Summary Utilization efficiency (LUE) of lightflecks by leaves increases with decreasing duration of the lightfleck, and depends on photosynthetic induction. Sun and shade leaves differ with respect to photosynthetic induction. Shade leaves may become fully induced by a series of light pulses, whereas photosynthetic induction of leaves from partial shade or full sun depends on continuous light. Additionally, shade leaves maintain a higher induction state over longer periods in dim light or darkness than sun leaves. Both features are advanta-geous to shade leaves in a highly dynamic light environment.
We determined whether pioneer plants and late-successional species differ in photosynthetic induction dynamics and LUE during the establishment phase when both plant types are growing in the shade of the understory. We also determined the effects of shade acclimation and successional position of spe-cies on photosynthetic induction and LUE. Results from tem-perate and tropical rain forests indicate a trade-off between leaf acclimation to shade and the successional position of species. Light acclimation is important, but in deep shade, late-succes-sional species maintain a higher induction state over longer periods than pioneer species.
Keywords: CO2 assimilation, Costa Rica, European beech forest, forest gap, India, photosynthetic induction, succes-sional position, sunfleck, tropical rain forest, understory, West-ern Ghats.
Introduction
Most forest trees start life in the understory, either awaiting gap formation as ‘‘oskars’’ (Silvertown 1987) or slowly but gradu-ally growing toward the canopy. During this phase of their life cycle, forest trees endure predominant shade more or less frequently interrupted by light- or sunflecks. We have adopted the definition of sunflecks proposed by Pearcy et al. (1994) as ‘‘fast excursions of high light above a dim light background.’’
We reserve the term lightfleck to the use of artificial light or a high intensity light pulse originating from indirect light. Sun-flecks and lightSun-flecks last from seconds up to (rarely) minutes (Chazdon and Fetcher 1984, Pfitsch and Pearcy 1989). In a variety of forests, sunflecks contribute between 30 and 80% to total daily photon flux, and it is likely that a large fraction of carbon gain is attributable to sunflecks.
Highly dynamic light conditions appear to be more frequent than conditions allowing for steady-state leaf gas exchange, not only in the understory but also in the sun-crown, because of effects of cloud cover, movement of leaves in wind having impacts on leaf temperature (Roden and Pearcy 1993), and movement of canopies. However, most of the modeling ap-proaches (e.g., Küppers and Schulze 1985, Caldwell et al. 1986) used in scaling from leaves to region (Ehleringer and Field 1993) are based on information obtained from steady-state conditions that clearly deviate from natural conditions (Pearcy et al. 1994). Only a few approaches model the re-sponses of leaf gas exchange to dynamic light conditions (Iino et al. 1985, Kirschbaum et al. 1988, Gross et al. 1991).
The first lightflecks after darkness or a prolonged dim light phase may not be fully utilized because photosynthetic carbon gain by a leaf is limited by low photosynthetic induction. This limitation is gradually removed during subsequent lightflecks (Chazdon and Pearcy 1986a, Schneider et al. 1991) so that lightflecks that occur later are utilized more efficiently (Chazdon and Pearcy 1986b, Küppers and Schneider 1993). Thus, the capacity of a leaf to assimilate carbon attributable to a sunfleck depends strongly on the rates at which photosyn-thetic induction is gained and lost. Although it is known that partial-shade and shade leaves differ in their response dynam-ics to sunflecks (Küppers and Schneider 1993), little is known about photosynthetic induction dynamics in a forest under-story or how efficiently co-occurring species utilize the highly dynamic light regime.
Effects of light environment and successional status on lightfleck use
by understory trees of temperate and tropical forests
MANFRED KÜPPERS,
1,2HANS TIMM,
1FRANK ORTH,
1JENS STEGEMANN,
1ROBERT
STÖBER
1, HANS SCHNEIDER,
1KAILASH PALIWAL,
3K. S. T. K. KARUNAICHAMY
3and
RODOLFO ORTIZ
41
Institut für Botanik, Technische Hochschule Darmstadt, Schnittspahnstrasse 10, D-64287 Darmstadt, Germany
2
Present address: Institut für Geobotanik und Botanischer Garten, Martin-Luther-Universität, Neuwerk 21, D-06108 Halle (Saale), Germany
3
School of Biological Sciences, Madurai Kamaraj University, Madurai 625 021, India
4
Escuela de Biologia, Universidad de Costa Rica, San José, Costa Rica
Received March 2, 1995
We examined leaf gas exchange in a pioneer species and a late-successional species that were co-occurring in the under-story and in an originally large but now mostly closed gap. We also compared leaf gas exchange of two series of species of different successional positions along gradients of light in which (a) pioneer species were growing at higher irradiances than successional species, and (b) pioneer species were grow-ing in similar or deeper shade than successional species. In addition, we compared leaf gas exchange of a series of species of different successional positions in which all plants origi-nated in the light regime of a refilled gap but were transplanted to deep shade so that all species had similarly shade-accli-mated leaves.
Study sites and plant materials
Tropical rain forest in Costa Rica
Experiments 1 and 2 were conducted from January to March 1994 in a primary tropical rain forest at the Reserva Forestal de San Ramon, 40 km NNW of San Ramon at 10°18′ N, 84°37′ W and 895 m above sea level. The locality is affected by NE winds and has a mean annual precipitation of 4460 mm and a mean annual temperature of 21 °C. It is characterized by tropical temperatures, hyperhumidity and a relatively dry pe-riod from January to March when monthly rainfall is about 120 mm.
