Geology, mineral chemistry and formation conditions of calc-silicate
minerals of Astamal Fe-LREE distal skarn deposit, Eastern Azarbaijan
Province, NW Iran
Saeid Baghban
a,⁎
, Mohammad Reza Hosseinzadeh
a, Mohsen Moayyed
a,
Mir Ali Asghar Mokhtari
b, Daniel Gregory
c aDepartment of Earth Science, Faculty of Natural Science, Tabriz University, 5166616471 Tabriz, Iran b
Department of Geology, Faculty of Science, University of Zanjan, 45371-38791 Zanjan, Iran c
ARC Centre of Excellence in Ore Deposits (CODES), School of Physical Sciences, University of Tasmania, Private Bag 79, Hobart, Tasmania 7001, Australia
a b s t r a c t
a r t i c l e
i n f o
Article history: Received 24 April 2014
Received in revised form 13 December 2014 Accepted 18 December 2014
The Astamal Fe-LREE skarn deposit is the largest iron skarn in NW Iran. It is a unique case, as a distal skarn deposit which crops out approximately 600 m from the associated Oligo-Miocene granodioritic pluton. This deposit formed in the south-southwest of the pluton where fractures and faults within the Upper Cretaceous volcano-sedimentary host rocks acted as conduits for the mineralizingfluids. The deposit contains three iron ore bodies: southern, north-ern and eastnorth-ern. The main mineral assemblage within the ore zones is characterized by magnetite, pyrrhotite and pyrite, with lesser quantities of chalcopyrite, hematite, goethite and limonite. The skarn minerals predominantly comprise garnet, epidote, actinolite, calcite, quartz, clinopyroxene and chlorite (in order of abundance). Retrograde alteration is strongly developed in the skarn zone where most of the garnet has been pervasively altered to second-ary minerals (e.g. epidote, calcite and quartz) both in the rims and the cores whereas the majority of the clinopyroxene has been replaced by a hydrous retrograde mineral assemblage (e.g. tremolite, actinolite and chlo-rite). Garnets with andradite–grossular compositions are the dominant mineral in the skarn zone, which are gener-ally isotropic with a narrow compositional range along the growth lines (Adr94.3–64.5Grs21.9–2.7Alm11.1–0.2). These
garnets are Fe-rich and have high Fe/(Fe + Al) ratios (between 0.96 and 0.78). Cu and Ni are enriched in the garnets. This suggests that these elements were enriched in the hydrothermalfluids from which the garnet precipitated. This is supported by the presence of chalcopyrite and Ni-bearing massive magnetite in the study area, which also sug-gests that Cu and Ni were enriched in the late stage ore-bearing hydrothermalfluids. Clinopyroxene with a hedenbergitic composition is generally homogenous and has particularly high Fe/Fe + Mg ratios (between 0.99 and 0.86) and is poor in TiO2, MnO and Cr2O3. High Zn concentrations were also detected in the clinopyroxenes
(up to 1044 ppm), despite an absence of significant Zn mineralization (such as sphalerite) in the district. Therefore, it is believed that the proportion of Zn in the hydrothermalfluids decreased significantly from the time of clinopyroxene formation to the period of the sulfide deposition phase. Allanite and LREE-bearing epidotes are the main LREE bearing minerals in this deposit. The epidote is also Fe-rich with high Fe/(Fe + Al) ratios (between 0.32 and 0.44). Due to a lack of replacement texture between both garnet and clinopyroxene and garnet and actin-olite (which is formed by alteration of clinopyroxene), it is believed that these two minerals have grown simulta-neously and are coexisting minerals. In the Astamal skarn, these minerals can be stable and coexisting at temperatures between 490 and 560 °C and LogƒO2=−16 to−31.
© 2014 Elsevier B.V. All rights reserved.
1. Introduction
The Astamal Fe-LREE skarn deposit is located in the Qara-Dagh– Sabalan metallogenic belt in northwest Iran and is considered to be part of lesser Caucasus in the Alpine–Himalayan orogenic belt. This area has long been of interest to geologists and explorers because of the well developed alteration zones and high prospectivity for mineral-ization. Volcanic activity, numerous intrusions and the influence of as-sociated hydrothermalfluids have led to the formation of a wide variety of deposits.
–
⁎ Corresponding author at: Abrasan Street, Pezeshkan, plaque 1.17, 5156845714 Tabriz, Iran.
E-mail addresses:Saeid.baghban_geomine@yahoo.com,SaeidBaghban@gmail.com (S. Baghban),MR-Hosseinzadeh@tabrizu.ac.ir(M.R. Hosseinzadeh),
Moayyed@Tabrizu.ac.Ir(M. Moayyed),Amokhtari@znu.ac.ir(M.A.A. Mokhtari), ddg@utas.edu.au(D. Gregory).
http://dx.doi.org/10.1016/j.oregeorev.2014.12.016 0169-1368/© 2014 Elsevier B.V. All rights reserved.
Contents lists available atScienceDirect
Ore Geology Reviews
The Qara-Dagh–Sabalan metallogenic belt and Caucasus Mountains formed during the post-collisional stage, after the closure of the Neotethys Ocean. During the Cenozoic era, intense magmatism, with a peak in the Eocene and Oligocene, occurred and the 1500 km2Qara-Dagh batholith
was emplaced. This batholith has been recognized to be responsible for the widespread mineralization in the region (Mehrpartou, 1997; Mokhtari, 2009).
