Polyamines and their cellular anti-senescence properties in honey
dew muskmelon fruit
G.E. Lester *
Kika de la Garza Subtropical Agricultural Research Center,Agricultural Research Ser6ice,United States Department of Agriculture, 2413E.Bus.Hwy.83,Weslaco,TX78596,USA
Received 25 April 2000; received in revised form 29 June 2000; accepted 15 August 2000
Abstract
Activated oxygen free radicals cause peroxidative damage to all membranes and hasten senescence. Polyamines (PAs) are effective scavengers of these free radicals produced by lipoxygenase (LOX) and phospholipase-D (PL-D). Five days prior to abscission (harvest), ‘Honey Brew’ (Cucumis meloL. (Inodorus group)) fruit have a change in the ratio of endogenous spermidine (SPD) to putrescine (PUT), from SPD\PUT to SPDBPUT, which coincides with the onset of fruit senescence. Hypodermal-mesocarp tissues from harvested fruit incubated in mannitol with exogenous SPD or spermine, at 0.25 or 0.5 M, had more chlorophyll (less senescence) following 6 or 48 h of darkness than tissues incubated in mannitol without PAs. Polyamine-incubated tissues versus no PA has less membrane peroxidation as indicated by less malondialdehyde production, and LOX and PL-D activities, and less plasma membrane perturbation as indicated by greater H+-ATPase activity, and protein and phospholipid
contents. Prolonging the duration of endogenous SPD content, whereby, it is greater then PUT content, in harvested (fully-ripened) ‘Honey Brew’ fruit, could delay melon senescence and promote a longer marketable life. Published by Elsevier Science Ireland Ltd.
Keywords:Chlorophyll;Cucumis melo(Inodorus group); Hypodermal-mesocarp; Malondialdehyde; Lipoxygenase; Phospholipase D
www.elsevier.com/locate/plantsci
1. Introduction
PAs: SPD, SPM and their diamine precursor PUT are ubiquitous in nearly all plant cells [1]. All, except PUT, are involved in many plant pro-cesses, including regulation of DNA replication, gene transcription, cell division, organ develop-ment, leaf senescence, fruit ripening, and in con-ferring membrane protective responses to environmental stresses [1]. Recent studies show that PAs reduce ethylene synthesis by inhibiting ACC synthase [2], and are effective in reducing leaf chlorophyll breakdown [3]. It has also been
shown that PAs greatest contribution to plants is their role in membrane stabilization, by function-ing as free radical scavengers [4], and directly interacting with membranes by inhibiting transbi-layer movement of phospholipids [5]. Polyamines also stabilize molecular complexes in membranes [6], and reduce membrane damage from lipoxyge-nase activity [7].
Postharvest senescence of many fruits and veg-etables is characterized by a loss of membrane phospholipids, destabilized membrane matrixes, and lipid peroxidation [8]. In muskmelon (Cucumis melo), both netted and honey dew fruits, mainte-nance of cellular membrane integrity within the H-M tissue is critical for regulating postharvest senescence [9]. The role of endogenous PAs, and the benefit of exogenous PAs, in muskmelon H-M membranes during postharvest senescence has not been reported. The objectives of this study were
Abbre6iations: Chl, total chlorophyll; DAA, days after anthesis;
H-ATPase, hydrogen-adenosine triphosphatase; H-M, hypodermal-mesocarp; LOX, lipoxygenase; MDA, malondialdehyde; PAs, Polyamines; PL, phospholipid; PL-D, phospholipase D; PM, plasma membrane; PUT, putrescine; SPD, spermidine; SPM, spermine.
* Tel.: +1-956-4476322; fax:+1-956-4476323.