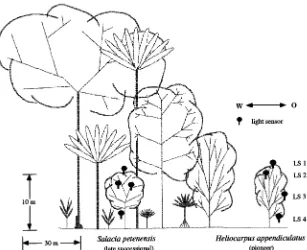
For Experiment 1, detailed measurements of leaf gas ex-change were made on three trees of the late-successional species Salacia petenensis Lundell (Hippocrataceae) growing in the understory. Each tree was about 2.5 m tall with a basal
stem diameter of 2 to 3 cm. The light gradient in the canopy was measured on an approximately 10-m tall S. petenensis tree (25 cm diameter at 1 m above the ground) (Figure 1). Compa-rable measurements were also made on two 3--4-year-old trees of the pioneer species Heliocarpus appendiculatus Turcz. (Tiliaceae) growing in a gap, each 10 m tall (Timm 1994, Stegemann 1994).
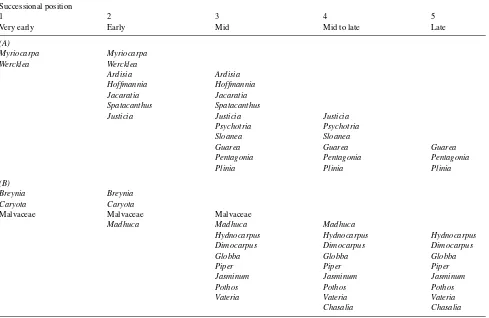
For an analysis of the effects of light regime versus sional position (Experiment 2), we studied a series of succes-sional species growing in their natural sites (Table 1A, for details see Orth 1995), where pioneer species were growing at higher irradiances than late-successional species.
Tropical rain forest in the Western Ghats, India
Experiment 3 was performed in a small plot of a primary tropical rain forest at the southern end of the Western Ghats, India, near the Kodayar Power Station (77°25′ E, 8°25′ N) at 330 m above sea level. Annual rainfall at the site is 2274 mm and mean annual temperature is 27.4 °C. The climate is char-acterized by a relatively dry period from December through March when monthly rainfall is about 30 to 80 mm. Measure-ments were performed in the rainy season in October 1993.
Experiment 3 was designed to determine the effect of small light gradients on leaf gas exchange of a series of successional species where pioneer species were growing in deeper shade than late-successional species. Measurements were made on early-, mid- and late-successional species (see Table 1B) grow-ing in a small, almost refilled, 5 to 10 m wide gap that received direct sunlight only in sunflecks. A diagramatic map of this gap showing the positions of the individual trees and the
projected light classes is shown in Figure 2. Light classes were estimated by eye (cf. Horn 1971), where light class I is com-parable to an intermediate of Figures 3C and 3D, light class II is an intermediate of Figures 3A and 3B, light class II--III is comparable to Figure 3A, light class III is comparable to Figure 3G, and light class III--IV is comparable to Figure 3F.
Temperate European beech forest in central Europe
In late summer 1991, eight co-occurring species (see Table 2 and Paliwal et al. 1994) in the seedling or sapling stage were dug out from the understory of a refilled gap in a central European beech forest near the Botany Institute, Darmstadt (Table 2), potted in their original soil and transferred to a greenhouse. The plants were cultivated in a greenhouse in a 12-h photoperiod providing approximately 4% of natural day-light at day/night air temperatures of 24/14 °C and a dew point of 20/10 °C. The plants were watered to field capacity every
second day. Under these conditions, all plants produced several new leaves that were completely acclimated to deep shade. Only these newly grown leaves were taken for leaf gas ex-change measurements for Experiment 4.
Classification of successional species
Species were classified into five successional categories (Ta-bles 1 and 2) on the basis of literature reports (Oberdorfer 1979, Janzen 1983, Küppers 1984, 1992, Oberbauer and Strain 1984, Oberbauer and Donnelly 1986, Pascal 1988) and our own observations. Most of the species occur across a range of successional states. When we plotted these categories on a linear scale (see Figures 8A and 9), we obtained a transforma-tion comparable to logarithmic normalizatransforma-tion of the true time of successional development, hence the abscissa in these fig-ures may be treated as a quasi-logarithmic scale.
Table 1. (A) Successional positions of selected species from the understory and refilling gaps of a tropical rain forest in Costa Rica. Species are:
Ardisia brenesii Standley (Myrsinaceae), Guarea glabra Vahl (Meliaceae), Hoffmannia dotae Standley (Rubiaceae), Jacaratia dolichaula
Woodson (Caricaceae), Justicia crenata Durkee (Acanthaceae), Myriocarpa longipes Liebmann (Urticaceae), Pentagonia costaricensis Burger & Taylor (Rubiaceae), Plinia salticola Mc Vaugh (Myrtaceae), Psychotria graciliflora Oersted (Rubiaceae), Sloanea faginea Standley (Elaeocar-paceae), Spatacanthus hoffmannii Lindau (Acanthaceae), and Wercklea insignis Pittier & Standley (Malvaceae). Allopulus psilospermus Radlkofer (Sapindaceae), Xylosma spp. (Flacourtiaceae) and the epiphyte Blakea gracilis Hemsley (Melastomataceae) are not included here but are included in the light classes of Table 4A. (B) Successional positions of selected species from the gap indicated in Figure 2, growing in the understory of a tropical rain forest of the Western Ghats in southern India. Species are: Breynia retusa Alston (Euphorbiaceae), Caryotaurens L. (Arecaceae),
Chasalia ophioxyloides (Wall.) Craib (Rubiaceae), Dimocarpus longan Lour var. longan (Sapindaceae), Globba orixensis Roxb. (Zingiberaceae),
Hydnocarpus pentandra (Buch. & Ham) Oken (Flacourtiaceae), Jasminum azoricum L. (Oleaceae), Madhuca nerifolia (Moon) Lam. (Sapotaceae),
Piperargyrophyllum Miquel (Piperaceae), Pothosscandens L. (Araceae), and Vateria indica L. (Dipterocarpaceae), as well as unknown species.