A variety of deposits are associated with this batholith and contem-poraneous intrusive bodies. These include: porphyry copper deposits (Sungun (Calagari, 2003, 2004), Saunajil (Hosseinzadeh, 2008), Haftcheshmeh (Hassanpour et al., 2011) and Masjed-Daghi porphyry copper–gold deposits (Akbarpour, 2005); skarn deposits (Sungun Cop-per (Calagari and Hosseinzadeh, 2006), Mazraeh Copper (Mollai, 1993), Anjerd Copper (Hosseinzadeh, 1999), Avan Copper–Iron (Mokhtari, 2009; Mokhtari et al., 2012), Astamal Iron (Baghban, 2013; Mokhtari and Hosseinzadeh, 2013), Gavdel Iron–Copper (Mahmoudinia, 2013), Kamtal Iron–Copper (Mokhtari et al., 2013), Pahnavar Iron (Mokhtari, 2012)) and epithermal gold deposits (Masjed-Daghi (Mohammadi,
2004), Mivehrood (Jamali et al., 2010), Zaglik (Heydarzadeh, 2007), and Nabijan (Baniadam, 2003)).
Also, various types of mineralization formed within the Qara-Dagh batholith such as: Aniq-Qarachilar gold–copper–molybdenum vein de-posit (Mokhtari et al., 2014), the Agarak and Kadjaran copper– molybde-num deposits (Zvezdov et al., 1993), the Zod epithermal gold deposit (Konstantinov et al., 2010; Kozerenko, 2004) and the Kapan, Alaverdi and Mehmana epithermal polymetallic deposits (Mederer et al., 2014). The Astamal–Nojehmehr alteration zone is one of the main features of this region. It consists of a widespread argillic, propylitic, sericitic and silicic alterations spanning a length of approximately 45 km. The alter-ation zone is also associated with a widespread Cu anomaly.
The Astamal Fe-LREE skarn deposit is one of the most significant deposits in this region. It was discovered two years ago and explora-tion is ongoing. It is the largest and richest iron deposit in northwest-ern Iran (Mokhtari and Hosseinzadeh, 2013) with an estimated 10 Mt resource of magnetite Fe ore with an average grade of approxi-mately 60%.
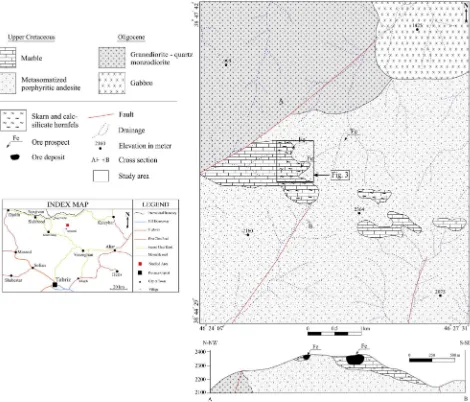
Fig. 2.Geological map of the study area showing the Astamal skarn location and its relationship with Qara-Dagh batholith. Modified afterMokhtari and Hosseinzadeh (2013).
In this paper, local and regional geology, petrographic studies of skarn zones, mineral chemistry and formation conditions of calc-silicate minerals are presented.
2. Geology
2.1. Regional geology
A specific group or formation or unit has not been identified in the Qara-Dagh area, so far. Lithostratigraphically, the oldest rocks are of Upper Cretaceous age and consist offlysch type rocks and mafic to inter-mediate submarine volcanic rocks. Theflysch rocks have been identified over a 30 km strike length and with a width of at least 15 km. They are composed of micritic limestone, sandstone, shale and mudstone. These sedimentary rocks have been folded and the calcareous layers decrease in abundance from west to east. Submarine volcanic activity is characterized by rocks of mafic to intermediate composition (andesite, basaltic andesite and pyroxene andesite) interlayered with the sedi-mentary sequence. The presence ofNummufallutiasp.,Hetrohelicidand Globotruncanasp. in the lower part of this sedimentary unit suggests a Santonian to Maastrichtian age (Mehrpartou, 1997). Rocks of Paleocene age are poorly represented in this region. The presence of red sandstone and microconglomerate layers at the base of this sequence exhibits epeirogenic movements of Laramian phase. Gray sandstone layers with limy interbeds progressively increase up section. The sandstone is overlain by Paleocene submarine andesitic volcanic rocks and felsic tuffs. The Paleocene and Upper Cretaceous rocks are unconformably overlain by Eocene strata. These Eocene rocks are 60 m thick with a
basal conglomerate layer overlain by sandstone. The presence of Nomolitein these layers (Mehrpartou, 1997) indicates a Mid-Eocene age. Upper Eocene facies overlie Mid-Eocene volcano-sedimentary rocks and outcrop south of Astamal village. The Upper Eocene rocks have a submarine andesitic to basaltic composition and form the south-ern and northsouth-ern limbs of the Avansar anticline (Mehrpartou, 1997).
The emplacement of Oligocene aged intrusive bodies played an im-portant role in this region (Fig. 1). The Qara-Dagh batholith is one of the largest intrusions of Oligocene–Miocene age covering an area of 1500 km2. Its intrusion into the Upper Cretaceous volcano-sedimentary
units resulted in widespread contact metamorphism and alteration. This batholith consists of gabbro, diorite, quartz-diorite to quartz-monzonite, granodiorite, monzogranite and granite porphyry (Mokhtari, 2009).
2.2. Local geology
Upper Cretaceous submarine andesitic rocks are the most prevalent units in the Astamal area (Fig. 2). These rocks have porphyritic textures with plagioclase phenocrysts and have been propylitically altered as a result of the Qara-Dagh batholith intrusion. The Upper Cretaceous sed-imentary sequence is widespread and consists of aflysch-type assem-blage including alternating thin to medium bedded sandstone, shale, marl and conglomerate covered by thick bedded to massive limestone. Thisflysch sequence has been thermally metamorphosed due to the Qara-Dagh batholith intrusion resulting in the development of skarn, hornfels and marble (Fig. 3). The iron mineralization predominantly oc-curs as high grade massive magnetite ore bodies at the Astamal deposit. Pyrrhotite, pyrite, chalcopyrite and minor hematite, malachite, azurite
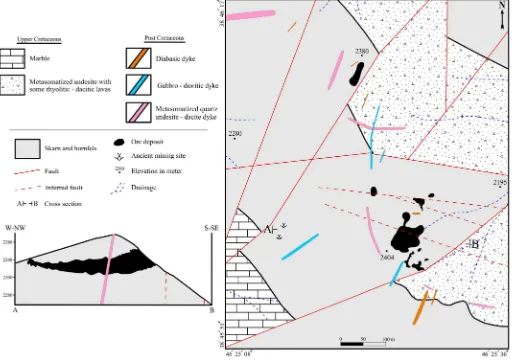
Fig. 3.Detailed geological map of the Astamal skarn deposit showing the distribution of ore bodies, host rocks, dykes, skarn and hornfels localities. The conceptual geometry of the ore body in the cross section is inferred from magnetic surveys discussed in the text.