E-mail address:[email protected] (G.E. Lester).
two-fold. First, establish the endogenous develop-mental profile of PAs in honey dew muskmelon (C. melo (Inodorus group)) H-M tissue during growth, maturation and at harvest. Second, dark incubate excised H-M tissue, of postharvest fruits, in different concentrations of exogenous SPM or SPD solutions with mannitol for 6 or 48 h, then evaluate and contrast plasma membrane lipid changes (H+-ATPase activity, and total protein
and total phospholipid contents), and membrane lipid peroxidation activities (lipoxygenase and phospholipase-D activity, malondialdehyde and chlorophyll concentrations) as measures of fruit senescence.
2. Materials and methods
2.1. Plant material and culture
‘Honey Brew’, a hybrid honey dew muskmelon (C.meloL. (Inodorus group)), plants were individ-ually grown in 16-l pots containing Sunshine c4 mix (Sun Gro Horticulture, Bellevue, WA) supple-mented with 30 ml Osmocote 14N-14-P-14K (The Scotts Co. Marysville, OH). During vegetative and fruit growth stages, plants were fertilized with Peters 20N-20-P-20K, (The Scotts Co. Marysville, OH), and during the flowering stage, with Peters 9N-45P-15K. Plants were maintained in a green-house under 300 mE s−1 m−2 radiation
supple-mental lighting from 07:00 to 19:00 h daily, supplied by 400 W, metal-halide lamps (Duro-Test Corp. Fairfield, NJ). Day/night average tempera-tures were 3098°C/2394°C and average humidi-ties were 72910%/9892%. Flowers were hand pollinated with one fruit per plant being allowed to develop. Fruit were harvested at 20, 30, 33, 35, 40, 47, 49 and 51 DAA and abscission. All fruit were harvested at 08:00 h.
2.2. Tissue isolation
All fruit were weighed immediately after harvest and the rind was surface sterilized in a 1.05% v/v solution of sodium hypochlorite prepared by a five-fold dilution of commercial bleach with Milli-Q H2O (Milli-Q; Millipore Corp., Bedford, MA).
The epidermis was removed, followed by the hy-podermal-mesocarp tissue with a sterilized veg-etable peeler. The hypodermal mesocarp (160 g
fresh weight) was added to a 1-l sterilized plastic tub containing 0.75 l of incubation solution. The containers were covered with plastic wrap then covered again with aluminum foil. Incubation was performed in the dark at 23°C for 6 or 48 h.
2.3. Incubation solutions
All incubation treatments contained 0.35 M mannitol as the osmoticurn with or without either 0.25 or 0.50 M spermidine or spermine. After incubation, all tissues were removed from the solu-tion, rinsed, patted dry in paper towels, and as-sayed immediately or sealed in plastic bags under N2, then stored at −80°C until analyses.
2.4. Chlorophyll, H+-ATPase, lipoxygenase,
malondialdehyde, phospholipase-D, phospholipid,
protein, polyamine, analyses
Chlorophyll a and b contents were determined in 80% acetone as previously described [10]. Lipoxygenase activity was determined spectropho-tometfically at 235 mn [11]; 1 Unit of lipoxygenase activity was defined as a change in 0.001 ab-sorbency units per min.
Malondialdehyde content was determined on non-frozen tissue according to [12]. Polyamines were extracted according to [13] and determined by high performance liquid chromatography using an Utrasphere ODS C-18, 45 mm×4.6 mm ID, 5 mm particle size column (Beckman – Coulter Co. Fullerton, CA) with a 64% methanolic mobile phase at 1 ml/min and detection at 254 nm [13]. Phospholipase-D activity was determined spec-trophotometrically at 500 nm according to [14] and expressed as nM choline/min. Total phospho-lipids, protein and H+-ATPase were extracted and
determined according to [15].