Successional position
1 2 3 4 5
Very early Early Mid Mid to late Late
(A)
Myriocarpa Myriocarpa
Wercklea Wercklea
Ardisia Ardisia
Hoffmannia Hoffmannia
Jacaratia Jacaratia
Spatacanthus Spatacanthus
Justicia Justicia Justicia
Psychotria Psychotria
Sloanea Sloanea
Guarea Guarea Guarea
Pentagonia Pentagonia Pentagonia
Plinia Plinia Plinia
(B)
Breynia Breynia
Caryota Caryota
Malvaceae Malvaceae Malvaceae
Madhuca Madhuca Madhuca
Hydnocarpus Hydnocarpus Hydnocarpus
Dimocarpus Dimocarpus Dimocarpus
Globba Globba Globba
Piper Piper Piper
Jasminum Jasminum Jasminum
Pothos Pothos Pothos
Vateria Vateria Vateria
Methods
Leaf gas exchange of the plants listed in Table 2 was measured as described by Küppers et al. (1987, 1993). All other meas-urements were performed in the field on uppermost fully expanded leaves of an individual plant with a temperature- and humidity-controlled CO2 porometer (CQP 130 and PMK-10,
modified for fast responses to CO2, WALZ, Effeltrich,
Ger-many; CO2/H2O gas analyzer, LI-6262, Li-Cor Inc., Lincoln,
NE; for details see Timm 1994). Artificial light was provided by Philips projection lamps (Type 13117). Photon irradiance was varied either by varying the distance between leaf and lamp or by using gray filters (Strand Lighting, Braunschweig, Germany). We followed the experimental procedures de-scribed by Schneider et al. (1993), Küppers and Schneider (1993) and Paliwal et al. (1994). All measurements were made between 0730 and 1830 h to minimize any diurnal change in leaf sensitivity to light gradients.
We characterized the light environment of the canopies by means of light sensors (Hamamatsu G1118 GaAsP photo-diodes, Bridgewater, NJ) (Pontailler 1990). We adopted the light fleck utilization efficiency (LUE) definition used by Chazdon and Pearcy (1986b).
Nitrogen and phosphate contents of leaves and carbon
dis-crimination values were determined on the same leaves used for the gas exchange measurements. Leaf samples were oven dried and digested with concentrated H2SO4 for 2 h at 400 °C.
Nitrogen and phosphorus in the extract were determined with an autoanalyzer (Technicon Instruments Corp., Tarrytown, NY). Carbon discrimination was measured by the technique described by Osmond et al. (1975).
Results and discussion
Characterization of light fluctuations in the understory and in a gap of a tropical rain forest
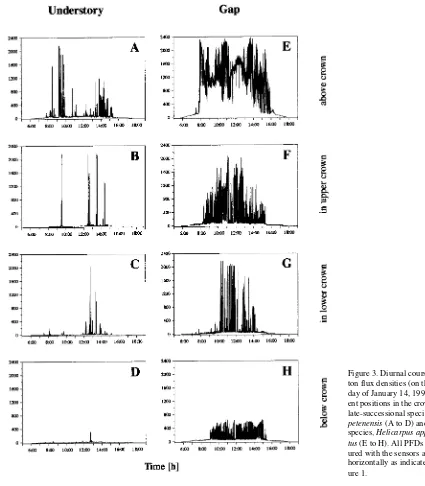
The late-successional species S. petenensis was growing in the understory and the pioneer species H. appendiculatus was growing at the edge of a 900 m2 wide gap. In the understory
above the canopy of the late-successional S. petenensis tree (Figure 1), long-lasting sunflecks (direct sunlight) were rare, but short sunflecks were frequent at certain times of day (Figure 3A), and they appeared in clusters (cf. Pearcy 1988). The number of sunflecks was significantly reduced inside the upper canopy compared with above the canopy (Figures 3B and 3C). No sunflecks were found below the canopy in deep shade (Figure 3D), but there were highly dynamic light fluc-tuations originating from indirect, very low intensity light (not shown but visible at a different scale of resolution). However, total daily light below the canopy could not support a positive daily carbon balance (cf. Timm 1994), which explains why no leaves were present.
Light fluctuations were more pronounced and the number of lightflecks was higher in the gap than in the understory (Fig-ures 3E and 3F). These fluctuations were caused by cloud cover, by movement of leaves in the wind, and by continuous movement of whole canopies in the neighborhood of the plant under investigation.
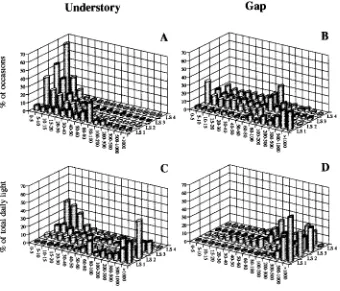
An analysis of sunflecks and lightflecks in terms of their daily frequencies, maximum photon flux density (PFD) and contribution to total daily light showed that very low PFDs prevailed most of the time in the understory (Figure 4A). In contrast, intermediate and high PFDs dominated in the gap; however, low PFDs were found in the leafy part of the gap (light sensor LS2), whereas lateral light penetrating the space below the leafy canopy resulted in higher light intensities of 60 to 80 µmol m−2 s−1 in the lower crown (Figure 4B, light sensor
LS3) and below it (Figure 4B, light sensor LS4).