and secondary iron oxide–hydroxide species accompany the magnetite in the ore bodies. Four iron ore bodies have been identified in the depos-it. The southern iron ore body has skarn (Fig. 4a) and the hornfels host-rocks (Fig. 4b). It is the main ore body and occurs between two faults, which demonstrate that the mineralization is controlled by fracturing and faulting. The existence of a consolidated hornfelsic component on the ore body is an indication of the low level erosion of the skarn (Fig. 4c). The western iron ore body (Fig. 4d) is located approximately 200 m west of the southern ore body and is only observed in subcrop. However, magnetic data (Jafari and Esmailzadeh, 2011) suggests that this ore body and the southern ore body are continuous at depth and form a single (200 m × 250 m × 70 m) massive ore zone (Fig. 3). The
geological cross-section of the ore body inFig. 3is also drawn based on the magnetic investigations. The northern iron ore body (10 m × 50 m × 40 m) (Fig. 4a, e) is hosted within calc-silicate hornfelsic rocks and is situated approximately 400 m north of the southern ore body. Ancient mining activity took place at a depth of 5 m on the eastern side of this ore body (Fig. 4e). The eastern iron ore body is the last one at the Astamal deposit. It is approximately 1 km east of the main ore body and is poorly exposed. Minor ancient mining activities were carried out at this deposit as well. In the eastern ore body, mineralization is either disseminated or occurs as low grade veins. LREE mineralization occurs in the deposit as LREE-bearing epi-dotes. These minerals are most common in the skarn and the Fe ore
Fig. 4.(a) Astamal skarn deposit showing the location of southern and northern iron ore bodies and their tectonically-controlled structures (view to the northwest). (b) Residue of calc-silicate hornfels host rock surrounded by magnetite ore (view to the west). (c) Hornfelsic cap rock of the iron ore body displaying low erosion of the mineralized zone (view to the north-west). (d) Outcrop features of western iron ore body and thick bedded marbles (view to the east). (e) Northern iron ore body in the vicinity of a strike-slip fault, quartz andesite–dacite and diabase dykes (view to the north). (f) Euhedral coarse-grained garnets in calcite and epidote matrix. (g) Subhedral very coarse-grained garnets in epidote matrix. (h) Massive garnetite consisting of euhedral to subhedral coarse-grained garnets. (i) Silicified zone within the andesitic rocks containing pyrite and secondary iron hydroxide minerals (view to the west). Ab-breviations: ad = quartz andesite–dacite dykes, db = diabasic dyke, Kuf
= Upper Cretaceous contact metamorphosedflysch type rocks (skarn and hornfels), Kuv
zones. The skarn and the hornfels zones are the most important lithol-ogies in the study area and are well developed and have appreciable economic value. Due to the interfingering of skarn and hornfels, their separation is very difficult even on the small scale geological map
shown inFig. 3. The skarn mineralogy predominantly consists of garnet pervasively altered to epidote and calcite. This mineral is observed as euhedral to subhedral coarse-grained crystals with brown, reddish brown and gray colors (Fig. 4f–g) and in some cases is the only
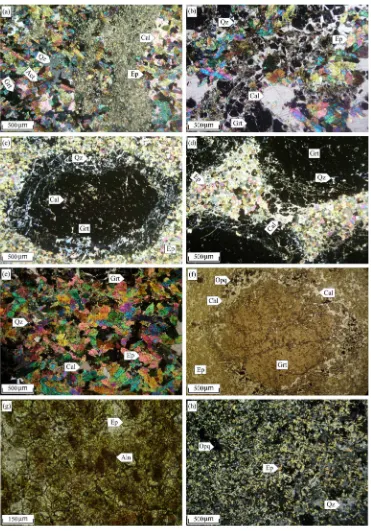
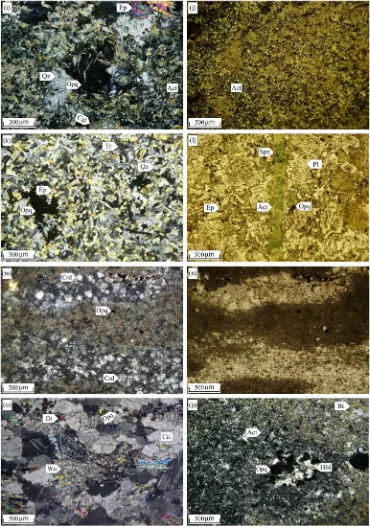
rock-Fig. 5.Photomicrographs of Astamal skarn rock specimens: (a) Mylonitized skarn zone with brecciated garnet, epidote, calcite and quartz crystals. (b) Astamal skarn containing garnet and epidote crystals in calcite and quartz matrix. (c) Replacement of garnet by calcite and quartz, both in the rim and the core, exhibiting the common intense alteration. (d) Epidote occurring as intergranular open-spacefill between coarse-grained euhedral garnet crystals. (e) Epidote pseudomorph after garnet with mosaic aggregate in calcite matrix. (f) Magnetite replacing garnet rims within the unmineralized zone. (g) REE mineralization occurring as allanite and LREE-bearing epidotes in the Astamal skarn (plane-polarized transmitted light). (h) Very strong epidote replacement due to the pervasive retrograde alteration in the skarn zone. (i) Fibrous and narrow acicular actinolite resulting from alteration of clinopyroxene. (j) Extensive actinolite formation in the skarn zone due to the pervasive retrograde alteration. (k) Elongated acicular tremolite aggregates with nematoblastic texture. (l) Late actinolite vein crosscutting the hornfels zone (plane-polarized transmitted light). (m) Occurrence of cordierite infine-grained quartz matrix within sandstone layers andfine-grained epidote and opaque minerals within marl layers from the metasedimentary sequence (plane-polarized transmitted light). (n) As the same as panel m but in non-polarized transmitted light. (o) Sparse to locally abundant radial texture wollastonite in the marble zone. (p) Aggregation of plagioclase, actinolite, opaque minerals and remnant hornblende with glomeroporphyro-blastic tex-ture showing hornblende hornfels contact metamorphism in the hornfels zone. Abbreviations: Act = actinolite, Aln = allanite, Bt = biotite, Cal = calcite, Chl = chlorite, Crd = cordierite, Di = diopside, Ep = epidote, Grt = garnet, Hbl = hornblende, Opq = opaque minerals, Pl = plagioclase, Qz = quartz, Spn = sphene, Tr = tremolite, Wo = wollastonite. Abbreviations fromWhitney and Evans (2010).