2.5. Statistics
Analysis of variance was used to evaluate treat-ment differences for chlorophyll, H+-ATPase,
lipoxygenase, malondialdehyde, protein, polyamine, phospholipase-D analyses data (SAS Inst. Cary, SC). Duncan’s multiple range test (P5
3. Results and discussion
3.1. De6elopmental profiles of PUT and SPM in
honey dew muskmelon H-M tissue during growth,
maturation and at har6est
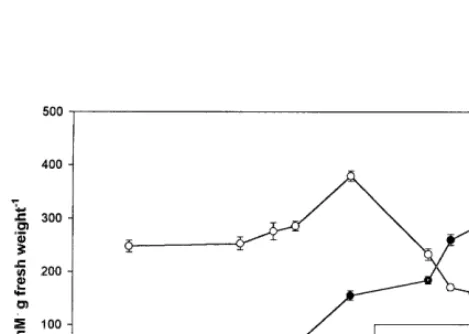
Only the polyamine SPM and its diamine pre-cursor PUT were detected in ‘Honey Brew’ H-M tissues (Fig. 1). At 20 DAA no PUT was de-tected but SPM was abundant. Both SPM and PUT gradually increased as fruit developed until 35 DAA. At 40 DAA a dramatic increase in both SPM and PUT (ca. 1.4- and 5.0-fold, re-spectively) occurred, which coincided with the known onset of ‘Honey Brew’ fruit physiological maturity [11]. After 40 DAA, SPM dropped pre-cipitously until fruit abscission (51 DAA). Pu-trescine, however, continued to increase with DAA and a second dramatic increase in PUT occurred between 45 and 50 DAA, which coin-cided with the continued sharp decline in SPM content. This loss in SPM content along with a concomitant increase in PUT, which occurred during ‘Honey Brew’ fruit ripening (40 – 51 DAA), has been shown to occur in other plant tissues [16]. In carnation flowers this concomitant change in PAs and PUT has been correlated with the onset of senescence [3]. Utilizing pear cells, it has been shown that very high levels of PAs must be present to trigger the biochemical
process leading to the ethylene climacteric [17]. Triggering the ethylene climacteric process occurs when a large build up of PAs suppress AdoMet decarboxylase, causing a shunt of AdoMet to the ethylene pathway. In ‘Honey Brew’ fruit the highest concentration of SPM occurred 10 days prior to abscission, which coincides with the ap-proximate onset of the ethylene climacteric in ‘Honey Brew’ fruit [18]. However, although high levels of PAs are required for triggering the on-set of the ethylene climacteric, ethylene produc-tion can only occur when PA levels are suppressed [19], otherwise ACC synthase will be inhibited [2]. The dramatic rise in PUT, shortly after the peak in PAs in fruit, which occurred in this study, is known to decrease in PA synthesis, thus promoting ethylene production and subse-quent fruit senescence [19].
3.2. Effects of incubating H-M tissue in SPM and SPD on changes in tissue PA le6els
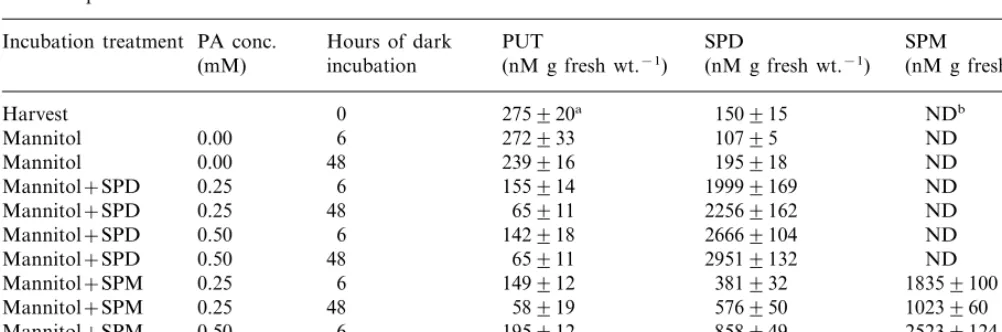