Despite these variations in PFDs, high PFDs contributed most to the total daily light flux in both the understory and the gap (Figures 4C and 4D), demonstrating the importance of sunflecks in both the gap and the understory. Although very few sunflecks reached PFDs above 1000 µmol m−2 s−1 in the
upper crown of Salacia (Figures 3B and 4A, LS2), sunflecks contributed 45% of the daily light dose (Figure 4C, LS2). Only in deep shade, where most plants have no leaves (LS4 in Figures 4A and 4C and Figure 3D), did sunflecks make little contribution to the total daily light flux.
Both the frequencies and relative contributions of different PFDs and the duration of those PFDs that contributed most to the total daily light flux were important for leaf gas exchange.
Table 2. Successional positions of selected species from the understory of a temperate European beech forest near Darmstadt, Germany. Species are: Clematis vitalba L. (Ranunculaceae), Cornusmas L. (Cornaceae), Fagussylvatica L. (Fagaceae), Hederahelix L. (Araliaceae), Prunus avium
L.subspp. avium (Rosaceae), Robinia pseudoacacia L. (Fabaceae), introduced from North America, Rubusfruticosus agg. (Rosaceae), and Viola reichenbachiana Jord. (Violaceae).
Successional position
1 2 3 4 5
Very early Early Mid Mid to late Late
Rubus Rubus
Robinia Robinia Robinia Robinia
Cornus Cornus
Clematis Clematis
Prunus Prunus Prunus
Hedera Hedera Hedera Hedera
Viola Viola Viola
Fagus Fagus
From Figure 5, it is evident that for those sunflecks contribut-ing the most to the total daily light flux, their duration de-creased with increasing shade. Below the crown, lightflecks lasting 30 to 60 s were significantly more important than lightflecks of other durations (Figure 5C), even though short lightflecks (1 to 5 s) are the most frequent (Pfitsch and Pearcy 1989). In the upper crown, lightflecks of intermediate duration were important but lightflecks lasting 60 to 300 s contributed even more to the total daily light flux. In our experiments, we applied 30-s lightflecks at PFDs just saturating carbon uptake.
Typical leaf responses to lightfleck regimes
Lightflecks of 1 or 5 s duration at a PFD just saturating for steady-state carbon uptake (1000 µmol m−2 s−1) resulted in a
large carbon gain (Figures 6A and 6B). Such lightflecks were frequent in the whole crown of the pioneer species (Figures 3F and 3G), and thus contributed significantly to total daily carb-on gain. However, if the lightfleck is of short duraticarb-on, most of the carbon will be assimilated after the lightfleck, i.e., post-il-luminative lightfleck CO2 fixation occurs. The longer the
light-fleck, the more carbon is fixed during the light phase (Figure 6C) but at a lower light use efficiency (Pearcy 1988, Küppers and Schneider 1993). At the end of the post-illuminative carb-on fixaticarb-on phase, a post-illuminative CO2 burst resulting from
photorespiration was detected (Figure 6C) (see Vines et al. 1983). A leaf that has been partially induced can be further induced by a subsequent lightfleck, as indicated by the steady increase in carbon gain during the light phase after the initial
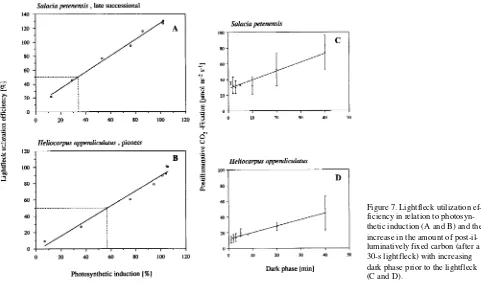
induction step (Figure 6D). Even in lightflecks as short as 1 s, a partial induction may take place (Küppers and Schneider 1993). The rate of induction differed between sun and shade leaves. The shade leaves of the late-successional S. petenensis became fully induced in response to pulses of light, whereas half-shade and sun leaves of the pioneer H. appendiculatis only became fully induced when exposed to continuous satu-rating light (cf. Küppers and Schneider 1993). Additionally, shade leaves maintained a higher induction state longer at lower PFDs than sun leaves, and therefore, shade leaves exhib-ited a higher LUE than sun leaves (Figure 7).
The relative contribution of post-illuminative CO2 fixation
to total carbon gain attributable to a lightfleck increased with decreasing duration of the lightfleck, and it also increased with decreasing induction state (cf. Figures 7C and 7D). Post-illu-minative carbon gain contributed more to the shade leaves of the late-successional Salacia than to the sun leaves of the pioneer Heliocarpus and may be an effect of either shade acclimation or successional status of the plants.
Depending on the time of measurements, we observed clear differences in leaf responses to transient light conditions with fastest rates of induction and slowest rates of induction loss at night and minimum variability during the day (Table 3).
Effect of partial shade gradients versus successional ranges of species
Experiment 2 was undertaken to determine whether the light environment contributes more to leaf responses than does the
successional position of species. Based on data from the tropi-cal rain forest in Costa Rica, where pioneer species were growing in the open and late-successional species were grow-ing in half to full shade (Table 4A), we found a significant negative correlation between light class and successional posi-tion of the species. The negative correlaposi-tion arises because, as a result of light acclimation, photosynthetic capacity was highly positively correlated with the light class in which the leaves were grown (Table 4B). Except for the fraction of
Figure 5. Relative contribution of lightflecks of certain durations to the total daily light dose in the understory (A) above the crown, (B) in the upper crown, and (C) below the crown of a Salaciapetenensis sapling.
