49, 1.48 and 1.09 mg albumin, IgG heavy chain, and IgG light chain. Magnetic bead purified
and concentrated sample contained 0.95 mg IgG
heavy chain and 0.91 mg IgG light chain, showing a 1:1 heavy:light chain ratio, which is as expected. Similar values were obtained during identical anal-ysis of whole pre-immune serum, supernatant, washes and magnetic bead purified pre-immune IgG samples (data not shown). Magnetic bead purified IgG had negligible contaminant serum protein as shown on gels using the very sensitive Sypro Red detection (Fig. 1(A, B)).
3.4. Western blot analysis
Western blot analysis of Pistia total soluble protein extracts separated via reducing SDS-PAGE gels and electroblotted to PVDF mem-branes (Fig. 2A) produced the following values when quantitatively analyzed against the BSA standard curve. Blots incubated with whole sera were compared to blots incubated with magnetic bead purified antibody eluant. Two bands were recognized in both blot preparations; a 66- and 45-kDa band. With whole serum, multiple non-specific bands were identified on the blot, which did not show up with the purified protein. Using magnetic bead purified anti-CRT IgG, a band with 1.38 mg protein was identified at 66 kDa in Pistiasoluble extract. A band of identical size was also found using whole anti-CRT serum, which detected 1.78 mg in Pistia soluble extract. An additional band of 1.93mg was observed in soluble extract at 45 kDa using magnetic bead purified anti-CRT. Whole anti-CRT serum also produced a 2.34-mg 45 kDa band in Pistia soluble extract. To determine if the 45-kDa band, seen in Fig. 2A, using magnetic bead purified ant-CRT was a CRT breakdown product or cross reaction with another protein, a new Pistia soluble protein extract was prepared and immediately run on a 10% SDS-PAGE gel, electroblotted to PVDF membrane, and incubated with magnetic bead purified anti-CRT as described above. The new anti-CRT blot also showed the presence of two bands; however, the 66-kDa band was by far the most dense, with only a faint secondary band visible (Fig. 2B).
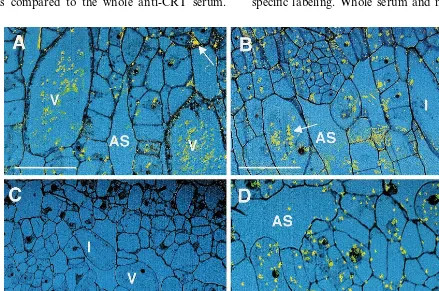
3.5. CRT immunolocalization
Immunolocalization patterns were compared for non-purified and purified pre-immune and anti-CRT IgG sera using confocal microscope imaging of silver-enhanced immunogold labeling (Fig. 3). Fig. 3A, B depict immunolocalization patterns using whole pimmune and anti-CRT sera re-spectively. Background is very high with both the pre-immune and anti-CRT whole sera. In particu-lar, the vacuoles and crystals of crystal idioblasts and the walls of many cells show labeling that is non-specific. In contrast as seen in Fig. 3C, the purified pre-immune IgG gave completely clean sections with no wall or vacuolar labeling. With purified anti-CRT IgG, labeling was restricted to the cytoplasm of all cells, and was particularly abundant in the cytoplasm of crystal idioblasts (Fig. 3D), while vacuolar and wall labeling were essentially eliminated. The amount of specific label was also much higher with the purified anti-CRT IgG as compared to the whole anti-CRT serum.
Thus, magnetic bead purified CRT IgG gave greatly increased signal to noise ratio for labeling on resin embedded sections, while background was eliminated when purified pre-immune IgG was used.
4. Discussion
Our results show magnetic bead protein purifi-cation appears to be a rapid, efficient, cost effec-tive method for purifying IgG. As demonstrated here, highly purified antibody produced by this technique can provide more specific data with less peripheral convolution of specific signals in both Western immunoblotting and immunocytochem-istry applications. This may have particular signifi-cance for plant tissue identification methodologies, where plant components such as wall polymers, glycoproteins, and polyphenolics may inhibit spe-cific antibody localization or lead to strong non-specific labeling. Whole serum and magnetic bead
purified anti-CRT IgG identified 4.45 and 1.57mg, respectively, total non-CRT protein in Western blots, corresponding to 2.83 times more secondary bands identified by whole serum than by magnetic bead purified anti-CRT IgG samples. Results from freshly prepared Pistia total soluble protein ex-tracts lead us to conclude the 45-kDa band is a CRT degradation product. It appears that even at
−80°C, storage of Pistia total soluble protein samples results in breakdown of CRT.