forming mineral present (Fig. 4h). Epidote is relatively abundant in the district as well and often occurs interstitially between the garnet crys-tals (Fig. 4g). Metasedimentary rocks are also observed near the skarn and include bedded marl, sandstone and conglomerate.
Unlike most skarn deposits, endoskarn is not observed at the Astamal skarn. Significant fracturing and faulting extends along a NE– SW trend from the Qara-Dagh batholith to the Astamal skarn aureole. It is suggested that the ore-bearing hydrothermalfluids moved from the Qara-Dagh batholith along these porous linear features and formed the Astamal Fe-LREE skarn as a distal occurrence within the Upper Cre-taceous volcano-sedimentary rocks, approximately 600 m from the Qara-Dagh batholith (Figs. 2 and 3). Silicic alteration accompanied by
sulfide mineralization and related supergene alteration is common along the shear zones (Fig. 4i). Marble of considerable extent and thick-ness occurs at the contact of the skarn–hornfels zone and the Upper Cre-taceous andesite (Fig. 4d). These cream to white colored thinly bedded, brecciated marbles are locally cross-cut by late coarse-grained calcite veinlets.
along the NE–SW fractures after the emplacement of the Qara-Dagh batholith.
3. Methodology
A total of 73 samples were collected from marble, skarn and hornfels rocks to undertake petrographic and mineral chemistry studies. Petro-graphic studies include identification of mineral assemblages, textures, alteration and metasomatic replacements. These were performed using an Olympus BX60 microscope at the ore-deposit laboratory at the University of Tabriz. Based on the optical microscope observations, 11 relatively unaltered samples were selected for electron microprobe analysis. The major oxide compositions of the calc-silicate minerals (e.g. garnet and clinopyroxene) were determined at the mineralogy di-vision of the Iranian Mineral Processing Research Center (IMPRC) using a Cameca SX-100 electron microprobe equipped with 5 wavelength-dispersive crystal spectrometers, operating with a 3μm beam diameter, 15 kV accelerating voltage, 15 nA sample current and 60 s counting time. Elements were calibrated against synthetic and natural standards. Chemical formulae and end-member proportions for the minerals anal-yses were calculated following the method ofDeer et al. (1992), and Fe+3was calculated based on ideal stoichiometric composition. X-ray
fluorescence microanalysis was completed to determine epidote chem-istry at the Kansaran Binaloud Mineral Research Corporation using a XGT-5000 Horiba (XRF). An accelerating voltage of 50 kV, incident X-ray beam of 10μm and counting time of 100 s were utilized in these analyses. The X-rayfluorescence was detected using an energy disper-sive high purity silicon detector cooled to−195.8 °C by liquid nitrogen. X-ray source and detection chamber were placed under vacuum in order to increase the X-ray beam intensity.
4. Petrography
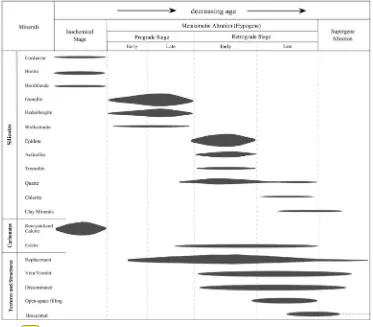
The main mineral assemblage at the Astamal skarn is, in order of abundance, garnet, epidote, actinolite, calcite, quartz, clinopyroxene and chlorite. These minerals have granoblastic, granonematoblastic, poikiloblastic, mega-porphyroblastic, and hornfelsic textures. In some areas these minerals have undergone cataclastic deformation and are locally mylonitized (Fig. 5a).
4.1. Garnet
Garnet (5–50%) is the most abundant mineral in the Astamal skarn and is present as euhedral to subhedralfine to coarse-grained (0.5– 1 cm) crystals, though they can be very coarse-grained (N3 cm) locally. The garnets are dominantly isotropic (Fig. 5b–c–d); however, the very coarse-grained crystals show weak diffusion and irregular zoning. Com-monly the garnet has been intensely retrograde altered to epidote, cal-cite and quartz. In rare cases the epidote forms pseudomorphs after garnet (Fig. 5e). Less altered garnets exhibit extensivefluid pressure fracturing. The fractures arefilled by late stage and retrograde mineral assemblages that include calcite, quartz, epidote and chlorite (Fig. 5c). Magnetite is also observed in the unmineralized skarn zone as replace-ments in the garnet rims andfissures (Fig. 5f).
4.2. Epidotes
Epidote occurs as euhedral to subhedral crystals (20–60%,b0.1–
1 mm) and is the most common retrograde calc-silicate mineral and is also the most important product of garnet alteration. The epidote is ob-served in several forms, such as: (1) massive aggregates in which
Fig. 6.Paragenetic sequence of minerals present in skarn, hornfels and marmorized zones in the Astamal skarn area. The thickness of the horizontal bars is related to the relative abundance of the minerals.