Figure 6. Responses of CO2 assimilation to lightflecks differing in
duration at saturating PFD (1800 µmol m−2 s−1). (A to C) Heliocarpus
post-illuminative carbon gain, none of the other response pa-rameters of CO2 assimilation was correlated with successional
position. This lack of correlation also appears to be a result of light acclimation rather than an effect of successional position. The fraction of post-illuminative carbon gain declined signifi-cantly with increasing light class (cf. Figures 7C and 7D). Thus, under natural conditions, the correlations between pho-tosynthetic parameters (both steady-state and dynamic) and the light class in which the plant grew were higher than the correlations between photosynthetic parameters and the suc-cessional position of the species.
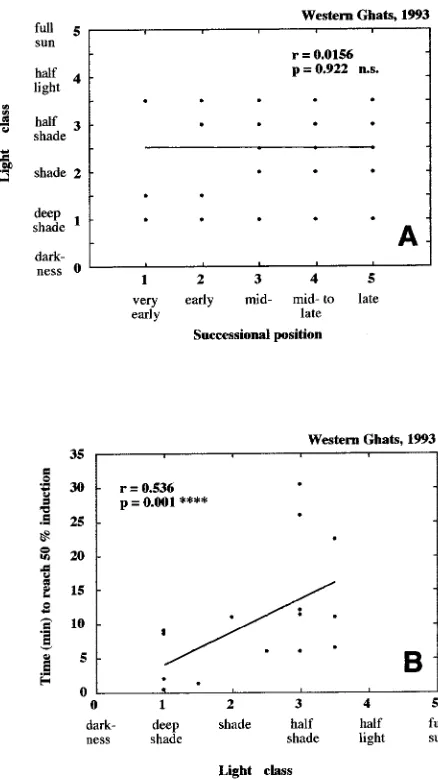
In a second data set, which originated from an almost
re-filled gap in the tropical rain forest of the Western Ghats, India, where pioneer species were growing in similar or deeper shade than late-successional species, we found that the light class of an individual’s site did not correlate with the individual’s successional position (Figure 8A). In this refilled gap, the light environment of a plant significantly affected its rate of photo-synthetic induction (Figure 8B), whereas successional position had no influence (Table 4B). Light acclimation clearly affected photosynthetic capacity (per unit of leaf area), the rate of photosynthetic induction and the minimal induction state, which was highest in the shade plants (significant negative slope, Table 4B).
Figure 7. Lightfleck utilization ef-ficiency in relation to photosyn-thetic induction (A and B) and the increase in the amount of post-il-luminatively fixed carbon (after a 30-s lightfleck) with increasing dark phase prior to the lightfleck (C and D).
Table 3. Photosynthetic induction and CO2 assimilation responses of two understory species at the Western Ghats site to transient light at different
times of the day and night.
Pothos scandens Hydnocarpus pentandra
Time (h) 0530 1240 2150 0830 1630 0120
Date (October 1993) 27 26 26 26 26 27
Time to reach 50% induction (min) 6 50 8 22 21 9
Time to reach 100% induction (min) 24 70 26 49 38 27
Fully induced leaves
Photosynthetic capacity 0.4 0.9 0.7 2.9 1.9
at ambient CO2 (µmol m−2 s−1)
Total CO2 gain attributable to 30-s 73 61 93 109 -- 107
lightfleck (µmol m−2 lightfleck−1)
LUE (%) attributable to 1-s lightfleck 120 124 122 134 138 195
LUE (%) attributable to 10-s lightfleck 112 111 117 119 112 120
LUE(%) attributable to 30-s lightfleck 103 108 115 108 72 (?) 112
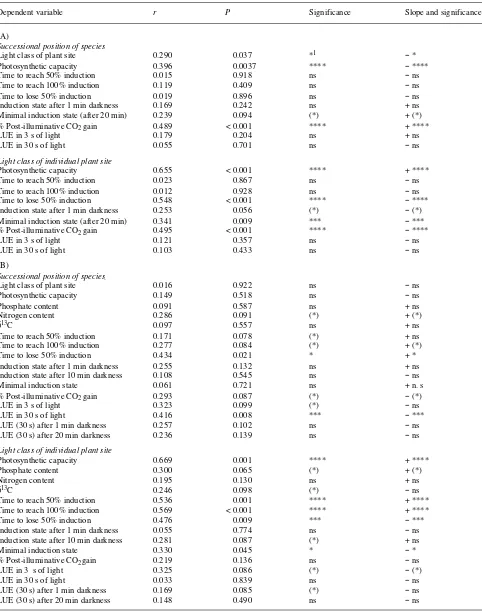
Table 4. (A) Correlations of parameters of dynamic photosynthesis with successional positions of species or the light classes at the individual plant sites for species in the understory of a tropical rain forest in Costa Rica. LUE = Lightfleck utilization efficiency, LQY = lightfleck quantum yield, and WUE = water use efficiency. (B) Correlations of parameters of dynamic photosynthesis with successional positions of species or the light classes at the individual plant sites (see Figure 2) for species in the understory of a tropical rain forest in the Western Ghats, India.