Incubation of ‘Honey Brew’ H-M tissue for 6 h in 0.35 M mannitol without PAs resulted in no change in PUT content (Table 1). However, H-M tissue incubated in mannitol for 6 h with PAs (0.25 M or 0.50 M SPD or SPM) resulted in a calculated averaged 41% decrease in PUT. After 48 h, all treatments showed a decrease in PUT, with the mannitol plus PA-treated tissues having a calculated averaged 78% decline in PUT, while, mannitol treated tissue without PAs had only a 13% decline compared to H-M tissue at harvest (Table 1). These PUT reduction data allowed two predictions. Firstly, maintaining ‘Honey Brew’ H-M tissue in an osmotic solution (equal to the osmotic conc. of the cell, data not shown) for 48 h, highly likely eliminated moisture loss (a stress) normally associated with this tissue in commercial stored melon fruits [20]. This sug-gests that under commercial storage, where fruit moisture loss will occur, detrimental levels of PUT should accumulate, accelerating fruit senes-cence. Secondly, increased PAs within posthar-vest ‘Honey Brew’ H-M tissue should lead to reduced PUT and thus result in improved cellu-lar membrane integrity (i.e. slowed senescence). In support of these two predictions, studies show that PUT accumulation is deleterious to cellular integrity [21]. For example, utilizing corn roots treated with PUT, a wound response like
Table 1
PUT, and PA: SPD, and SPM content in honey dew hypodermal-mesocarp tissue following dark incubation at 23°C in 0.35 M mannitol plus or minus SPD or SPM
SPD Hours of dark
Incubation treatment PA conc. PUT SPM (nM g fresh wt.−1)
Mannitol+SPD ND
0.25
Mannitol+SPD 48 65911 22569162 ND 6 142918
Mannitol+SPD 0.50 26669104 ND
48 65911
0.50 29519132
Mannitol+SPD ND
0.25
Mannitol+SPM 25239124
48 5396
Mannitol+SPM 0.50 10719105 19219183
aStandard error. bNone detected.
tom takes place, whereby, plasma membrane de-polarization occurs and is accompanied by an increase in potassium leakage due to PUT stimu-lated apoplastic diamine oxidase activity generat-ing oxidation products, which induce membrane senescence [21].
3.3. Effect of incubating H-M tissues in SPD and SPM on chlorophyll retention, MDA content and LOX acti6ity
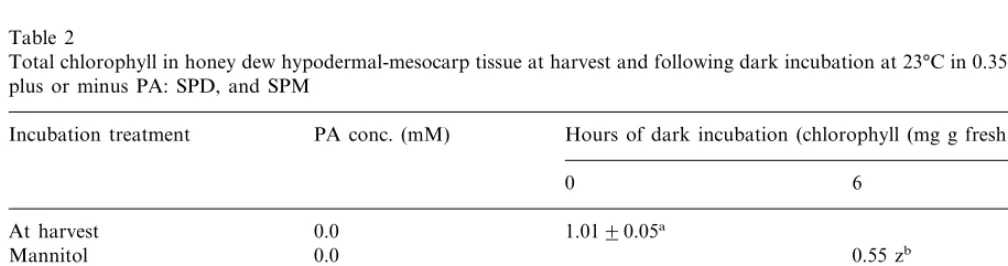
Chlorophyll in H-M tissue incubated in manni-tol without PAs declined 46 and 69% after 6 and 48 h, respectively, compared to H-M tissue at harvest (Table 2). In contrast, chlorophyll in H-M tissues incubated in mannitol plus PAs declined a calculated averaged 21 and 33% after 6 and 48 h, respectively, compared to tissues at harvest. The role of PAs in retarding chlorophyll loss involves reducing the hydrolytic activity of chloroplast thy-lakoid membranes [22].