86 S. Baghban et al. / Ore Geology Reviews 68 (2015) 79–96
Edited by Foxit Reader
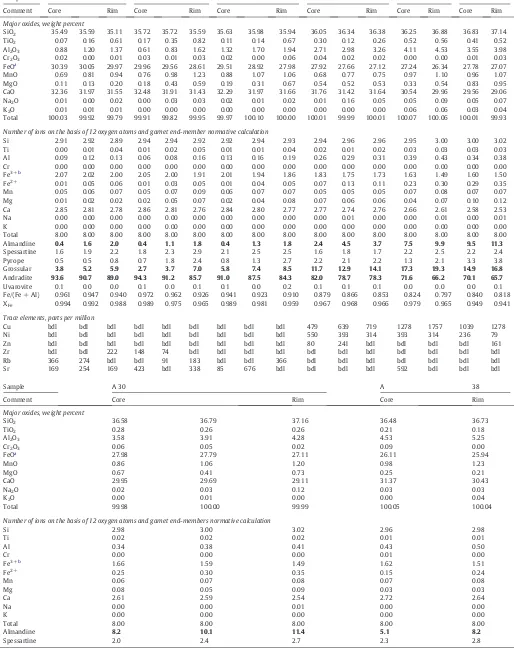
Table 1
Representative electron microprobe (EPMA) data of garnets from Astamal skarn.
Sample A 12 A 13 A 14 A 15 A 16 A 17
Comment Core Rim Core Rim Core Rim Core Rim Core Rim Core Rim
Major oxides, weight percent
SiO2 35.49 35.59 35.11 35.72 35.72 35.59 35.63 35.98 35.94 36.05 36.34 36.38 36.25 36.88 36.83 37.14
TiO2 0.07 0.16 0.61 0.17 0.35 0.82 0.11 0.14 0.67 0.30 0.12 0.26 0.52 0.56 0.41 0.52
Al2O3 0.88 1.20 1.37 0.61 0.83 1.62 1.32 1.70 1.94 2.71 2.98 3.26 4.11 4.53 3.55 3.98
Cr2O3 0.02 0.00 0.01 0.03 0.01 0.03 0.02 0.00 0.06 0.04 0.02 0.02 0.00 0.00 0.01 0.03
FeOa
30.39 30.05 29.97 29.96 29.56 28.61 29.51 28.92 27.98 27.92 27.66 27.12 27.24 26.34 27.78 27.07
MnO 0.69 0.81 0.94 0.76 0.98 1.23 0.88 1.07 1.06 0.68 0.77 0.75 0.97 1.10 0.96 1.07
MgO 0.11 0.13 0.20 0.18 0.43 0.59 0.19 0.31 0.67 0.54 0.52 0.53 0.33 0.54 0.83 0.95
CaO 32.36 31.97 31.55 32.48 31.91 31.43 32.29 31.97 31.66 31.76 31.42 31.64 30.54 29.96 29.56 29.06
Na2O 0.01 0.00 0.02 0.00 0.03 0.03 0.02 0.01 0.02 0.01 0.16 0.05 0.05 0.09 0.05 0.07
K2O 0.01 0.01 0.01 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.06 0.06 0.03 0.04
Total 100.03 99.92 99.79 99.91 99.82 99.95 99.97 100.10 100.00 100.01 99.99 100.01 100.07 100.06 100.01 99.93
Number of ions on the basis of 12 oxygen atoms and garnet end-member normative calculation
Si 2.91 2.92 2.89 2.94 2.94 2.92 2.92 2.94 2.93 2.94 2.96 2.96 2.95 3.00 3.00 3.02
Ti 0.00 0.01 0.04 0.01 0.02 0.05 0.01 0.01 0.04 0.02 0.01 0.02 0.03 0.03 0.03 0.03
Al 0.09 0.12 0.13 0.06 0.08 0.16 0.13 0.16 0.19 0.26 0.29 0.31 0.39 0.43 0.34 0.38
Cr 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
Fe3+b
2.07 2.02 2.00 2.05 2.00 1.91 2.01 1.94 1.86 1.83 1.75 1.73 1.63 1.49 1.60 1.50
Fe2+ 0.01 0.05 0.06 0.01 0.03 0.05 0.01 0.04 0.05 0.07 0.13 0.11 0.23 0.30 0.29 0.35
Total 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00 8.00
Almandine 0.4 1.6 2.0 0.4 1.1 1.8 0.4 1.3 1.8 2.4 4.5 3.7 7.5 9.9 9.5 11.3
Spessartine 1.6 1.9 2.2 1.8 2.3 2.9 2.1 2.5 2.5 1.6 1.8 1.7 2.2 2.5 2.2 2.4
Pyrope 0.5 0.5 0.8 0.7 1.8 2.4 0.8 1.3 2.7 2.2 2.1 2.2 1.3 2.1 3.3 3.8
Grossular 3.8 5.2 5.9 2.7 3.7 7.0 5.8 7.4 8.5 11.7 12.9 14.1 17.3 19.3 14.9 16.8
Andradite 93.6 90.7 89.0 94.3 91.2 85.7 91.0 87.5 84.3 82.0 78.7 78.3 71.6 66.2 70.1 65.7
Uvarovite 0.1 0.0 0.0 0.1 0.0 0.1 0.1 0.0 0.2 0.1 0.1 0.1 0.0 0.0 0.0 0.1
Fe/(Fe + Al) 0.961 0.947 0.940 0.972 0.962 0.926 0.941 0.923 0.910 0.879 0.866 0.853 0.824 0.797 0.840 0.818 XFe 0.994 0.992 0.988 0.989 0.975 0.965 0.989 0.981 0.959 0.967 0.968 0.966 0.979 0.965 0.949 0.941
Trace elements, parts per million
Cu bdl bdl bdl bdl bdl bdl bdl bdl bdl 479 639 719 1278 1757 1039 1278
Ni bdl bdl bdl bdl bdl bdl bdl bdl bdl 550 393 314 393 314 236 79
SiO2 36.58 36.79 37.16 36.48 36.73
TiO2 0.28 0.26 0.26 0.21 0.18
Al2O3 3.58 3.91 4.28 4.53 5.25
Cr2O3 0.06 0.05 0.02 0.09 0.00
FeOa
27.98 27.79 27.11 26.11 25.94
MnO 0.86 1.06 1.20 0.98 1.23
MgO 0.67 0.41 0.73 0.25 0.21
CaO 29.95 29.69 29.11 31.37 30.43
Na2O 0.02 0.03 0.12 0.03 0.03
K2O 0.00 0.01 0.00 0.00 0.04
Total 99.98 100.00 99.99 100.05 100.04
Number of ions on the basis of 12 oxygen atoms and garnet end-members normative calculation
Si 2.98 3.00 3.02 2.96 2.98
Total 8.00 8.00 8.00 8.00 8.00
Almandine 8.2 10.1 11.4 5.1 8.2
Spessartine 2.0 2.4 2.7 2.3 2.8
epidote has replaced both the rim and the core of the garnets (Fig. 5b), (2) mosaic aggregates in which epidote is closely packed with a granoblastic texture (Fig. 5e) and (3) as open-spacefilling aggregates in which epidote occurs as intergranular-masses which havefilled voids between coarse-grained garnet crystals (Fig. 5d). A locally abun-dant allanite is distinguished from epidote by its brownish color, and is occasionally rimmed by epidote (Fig. 5g). A lack of metamictization and anastomosing cracks within the allanite exhibit its low radioactive element content. Extensive epidote alteration is common throughout the skarn zone (Fig. 5h).