A similar increase in anti-CRT IgG specificity was seen for immunocytochemistry. Magnetic bead purified anti-CRT IgG elicited specific signal of greater density with non-detectable background compared to whole anti-CRT serum, which showed high levels of non-specific labeling. Impor-tantly, while whole pre-immune sera gave very high non-specific labeling, magnetic bead purified pre-immune IgG was absolutely clean. The lack of CRT recognition by magnetic bead purified pre-immune serum IgG indicates the CRT anti-body was specifically produced during immuniza-tion, and that no anti-CRT antibodies pre-existed in the rabbit used for antibody production, con-trary to what whole serum pre-immune results might have suggested. The high levels of non-spe-cific signal using whole pre-immune and anti-CRT sera may be due to the presence of secondary protein contaminants, which are removed by the purification process. These secondary proteins ap-pear to bind non-specific tissue targets such as cell walls and calcium oxalate crystals found within crystal idioblast vacuoles.
Coomassie-stained SDS-PAGE gels were ini-tially used for visually estimating heavy and light chain pre-immune and anti-CRT IgG bands from total whole serum preparations. Coomassie-based staining methods are not as sensitive as Sypro Red fluorescent staining, and may contribute to dis-crepancies between expected and observed IgG amounts. This may have also contributed to mag-netic bead overload as a result of underestimating IgG amounts in serum. Interestingly, IgG eluant purity using bead aliquots overloaded six times the stated bead capacity was excellent, with very low levels of secondary contaminants (Fig. 1, lane MB). Excess IgG not bound by the beads was retained in the supernatant (Fig. 1(A, B), lane Super) while bound IgG was retained throughout subsequent wash steps (Fig. 1, lanes Wash 1 – 3). IgG yield differences between normal and
over-loaded bead aliquots were also minimal (Table 1), indicating the magnetic beads’ ability to purify IgG from serum is not compromised by excess target load, which may not be the case with other purification systems. Bead aliquots loaded with IgG amounts at stated bead capacity had no
de-tectable secondary contaminants (data not
shown). Repeated use of magnetic bead aliquots showed total IgG yield decreases of 43 and 29% for pre-immune and anti-CRT IgG, respectively. Yield decreases were significant after seven uses and dropped quickly after ten uses. However, antibody yields remained above 50% after ten uses for several additional purifications (data not shown).
Discrepancies between expected and observed IgG amounts recovered in the final eluant concen-trate may be attributed to centrifugal concentra-tion of the sample. Centrifugal concentraconcentra-tion devices used for this study were neither pretreated nor non-specific protein binding sites blocked to reduce protein loss. SDS-PAGE gels used to ex-amine final product purity used magnetic bead purified anti-CRT IgG eluant concentrated in two steps; once with a 30-kDa and once with a 3-kDa MW cutoff concentrator. This procedure was ini-tially necessary to obtain a sample concentrated enough to be effectively gel-analyzed. Calculations estimate a 30% IgG loss from non-concentrated protein eluant (data not shown). Literature pro-vided by the manufacturer indicates without pre-treatment of spin concentrator membranes, a 15% protein yield reduction can be expected. This 15% reduction, occurring with both the 30- and 3-kDa cutoff concentrators, would entirely account for observed yield losses. Taking sample concentra-tion losses in account, calculated IgG amounts in the magnetic bead supernatant and eluant are within 5% of IgG originally loaded on the mag-netic beads (data not shown). This yield efficiency is further evidence of the value of magnetic bead purification.
An estimate of the economic utility of magnetic bead anti-CRT IgG purification is as follows. Pre-coated magnetic beads and bi-directional mixer:
$230+$375=$600. Average number of uses for
single bead aliquot: 10. Number of bead aliquots per package: 4. Average percent anti-CRT IgG yield over bead aliquot usable lifespan: 78%. Max-imum IgG binding capacity of magnetic beads
anti-CRT IgG production of magnetic beads over us-able lifespan with average anti-CRT yield: 117 mg. Cost of purifying anti-CRT IgG for entire pack-age: $0.78 per mg. Utilizing a bi-directional mixer has an appreciable increasing effect on antibody yield as compared to previous work [16]. This difference is not thought to be due to differences in antibody class. Magnetic beads may also be used beyond ten uses, further decreasing overall cost. Additionally, initial cost of the bi-directional mixer is distributed over subsequent purchases of magnetic beads. Given differences in costs, yield and time, magnetic bead isolation of antibody fractions should prove a useful tool for plant biology research utilizing immunological tech-niques.