Another measure of PAs effects on stabilizing the membrane matrix was further investigated by measuring lipid peroxidation as estimated by MDA production (Table 3). Malondialdehyde content in H-M tissue incubated in mannitol with-out PAs was 1.5- and 2.2-fold higher, after 6 and 48 h, respectively, compared to MDA in H-M tissue at harvest. Malondialdehyde content in H-M tissues following incubation in mannitol with PAs averaged 1.2- and 1.6-fold higher, after 6 and
48 h, respectively compared to the MDA in H-M tissues at harvest. Demonstrating that PAs had a significant influence on the reduction in lipid per-oxidation, and most likely did so by scavenging free radicals [23].
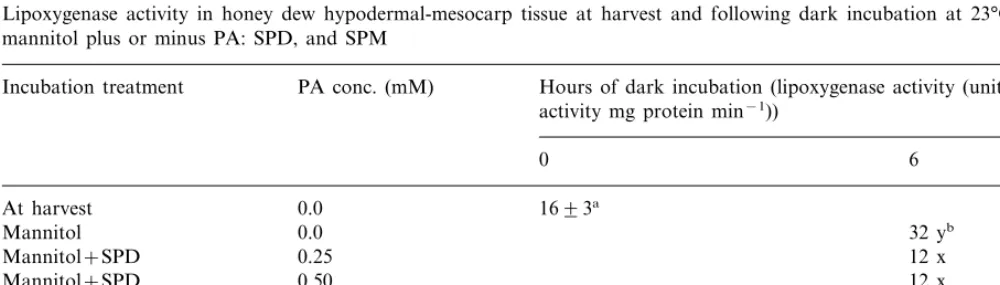
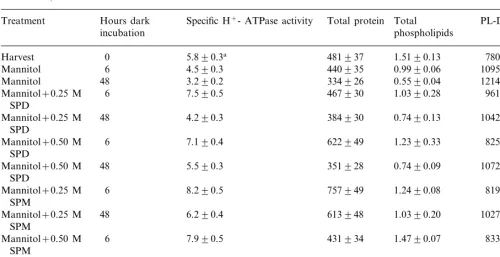
Lipid peroxides generated by LOX, which uses molecular oxygen to transform cis, cis -1,4-penta-diene structured fatty acids (linoleic and linolenic) into peroxides, are further transformed into free radicals, MDA, and jasmonic acid [24]. Lipoxyge-nase activity is strongly associated with ‘Honey Brew’ H-M tissue plasma membrane peroxidation during senescence [11]. Lipoxygenase activity in ‘Honey Brew’ H-M tissue following incubation in mannitol with or without PAs, is shown in Table 4. After incubation for 6 h without PAs, LOX activity more than doubled compared to LOX activity in H-M tissues at harvest. While with PAs, LOX activity showed no increase. After 48 h of incubation, without PAs, LOX activity was three-fold greater than in H-M tissues at harvest, while with PAs LOX activity increased but the increase was not significantly greater than LOX activity at harvest. Lipoxygenase-generated free radicals are known to disrupt membrane selective permeabil-ity, via peroxidation of membrane phospholipids (PL), resulting in membrane leakage [25], reduced H+-ATPase selective ion pumping activity, and
3.4.Effects of incubation of H-M tissues in SPD and SPM on plasma membrane H+-ATPase and PL-D
acti6ities and total protein mid total PL content
Increased membrane leakage results in increased cytoplasmic calcium and hydrogen ion contents due to a decrease in membrane localized Ca2+-ATPase
and H+-ATPase pumping systems [27]. Elevated
cytoplasmic calcium content promotes cellular de-compartmentation, causing an increase in hydrogen ions, furthering cytoplasmic acidity, and stimulat-ing PL-D activity. PL-D activity in H-M tissue incubated in mannitol without PAs increased 1.4-and 1.6-fold after 6 1.4-and 48 h, respectively, com-pared to PL-D in H-M tissue at harvest (Table 5). In contrast, incubation in mannitol plus PAs, PL-D average only a 1.1- and 1.3-fold increase after 6 and 48 h, respectively, compared to PL-D in H-M tissue at harvest. The PA treatment affect, in retarding PL-D activity, was manifested in reduced perturba-tion of the plasma membrane as measured by specific H+-ATPase activity, and membrane
protein and PL contents. Specific H+-ATPase