4.3. Tremolite–actinolite
Tremolite and actinolite are products of clinopyroxene alteration that occur both asfibrous and acicular forms (Fig. 5i, j). They range from 10–35% of the rock, range in size fromb0.1–1 mm and have a
nematoblastic texture (Fig. 5k). In some areas, actinolite is observed as veinlets which are different from the metasomatic actinolite. The veinlet
actinolite appears to have precipitated directly from hydrothermal fluids (Fig. 5l) that postdate the skarn formation.
4.4. Calcite
Three types of calcite are found at the Astamal skarn. Two formed ei-ther from metamorphism of the limestone or alteration of the garnet crystals (Fig. 5b–c). These are usually 15–20% of the rock and the crys-tals areb1 mm in diameter. Late stage calcite veins are also common in the marble zone and calcite crystals are generally 1 cm in diameter.
4.5. Clinopyroxene
Clinopyroxene crystals are commonly formed in the isochemical and prograde stages of iron skarn deposit formation. Clinopyroxene results from the interactions of ore-bearing metasomaticfluids with the calcareous host rocks and is found in association with gar-net. Together clinopyroxene and garnet are usually the predominant Table 1(continued)
Sample A 30 A 38
Comment Core Rim Core Rim
Pyrope 2.7 1.6 2.9 1.0 0.8
Grossular 14.9 16.4 17.9 19.3 21.9
Andradite 72.1 69.3 65.1 72.1 66.2
Uvarovite 0.2 0.1 0.1 0.3 0.0
Fe/(Fe + Al) 0.842 0.827 0.807 0.797 0.781
XFe 0.959 0.974 0.954 0.983 0.986
Trace elements, parts per million
Cu 879 959 1118 1198 1997
Ni bdl bdl 393 236 157
Zn bdl bdl bdl bdl bdl
Zr bdl bdl bdl bdl bdl
Rb bdl bdl bdl bdl bdl
Sr bdl bdl bdl 507 254
bdl: below detection limit. a
Total iron as FeO. b
Recalculated from stoichiometry.
Fig. 7.Composition of Astamal skarn garnets in the andradite–grossularite–pyralspite ternary diagram. Arrows indicate the growth trends in the garnets from the core to the rim. Repre-sentative EPMA data are given inTable 1.
minerals in calcic iron skarn deposits. In the Astamal skarn, clinopyroxene forms 0–3% of the rock and crystals are b0.1– 0.5 mm in diameter. The clinopyroxene has almost been completely destroyed by extensive retrograde alteration and has been replaced by hydrous calc-silicate minerals such as tremolite, actinolite, chlorite and opaque minerals (Fig. 5k–i). When observed, clinopyroxene occurs as relict crystals between the different retro-grade assemblages.
4.6. Other minerals
Quartz is usuallyfine-grained and present as a replacement mineral in the rims of coarse-grained garnets and is believed to have formed by alteration of the garnet and clinopyroxene (Fig. 5c). Occasionally, itfills fractures and interstices as a retrograde anhedral mineral in the late-stage mineralization. Chlorite is a retrograde alteration product of the garnet and actinolite that occurs as sheet-like rims around garnet and
Table 2
Electron microprobe (EPMA) data of clinopyroxenes from Astamal skarn.