Dependent variable r P Significance Slope and significance
(A)
Successional position of species
Light class of plant site 0.290 0.037 *1 − *
Photosynthetic capacity 0.396 0.0037 **** − ****
Time to reach 50% induction 0.015 0.918 ns − ns
Time to reach 100% induction 0.119 0.409 ns − ns
Time to lose 50% induction 0.019 0.896 ns − ns
Induction state after 1 min darkness 0.169 0.242 ns + ns
Minimal induction state (after 20 min) 0.239 0.094 (*) + (*)
% Post-illuminative CO2 gain 0.489 < 0.001 **** + ****
LUE in 3 s of light 0.179 0.204 ns + ns
LUE in 30 s of light 0.055 0.701 ns − ns
Light class of individual plant site
Photosynthetic capacity 0.655 < 0.001 **** + ****
Time to reach 50% induction 0.023 0.867 ns − ns
Time to reach 100% induction 0.012 0.928 ns − ns
Time to lose 50% induction 0.548 < 0.001 **** − ****
Induction state after 1 min darkness 0.253 0.056 (*) − (*)
Minimal induction state (after 20 min) 0.341 0.009 *** − ***
% Post-illuminative CO2 gain 0.495 < 0.001 **** − ****
LUE in 3 s of light 0.121 0.357 ns − ns
LUE in 30 s of light 0.103 0.433 ns − ns
(B)
Successional position of species
Light class of plant site 0.016 0.922 ns − ns
Photosynthetic capacity 0.149 0.518 ns − ns
Phosphate content 0.091 0.587 ns + ns
Nitrogen content 0.286 0.091 (*) + (*)
δ13C 0.097 0.557 ns + ns
Time to reach 50% induction 0.171 0.078 (*) + ns
Time to reach 100% induction 0.277 0.084 (*) + (*)
Time to lose 50% induction 0.434 0.021 * + *
Induction state after 1 min darkness 0.255 0.132 ns + ns
Induction state after 10 min darkness 0.108 0.545 ns − ns
Minimal induction state 0.061 0.721 ns + n. s
% Post-illuminative CO2 gain 0.293 0.087 (*) − (*)
LUE in 3 s of light 0.323 0.099 (*) − ns
LUE in 30 s of light 0.416 0.008 *** − ***
LUE (30 s) after 1 min darkness 0.257 0.102 ns − ns
LUE (30 s) after 20 min darkness 0.236 0.139 ns − ns
Light class of individual plant site
Photosynthetic capacity 0.669 0.001 **** + ****
Phosphate content 0.300 0.065 (*) + (*)
Nitrogen content 0.195 0.130 ns + ns
δ13C 0.246 0.098 (*) − ns
Time to reach 50% induction 0.536 0.001 **** + ****
Time to reach 100% induction 0.569 < 0.001 **** + ****
Time to lose 50% induction 0.476 0.009 *** − ***
Induction state after 1 min darkness 0.055 0.774 ns − ns
Induction state after 10 min darkness 0.281 0.087 (*) + ns
Minimal induction state 0.330 0.045 * − *
% Post-illuminative CO2 gain 0.219 0.136 ns − ns
LUE in 3 s of light 0.325 0.086 (*) − (*)
LUE in 30 s of light 0.033 0.839 ns − ns
LUE (30 s) after 1 min darkness 0.169 0.085 (*) − ns
LUE (30 s) after 20 min darkness 0.148 0.490 ns − ns
In the almost refilled gap at Western Ghats, there were only weak correlations between successional position of the species and the dynamic response parameters studied (Table 4B). Thus, we observed significant correlations between succes-sional position and nitrogen content, time to reach 100% induction, % post-illuminative CO2 gain, and LUE in response
to 30 s of light (Table 4B). All of these correlations, which contrast with those normally obtained, are a consequence of light acclimation in the reverse situation, i.e., the pioneer species were growing in similar or deeper shade than the late-successional species. However, in agreement with the observation of Poorter and Oberbauer (1993) and our observa-tions at the Costa Rica site, the late-successional species main-tained a higher induction status longer than the pioneer species (Table 4B). We also observed highly significant positive corre-lations between photosynthetic capacity and phosphate or ni-trogen content (P < 0.0005 and P = 0.017, respectively), and a negative correlation between photosynthetic capacity and δ13C
values (P = 0.001; data not shown).
Effect of successional range of species originating from the same understory and cultivated in identical shade
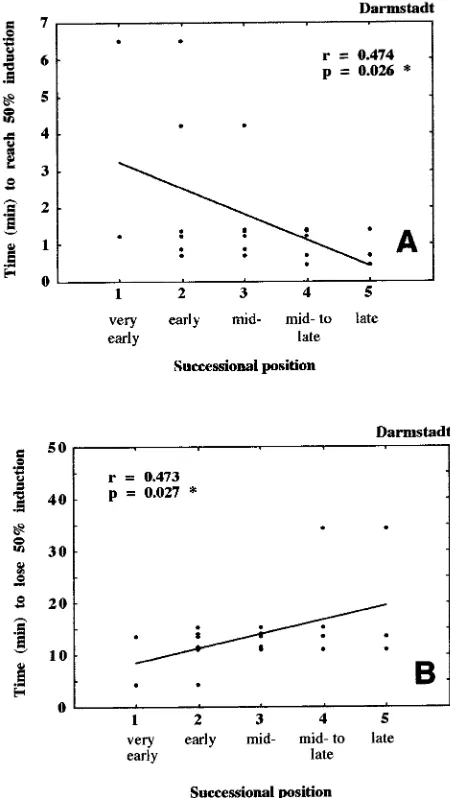
Results from Experiments 1--3 clearly indicate the importance of the light regime relative to the successional position of the species on leaf responses. To determine whether successional position has any detectable influence on leaf gas exchange, we selected plants growing in the understory of a refilled gap in a beech forest, potted the plants in their original soil and main-tained them in shade in a greenhouse. We then made measure-ments on the newly grown shade leaves. There was no correlation between photosynthetic capacity, minimal induc-tion state and successional posiinduc-tion of species. All other pa-rameters studied were significantly or weakly significantly correlated (Table 5). Thus, rates of induction in high PFDs were faster in late-successional plants than in early-succes-sional plants (Figure 9A, significant negative slope, and Table 5), whereas rates of induction loss in low PFDs were slower in late-successional plants than in early-successional plants (Fig-ure 9B, significant positive slope). The minimum saturating light required for steady-state conditions was lower and light-fleck quantum yield, LQY (comparable to LUE; see Küppers
Figure 8. (A) Correlation between the light classes of individual plants of several species growing in the gap depicted in Figure 2 and their successional positions. (No significant correlation (ns) was found.) (B) Correlation between the time necessary for leaves to become half induced (by a step from darkness to continuous saturating light) and the light classes of individual plants of several species growing in the gap depicted in Figure 2.