Acknowledgements
This work was supported by MES and NSF grant MCB9632027 to V.R. Franceschi. The au-thors thank Rosemary Travato and Dynal, Inc. for their support. T.A. Kostman gratefully ac-knowledges a N. Higinbotham Foundation grant and use of the University of Idaho WAMI Pro-gram confocal microscope.
References
[1] K.H. Krause, M. Michalak, Calreticulin, Cell 88 (1997) 439 – 443.
[2] M. Opas, S. Tharin, R.E. Milner, M. Michalak, Identifi-cation and localization of calreticulin in plant cells, Pro-toplasma 191 (1996) 164 – 171.
[3] M. Michalak, R.E. Milner, K. Burns, M. Opas, Calreti-culin, Biochem. J. 285 (1992) 681 – 692.
[4] A.M. Hassan, C. Wesson, W.R. Trumble, Calreticulin is the major Ca2+ storage protein in the endoplasmic
reticulum of the pea plant (Pisum sati6um), Biochem.
Biophys. Res. Commun. 211 (1995) 54 – 59.
[5] J. Denecke, L.E. Carlsson, S. Vidal, A.S. Hoglund, B. Ek, M.J. van Zijl, K.M.C. Sinorgo, E.T. Palva, The tobacco homologue of mammalian calreticulin is present in protein complexes in vivo, Plant Cell 7 (1995) 391 – 406.
[6] F.H. Chen, P.M. Hayes, D.M. Mulrooney, A. Pan, Identification and characterization of cDNA clones en-coding plant calreticulin in barley, Plant Cell 6 (1994) 835 – 843.
[7] N.S. Allen, S.C. Tiwari, Localization of calreticulin, a major calcium binding protein, in plant cells, Plant Phys-iol. 96 (1991) 42.
[8] A.J. Crofts, J. Denecke, Calreticulin and calnexin in plants, Trends Plant Sci. 3 (1998) 396 – 399.
[9] V.R. Franceschi, X. Li, D. Zhang, T.O. Okita, Calse-questrin-like calcium binding protein is expressed in cal-cium accumulating cells of Pistia stratiotes, Proc. Natl. Acad. Sci. 90 (1993) 6986 – 6990.
[10] V.R. Franceschi, H.T. Horner, Calcium oxalate crystals in plants, Bot. Rev. 486 (1980) 361 – 427.
[11] H.T. Horner, B.L. Wagner, Calcium oxalate formation in higher plants, in: S.R. Khan (Ed.), Calcium Oxalate in Biological Systems, Vol. 1, CRC Press, 1995, pp. 53 – 72. [12] R.B. Knox, Methods for locating and identifying anti-gens in plant tissues, in: G.R. Bullock, P. Petrusz (Eds.), Techniques in Immunocytochemistry, Vol. 1, Academic Press, 1982, pp. 205 – 238.
[13] E.M. Herman, Colloidal gold labeling of acrylic embed-ded plant tissues, in: M.A. Hayat (Ed.), Colloidal Gold: Principles, Methods and Applications, Vol. 2, Academic Press, 1989, pp. 304 – 321.
[14] V.P. Knutson, R.A. Buck, R.M. Moreno, Purification of a murine monoclonal antibody of the IgM class, J. Immunol. Methods 136 (1991) 151 – 157.
[15] C. Lucas, C. Nelson, M.L. Peterson, S. Frie, D. Vetter-lein, T. Gregory, A.B. Chen, Enzyme-linked immunosor-bent assays (ELISAs) for the determination of contaminants resulting from the immunoaffinity purifica-tion of recombinant proteins, J. Immunol. Methods 113 (1988) 113 – 122.
[16] I.J. Quitadamo, M.E. Schelling, Efficient purification of mouse anti-FGF receptor IgM monoclonal antibody by magnetic beads, Hybridoma 17 (1998) 199 – 207. [17] U.K. Laemmli, Cleavage of structural proteins during
the assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
.