Sample A 12 A 13 A 42 A 43
Comment Core Rim Core Rim Core Rim Core Rim
Major oxides, weight percent
SiO2 48.88 49.19 49.46 49.13 48.98 48.91 48.85 48.77 49.96 49.11
Al2O3 0.03 0.05 0.21 0.00 0.04 0.09 0.07 0.11 0.12 0.16
TiO2 0.67 0.81 0.99 1.3 1.27 1.7 1.33 0.74 1.85 0.17
Cr2O3 0.00 0.01 0.03 0.00 0.02 0.03 0.02 0.00 0.01 0.00
FeOa 27.9 27.79 27.72 27.71 27.87 27.66 24.82 23.3 21.36 20.62
MnO 0.34 0.29 0.27 0.18 0.23 0.25 0.51 0.45 0.87 1.05
MgO 0.07 0.19 0.19 0.56 0.53 0.57 1.22 1.1 1.96 1.8
CaO 21.99 21.43 20.81 21.13 20.82 20.66 23.11 25.14 23.65 26.91
Na2O 0.09 0.09 0.01 0.00 0.03 0.00 0.03 0.36 0.29 0.47
K2O 0.00 0.07 0.23 0.03 0.04 0.03 0.09 0.21 0.16 0.05
Total 99.97 99.92 99.92 100.04 99.83 99.9 100.05 100.18 100.23 100.34
Number of ions on the basis of 6 oxygen atoms and clinopyroxene end-members normative calculation
Si 2.01 2.02 2.03 2.01 2.01 2.00 1.98 1.99 2.00 1.96
Al 0.00 0.00 0.01 0.00 0.00 0.00 0.00 0.00 0.00 0.00
Ti 0.03 0.04 0.05 0.06 0.06 0.08 0.06 0.03 0.088 0.01
Cr 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
Fe3+b
0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.06 0.00 0.09
Fe2+
0.96 0.96 0.95 0.95 0.96 0.95 0.84 0.72 0.72 0.59
Mn 0.01 0.01 0.01 0.01 0.01 0.01 0.02 0.01 0.03 0.04
Mg 0.00 0.01 0.01 0.03 0.03 0.03 0.07 0.07 0.12 0.11
Ca 0.97 0.94 0.92 0.93 0.92 0.91 1.00 1.08 1.01 1.15
Na 0.01 0.01 0.00 0.00 0.00 0.00 0.00 0.03 0.02 0.04
K 0.00 0.00 0.01 0.00 0.00 0.00 0.00 0.01 0.01 0.00
Total 4.00 4.00 4.00 4.00 4.00 4.00 4.00 4.00 4.00 4.00
X En 0.002 0.006 0.006 0.017 0.016 0.017 0.038 0.035 0.061 0.058
X Wo 0.510 0.520 0.537 0.520 0.525 0.528 0.448 0.386 0.409 0.321
X Fs 0.488 0.474 0.457 0.463 0.459 0.455 0.515 0.579 0.530 0.622
Fe/(Fe + Mg) 0.996 0.988 0.988 0.965 0.967 0.965 0.919 0.922 0.859 0.865
Mn/Fe 0.012 0.011 0.010 0.007 0.008 0.009 0.021 0.020 0.041 0.052
Q 2.0 2.0 2.0 2.0 2.0 2.0 2.0 1.9 1.9 1.9
J 0.0 0.0 0.0 0.0 0.0 0.0 0.0 0.1 0.1 0.1
Trace elements, parts per million
Cu 240 bdl 80 bdl 160 bdl 399 bdl bdl bdl
Ni 79 bdl bdl 236 bdl bdl 550 472 bdl bdl
Zn 723 482 1044 bdl 884 562 bdl 321 629 bdl
As 379 303 530 bdl bdl bdl bdl 277 bdl bdl
Ge 139 bdl bdl 278 bdl bdl bdl 208 bdl bdl
Sr 338 676 bdl 1099 930 1015 507 338 423 254
bdl: below detection limit. a
Total iron as FeO. b
Recalculated from stoichiometry.
actinolite (Fig. 5c). Fine-grained spindle-shape sphene (Fig. 5l) and tab-ular apatite are rare accessory minerals in the skarn zone. Cordierite with low birefringence and butterfly twinning occurs in the matrix of fine-grained quartz (Fig. 5m and n) in the metasedimentary rocks. Wol-lastonite with parallel extinction, elongated and radial texture (Fig. 5o) can be observed in the marble. Biotite is rarely observed in the skarn zone, but it is a common mineral in the metasomatized andesitic rocks where it occurs asfine-grained disseminatations along with trem-olite–actinolite and remnant hornblende. These are indicators of the hornblende hornfels metamorphic facies (Fig. 5p). In samples that have been intensively altered, clay minerals can be observed on the mineral surfaces.Fig. 6represents a paragenetic sequence of minerals in the Astamal skarn, hornfels and marmorized zones.
Based on the observed mineral assemblages, it is believed that the host rock of the skarn zones has been chiefly composed of marl, calcar-eous marl and argillaccalcar-eous limestone.
5. Mineral chemistry
5.1. Garnet
Microprobe analyses, formulae and end-member compositions of garnet crystals are given inTable 1. Most of the garnets are generally isotropic and no specific zoning is observed along the growth lines (i.e. there is little variation in overall range of composition) most fall within a narrow compositional range (Adr94.3–64.5Grs21.9–2.7Alm11.1–
0.2). The amount of spessartine is minor, the amount of pyrope is
negli-gible (total less than 4 mol%) and the amount of uvarovite is usually below detection limits. Therefore, the garnets are classified as being on the andradite–grossular solid solution series with andradite as the dominant end-member (Table 1,Fig. 7).
The chemical formulae vary slightly and are as follows:
Na(0–0.01)Ca(2.53–2.86)Mg(0.01–0.12)Mn(0.05–0.09)Cu(0–0.11)Fe2 +(0.01–0.35)
Fe3+
(1.49–2.07)Ni(0–0.12)Cr(0–0.01)Al(0.06–0.50)Ti(0–0.05)Si(2.89–3.02)O12.
Though there is no distinct zone boundaries there are minor compo-sitional variations from the core to the rim of the garnet. Silicon, Fe2+,
Mn, Mg, Cu and Ti contents increase and Al, Fe3+, Ca and Ni contents
de-crease from the core to the rim. Calcium enrichment in the garnets is as-sociated with proximity to calcareous sedimentary host-rocks,fluid infiltration and chloritization of open cracks within the grains (Hwang et al., 2003). Very low content of TiO2in the garnet crystals of at the
Astamal skarn implies a high activity of SiO2during their formation
(Dingwell and Brearley, 1985). These low values are a clear difference between skarn garnets and igneous garnets. For example garnets of analcime-bearing syenitic bodies in the Kaleybar (Ashrafiet al., 2009), melanite-bearing volcanic rocks of Alberta (Dingwell and Brearley, 1985), garnets in the Rugged Mountain trachytic dykes of the Canadian Cordillera (Russell et al., 1999) and garnets in the Kaiserstuhl phonolitic dykes of Germany (Armbruster et al., 1998) all have relatively high TiO2
compared to skarn garnets. Some exceptions are present, including tita-nium andradite (TiO2= 12.4%) from skarn of the Northern Red Sea Hill
Fig. 9.Compositions of Astamal skarn clinopyroxenes in the ferrosilite–enstatite–wollastonite ternary diagram (Deer et al., 1978). Arrows indicate the growth trends in the clinopyroxenes from the core to the rim. EPMA data are given inTable 3.