ac-tivity in H-M tissue incubated in mannitol plus PAs actually demonstrated an averaged 1.3-fold in-crease after 6 h and only a 13% dein-crease after 48 h, compared to H-M tissue at harvest. In H-M tissue incubated in mannitol without PAs, specific H+-ATPase activity decreased 22 and 45% after 6
and 48 h, respectively, compared to H-M tissue at harvest. Plasma membrane protein and PL contents in H-M tissues incubated in mannitol without PAs decreased 8 and 34% after 6 h incubation, respec-tively, compared to H-M tissue at harvest. And
after 48 h of incubation, protein and PL content decreased 31 and 64%, respectively, compared to H-M tissue at harvest. However, after incubation of H-M tissues in mannitol with PAs for 6 h, plasma membrane protein content actually increased 1.2-fold, and after 48 h only decreased 10%. The phospholipid content from the plasma membrane of H-M tissue incubated in mannitol with PAs, slightly decreased an averaged 18 and 45% after 6 and 48 h, respectively, compared to H-M tissues at harvest.
In conclusion, senescence in ‘Honey Brew’ fruit is highly likely associated with the rapid loss of SPD and the concomitant dramatic increase in PUT, which occurs within the last 5 – 6 days of fruit maturation, prior to abscission (harvest). This change in endogenous SPM content, from greater than, to less than the PUT content is considered a trigger for ethylene initiation [17]. ‘Honey Brew’ H-M tissues from abscissed fruit and incubated in mannitol with either SPD or SPM at either 0.25 or 0.5 M were less senescent following either 6 or 48 h of dark incubation then H-M tissues incubated in mannitol without PAs. Polyamine treated H-M tissues, compared to non-PA-treatment, had higher chlorophyll retention, which is an indicator of reduced senescence rate. Also, PA-treated H-M tissues demonstrated less membrane peroxidation then non-PA-treated tissues as indicated by lowered MDA production. Non-PA-treated H-M tissues also had lowered LOX and PL-D activities, which likely contributed to lowered membrane peroxida-tion. ‘Honey Brew’ H-M tissues treated with
Table 2
Total chlorophyll in honey dew hypodermal-mesocarp tissue at harvest and following dark incubation at 23°C in 0.35 M mannitol plus or minus PA: SPD, and SPM
PA conc. (mM)
Incubation treatment Hours of dark incubation (chlorophyll (mg g fresh wt.−1))
0 6 48
0.0 1.0190.05a
At harvest
0.31 z 0.0
Mannitol 0.55 zb
0.25
Mannitol+SPD 0.77 y 0.64 y
Mannitol+SPD 0.50 0.73 y 0.69 x
Mannitol+SPM 0.25 0.86 x 0.64 y
0.50
Mannitol+SPM 0.84 x 0.74 x
0.08 0.09
PB0.05
aStandard error.
Table 3
Malondialdehyde, a measure of lipid peroxidation, in honey dew hypodermal-mesocarp tissue at harvest and following dark incubation at 23°C in 0.35 M mannitol plus or minus PA: SPD, and SPM
Incubation treatment PA conc. (mM) Hours of dark incubation (malondialdehyde (nM g fresh wt.−1))
0 6 48
At harvest 0.0 1.1290.3a
Mannitol 0.0 1.6 yzb 2.4 z
1.7 z
0.25 2.0 y
Mannitol+SPD
0.50
Mannitol+SPD 1.1 x 1.6 x
1.3 xy
Mannitol+SPM 0.25 2.0 y
1.1 x
0.50 1.4 y
Mannitol+SPM
PB0.05 0.3 0.3
aStandard error.
bMeans within columns followed by the same letter are not significantly different according to Duncan’s Multiple Range Test.