Table 5. Correlations of parameters of dynamic photosynthesis with successional positions of species for species from the understory of a temperate European beech forest. LQY = Lightfleck quantum yield, and WUE = water use efficiency.
Dependent variable r P Significance Slope and significance
Successional position of species
Photosynthetic capacity 0.083 0.713 ns1 − ns
Time to reach 50% induction 0.474 0.026 * − (*)
Time to reach 100% induction 0.378 0.076 (*) − (*)
Time to lose 50% induction 0.473 0.027 * + *
Induction state after 1 h of dim light 0.243 0.142 ns + ns
Minimal saturating light 0.522 0.013 ** − **
Total CO2 gain (30-s lightfleck) 0.165 0.085 (*) − ns
LQY (30-s lightfleck) 0.280 0.085 (*) + (*)
WUE (30-s lightfleck) 0.442 0.041 * − **
and Schneider 1993), higher in the late-successional species than in early-successional species, whereas water use effi-ciency attributable to lightflecks was lower in late-succes-sional species than in early-succeslate-succes-sional species (Table 5).
Late-successional species maintained more shade leaf acteristics and pioneer species maintained more sun leaf char-acteristics when grown in deep shade. However, the correla-tions were not as significant as those found for the effects of light regime on the leaf response patterns to lightflecks.
Based on our results from temperate and tropical rain for-ests, we conclude that there is a trade-off between leaf accli-mation to shade and the successional position of species. Light acclimation is important, but in deep shade, late-successional species maintain a higher induction state over longer periods than pioneer species, and this marginal difference will
eventu-ally lead to advantages in carbon gain (cf. Cowan 1982). Poorter and Oberbauer (1993) came to a similar conclusion based on transplantation experiments with a tropical pioneer species and a late-successional species.
Acknowledgments
We thank the Institut Français de Pondichéry, Madras, for help in identifying species from the understory of the Western Ghats and Prof. Dr. Hubert Ziegler, Munich, for the carbon discrimination analyses. We also gratefully acknowledge support from the Deutsche For-schungsgemeinschaft to M.K. and K.P. and a fellowship by the Studi-enstiftung des Deutschen Volkes to H.S. We very much appreciate the help provided by Prof. R.W. Pearcy (UC Davis) while on a visit to Darmstadt.
References
Caldwell, M.M., H.P. Meister, J.D. Tenhunen and O.L. Lange. 1986. Canopy structure, light microclimate and leaf gas exchange of
Quercus coccifera L. in a Portuguese macchia: measurements in different canopy layers and simulations with a canopy model. Trees 1:25--41.
Chazdon, R.L. and N. Fetcher. 1984. Photosynthetic light environ-ments in a lowland tropical forest in Costa Rica. J. Ecol. 72:553--564.
Chazdon, R.L. and R.W. Pearcy. 1986a. Photosynthetic responses to light variation in rain forest species. I. Induction under constant and fluctuating light conditions. Oecologia 69:517--523.
Chazdon, R.L. and R.W. Pearcy. 1986b. Photosynthetic responses to light variation in rain forest species. II. Carbon gain and photosyn-thetic efficiency during lightflecks. Oecologia 69:524--531. Cowan, I.R. 1982. Regulation of water use in relation to carbon gain
in higher plants. In Physiological Plant Ecology II. Water Relations and Carbon Assimilation. Eds. O.L. Lange, P.S. Nobel, C.B. Os-mond and H. Ziegler. Encyclopedia of Plant Physiology, Vol. 12B, Springer-Verlag, Berlin, pp 589--613.
Ehleringer, J.R. and C.B. Field. 1993. Scaling physiological proc-esses: leaf to globe. Academic Press, San Diego, New York, 388 p. Gross, L.J., M.U.F. Kirschbaum and R.W. Rearcy. 1991. A dynamic model of photosynthesis in varying light taking account of stomatal conductance, Calvin cycle intermediates, photorespiration and Ru-bisco activation. Plant Cell Environ. 14:881--893.
Horn, H.S. 1971. The adaptive geometry of trees. Princeton University Press, Princeton, NJ, pp 144.
Iino, M., T. Ogawa and E. Zeiger. 1985. Kinetic properties of the blue-light response of stomata. Proc. Natl. Acad. Sci. 82:8019--8023.
Janzen, D.H. 1983. Costa Rican natural history. The University of Chicago Press, Chicago, London, 816 p.
Kirschbaum, M.U.F., L.J. Gross and R.W. Pearcy. 1988. Observed and modelled stomatal responses to a dynamic light environment in the shade plant Alocasia macrorrhiza. Plant Cell Environ. 11:111--121. Küppers, M. 1984. Kohlenstoffhaushalt, Wasserhaushalt, Wachstum und Wuchsform von Holzgewächsen im Konkurrenzgefüge eines Heckenstandortes. In Die pflanzenökologische Bedeutung und Bewertung von Hecken. Eds. E.-D. Schulze, A. Reif and M. Küp-pers. Akademie für Naturschutz und Landschaftspflege, Suppl. 3, Part 1, Laufen/Salzach, Germany, pp 10--102.