Table 4
Lipoxygenase activity in honey dew hypodermal-mesocarp tissue at harvest and following dark incubation at 23°C in 0.35 M mannitol plus or minus PA: SPD, and SPM
PA conc. (mM) Hours of dark incubation (lipoxygenase activity (units of Incubation treatment
activity mg protein min−1))
0 6 48
1693a
At harvest 0.0 0.0
Mannitol 32 yb 49 z
Mannitol+SPD 0.25 12 x 15 x
Mannitol+SPD 0.50 12 x 19 x
14 x
0.25 10 x
Mannitol+SPM
13 x
Mannitol+SPM 0.50 19 x
8 9
PB0.05
aStandard error.
bMeans within columns followed by the same letter are not significantly different according to Duncan’s Multiple Range Test.
PAs also had higher plasma membrane protein and phospholipid contents.
Although, accelerated senescence was not tested by incubating ‘Honey Brew’ H-M tissue in exoge-nous PUT, it is possible to concluded that main-taining a higher ratio of SPD:PUT in postharvest melons, could retard senescence. Further studies using either transgenic or mutigenic fruit with reduced PUT or with fruit containing a naturally high ratio of SPD to PUT postharvest are needed to resolve this issue. Also, necessary electrolyte leakage studies can be afforded when tissue is not incubated.
Acknowledgements
Table 5
H+-ATPase activity (mM Pi mg protein h−1), total protein (mg), total phospholipids (mM) and PL-D activity (nM Choline
min−1) in honey dew hypodermal-mesocarp tissue at harvest and following dark incubation at 23oC in 0.35 M mannitol plus or
minus SPD, and SPM
Hours dark Specific H+- ATPase activity Total protein
Treatment Total PL-D activity
phospholipids
Mannitol 1095994
3.290.2 334926
Mannitol 48 0.5590.04 12149104
6 7.590.5 467930 1.0390.28
[1] A. Bouchereau, A. Aziz, F. Larher, J. Martin-Tanguy, Review: polyamines and environmental changes: recent developments, Plant Sci. 140 (1999) 103 – 125.
[2] P.J. Davies, R. Rostogi, D.M. Law, Polyamines and their metabolism in ripening tomato fruit, in: H.E. Flo-res, R.N. Arteca, J.C. Channon (Eds.), Polyamines and Ethylene, Biochemistry, Physiology and Interactions, Curr. Topics Plant Physiol. 5 (1990) 112 – 125.
[3] M.M. Kushad, E.B. Dumbroff, Metabolic and physio-logical relationships between the polyamine and ethylene biosynthetic pathways, in: R.D. Slocum, H.E. Flores (Eds.), The Biochemistry and Physiology of Polyamines in Plants, CRC Press, Boca Raton, FL, 1991, pp. 78 – 89. [4] N. Bors, C. Langebartels, C. Mitchel, H. Sanderman, Jr, Polyamines as radical scavengers and protectants against ozone damage, Phytochem. 28 (1989) 1589 – 1595. [5] D.L. Bratton, Polyamine inhibition of transbilayer
movements of plasma membrane phospholipids in the erythrocyte ghost, J. Biol. Chem. 269 (1994) 22517 – 22523.
[6] R.T. Besford, C.M. Richardson, J.L. Campos, A.F. Tiburico, Effect of polyamines in stabilization of molec-ular complexes of thylakoid membranes of osmotically stressed oat leaves, Planta 189 (1993) 201 – 206. [7] A.F. Tiburico, R. Kaur-Sawhney, A.W. Galston,
Polyamine metabolism of plants, in: B.J. Miflin, P.J. Lea (Eds.), The Biochemistry of Plants, vol. 16, Academic Press, New York, 1990, pp. 283 – 325.
[8] Y.Y. Lesham, Plant Membranes: A Biophysical Ap-proach to Structure Development and Senescence, Kluwer, Dordredt, 1992, pp. 174 – 188.
[9] G.E. Lester, M.A. Grusak, Postharvest application of calcium and magnesium to honey dew and netted muskmelons: effect on tissue ion concentrations, quan-tity and senescence, J. Am. Soc. Hort. Sci. 124 (1999) 545 – 552.
[10] A.R. Wellburn, H. Lichtenthaler, Formulae and pro-gram to determine to carotenoids and chlorophylls A and B of leaf-extracts in different solvents, in: C. Sybesma (Ed.), Advances in Photosynthesis Research, vol. 11, Martinus Nijhoff/Dr. W. Junk Pub, The Hague, 1984, pp. 9 – 12.
[11] G.E. Lester, Physicochernical characterization of hybrid honey dew muskmelon fruit (Cucumis melo L var. in-odorus Naud.) following maturation, abscission, and postharvest storage, J. Am. Soc. Hort. Sci. 123 (1998) 126 – 129.
[12] D.M. Hodges, J.M. DeLong, C.F. Forney, R.P. Prange, Improving the thiobarbituric acid reactive-substance as-say for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering com-pounds, Planta 207 (1999) 604 – 611.
[13] H.E. Flores, A.W. Galston, Analysis of polyamines in higher plants by high performance liquid chromatogra-phy, Plant Physiol. 69 (1982) 701 – 706.
[15] G.E. Lester, B. Whitaker, Gamma-ray-induced changes in hypodermal mesocarp tissue plasma membrane of pre- and post-storage muskmelon, Physiol. Plant 98 (1996) 265 – 270.
[16] M. Egea-Cortines, Y. Mizrahi, Polyamines in cell divi-sion, fruit set and development, and seed germination, in: R.D. Solcurn, H.E. Flores (Eds.), The Biochemistry and Physiology of Polyarnines in Plants, CRC Press, Boca Raton, FL, 1991, pp. 144 – 154.
[17] D. Ke, R.J. Romani, Effects of spermidine on ethylene production and, the senescence of suspension-cultured pear fruits cells, Plant Physiol. Biochem. 26 (1988) 109 – 116.
[18] H.K. Pratt, J.D. Goeschl, F.W. Martin, Fruits growth and development, ripening and the role of ethylene in the ‘Honey Dew’ muskmelon, J. Am. Soc. Hort. Sci. 102 (1977) 203 – 210.
[19] M.A. Smith, P.J. Davies, Effect of photoperiod on polyamine metabolism in apical buds of G-2 peas in relationship to the induction of apical senescence, Plant Physiol. 79 (1985) 400 – 405.
[20] G.E. Lester, B.D. Bruton, Relationship of netted muskmelon fruit water loss to postharvest storage, J. Am. Soc. Hort. Sci. 111 (1986) 727 – 731.
[21] J.M. DiTomaso, J.E. Shaff, L.V. Kochian, Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings, Plant Physiol. (1989) 988 – 995.
[22] R.B. Popovic, D.J. Kyle, A.S. Cohen, S. Zalik, Stabiliza-tion of thylakoid membranes by spermine during stress induced senescence of barley leaf discs, Plant Physiol. 64 (1979) 721 – 726.
[23] G. Drolet, E.B. Dumbroff, R.L. Legge, J.E. Thompson, Radicle scavenging properties of polyamines, Phy-tochem. 25 (1986) 367 – 371.
[24] B. Vick, D.C. Zimmerman, Metabolisms of fatty acid hydroperoxides by Chorella pyrenoidosa, Plant Physiol. 90 (1989) 125 – 132.
[25] J.E. Thompson, R.L. Legge, R.F. Barber, The role of free radicles in senescence and wounding, New Phytol. 105 (1987) 317 – 344.
[26] G.E. Lester, E. Stein, Plasma membrane physicochemi-cal changes during maturation and postharvest storage of muskmelon fruit, J. Am. Soc. Hort. Sci. 118 (1993) 223 – 227.