Küppers, M. 1992. Changes in plant ecophysiology across a central European hedgerow ecotone. In Landscape Boundaries----Conse-quences for Biotic Diversity and Ecological Flows. Eds. A.J. Han-sen and F. di Castri. Ecological Studies 92, Springer-Verlag, Berlin, pp 285--303.
Küppers, M. and H. Schneider. 1993. Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: effects of fleck length and leaf temperature in leaves grown in deep and partial shade. Trees 7:160--168.
Küppers, M. and E.-D. Schulze. 1985. An empirical model of net photosynthesis and leaf conductance for the simulation of diurnal courses of CO2 and H2O exchange. Aust. J. Plant Physiol.
12:513--526.
Küppers, M., H. Schneider and A.G. Swan. 1993. Leaf gas exchange of beech (Fagus sylvatica L.) seedlings in lightflecks: a system for measuring rapid changes in CO2 partial pressures. Photosynth. Res.
35:299--304.
Küppers, M., A.G. Swan, D. Tompkins, W.C.L. Gabriel, B.I.L. Küp-pers and S. Linder. 1987. A field portable system for the measure-ment of gas exchange of leaves under natural and controlled conditions. Plant Cell Environ. 10:425--435.
Oberbauer, S.F. and M.A. Donnelly. 1986. Growth analysis and suc-cessional status of Costa Rican rain forest trees. New Phytol 104:517--521.
Oberbauer, S.F. and B.R. Strain. 1984. Photosynthesis and succes-sional status of Costa Rican rain forest trees. Photosynth. Res. 5:227--232.
Oberdorfer, E. 1979. Pflanzensoziologische Exkursionsflora. Eugen Ulmer, Stuttgart, 997 p.
Orth, F. 1995. Der Einfluß des Lichtklimas am natürlichen Standort im tropischen Regenwald von Costa Rica und der sukzessionalen Stel-lung der Arten auf das Photosynthese-Verhalten in Lichtflecken. Diplomarbeit, Tech. Univ. Darmstadt, 108 p.
Osmond, C.B., H. Ziegler, W. Stichler and P. Trimborn. 1975. Carbon isotope discrimination in alpine succulent plants supposed to be capable of crassulacean acid metabolism (CAM). Oecologia 18:209--217.
Paliwal, K., M. Küppers and H. Schneider. 1994. Leaf gas exchange in lightflecks of plants of different successional range in the under-story of a central European beech forest. Curr. Sci. 67:29--33. Pascal, J.P. 1988. Wet evergreen forests of the Western Ghats of
India----ecology, structure, floristic composition and succession. Institut Français de Pondichéry, Sri Aurobindo Ashram Press, Pon-dicherry, India, 345 p.
Pearcy, R.W. 1988. Photosynthetic utilization of lightflecks by under-story plants. Aust. J. Plant Physiol. 15:223--238.
Pearcy, R.W., R.L. Chazdon, L.J. Gross and K.A. Mott. 1994. Photo-synthetic utilization of sunflecks: a temporally patchy resource on a time scale of seconds to minutes. In Exploitation of Environ-mental Heterogeneity by Plants----Ecophysiological Processes Above- and Belowground. Eds. M.M. Caldwell and R.W. Pearcy. Academic Press, San Diego, New York, pp 175--208.
Pfitsch, W.A. and R.W. Pearcy. 1989. Daily carbon gain by Adenocau-lon bicolor (Asteraceae), a redwood forest understory herb, in relation to its light environment. Oecologia 80:465--470.
Pontailler, J.Y. 1990. A cheap quantum sensor using a galium arsenide photodiode. Funct. Ecol. 4:591--596.
Poorter, L. and S.F. Oberbauer. 1993. Photosynthetic induction re-sponses of two rain forest tree species in relation to light environ-ment. Oecologia 96:193--199.
Roden, J.S. and R.W. Pearcy. 1993. The effect of flutter on the temperature of poplar leaves and its implications for carbon gain. Plant Cell Environ. 16:571--577.
Schneider, H., M. Küppers and A.G. Swan. 1991. Nettophotosynthese-und Stomataverhalten der Blätter von Buchenkeimlingen (Fagus sylvatica L.) in Lichtflecken. Verh. Ges. Oekol. 20:967--972. Schneider, H., K. Paliwal and M. Küppers, M. 1993.
Blattgasaus-tausch in Lichtflecken von Jungpflanzen unterschiedlicher sukzes-sionaler Stellung aus dem Unterwuchs eines mitteleuropäischen Buchenwaldes - eine analytische Grundlage für die Ellenbergschen Licht-Zeigerwerte? Verh. Ges. Oekol. 22:439--442.
Silvertown, J.W. 1987. Introduction to plant population ecology. Longman Scientific & Technical, Harlow, England, 229 p. Stegemann, J. 1994. Blattgaswechsel der Pionier-Baumart
Heliocar-pus appendiculatus (Turcz.) unter den dynamischen Lichtverhält-nissen am natürlichen Standort im tropischen Regenwald von Costa Rica. Diplomarbeit, Tech. Univ. Darmstadt, 82 p.
Timm, H.-C. 1994. Blattgasaustausch und Kohlenstoffökonomie einer ‘‘Klimax’’-Baumart am Beispiel von Salacia petenensis (Lundell) im tropischen Regenwald von Costa Rica. Diplomarbeit, Tech. Univ. Darmstadt, 70 p.
Vines, M.H., Z.-P. Tu, A.M. Armitage, S. Chen and C.C. Black. 1983. Environmental responses of the post-lower illumination CO2 burst