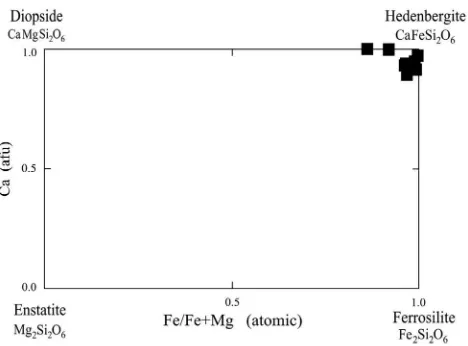
Fig. 10.Ca (afu) vs. Fe/(Fe + Mg) (atomic) for clinopyroxenes in the Astamal skarn. AfterPapike et al. (1998).
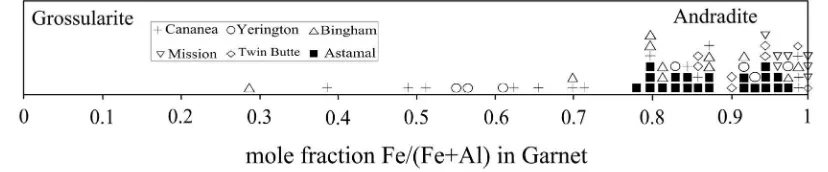
in Sudan (Huggins et al., 1977) and hydrothermal titanian andradite (TiO2= 2.0%) from Galore Creek in the Canadian Cordillera (Russell et al., 1999; Micko et al., 2014). The latter skarns are associated with al-kali plutonic and volcanic rocks.Fig. 8shows similarities and differences between Astamal skarn garnets and the Cananea, Mission, Yerington, Bingham and Twin Butte skarn deposits.
5.2. Clinopyroxene
Clinopyroxene has been intensely altered and is rarely observed as an intact mineral. Therefore, only 4 relatively unaltered clinopyroxenes were able to be analyzed in this study.Table 2presents microprobe analyses of
the clinopyroxenes from the Astamal skarn. Based on these data, the clinopyroxene has a hedenbergitic composition (Figs. 9 and 10), in which hedenbergite values are slightly decreased and ferrosalite and wol-lastonite proportions are slightly increased from the core to the rim (Fig. 9).
Similar to the garnets, the clinopyroxene is generally homogenous and has particularly high Fe/Fe + Mg ratios and low TiO2, MnO and Cr2O3
(Table 3). As shown inFig. 11, there is significantly lower contents of Ti (0.008–0.087 a.p.f.u), Cr (N0.001 a.p.f.u), Na (N0.036 a.p.f.u) and Al
(N0.007 a.p.f.u.) indicating that the clinopyroxene is of metamorphic ori-gin. A wide variety of ionic substitutions occur in the clinopyroxene. The substitution of Fe and Mg by Mn is common because diopside– Table 3
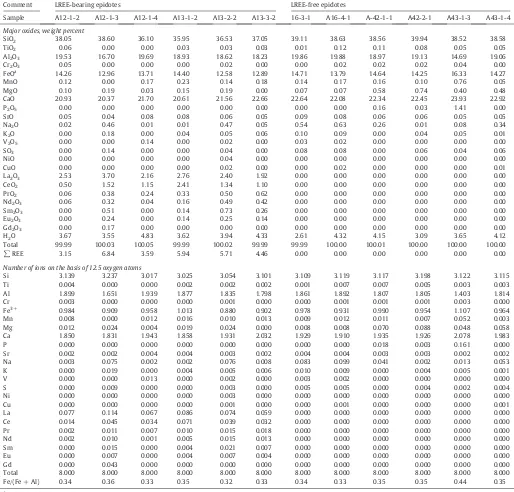
Representative X-ray microanalysis of epidotes from Astamal skarn.
Comment LREE-bearing epidotes LREE-free epidotes
Sample A12-1-2 A12-1-3 A12-1-4 A13-1-2 A13-2-2 A13-3-2 16-3-1 A16-4-1 A-42-1-1 A42-2-1 A43-1-3 A43-1-4
Major oxides, weight percent
SiO2 38.05 38.60 36.10 35.95 36.53 37.05 39.11 38.63 38.56 39.94 38.52 38.58
TiO2 0.06 0.00 0.00 0.03 0.03 0.03 0.01 0.12 0.11 0.08 0.05 0.05
Al2O3 19.53 16.70 19.69 18.93 18.62 18.23 19.86 19.88 18.97 19.13 14.69 19.06
Cr2O3 0.05 0.00 0.00 0.00 0.02 0.00 0.00 0.02 0.02 0.02 0.04 0.00
FeOa
14.26 12.96 13.71 14.40 12.58 12.89 14.71 13.79 14.64 14.25 16.33 14.27
MnO 0.12 0.00 0.17 0.23 0.14 0.18 0.14 0.17 0.16 0.10 0.76 0.05
MgO 0.10 0.19 0.03 0.15 0.19 0.00 0.07 0.07 0.58 0.74 0.40 0.48
CaO 20.93 20.37 21.70 20.61 21.56 22.66 22.64 22.08 22.34 22.45 23.93 22.92
P2O5 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.16 0.03 1.41 0.00
SrO 0.05 0.04 0.08 0.08 0.06 0.05 0.09 0.08 0.06 0.06 0.05 0.05
Na2O 0.02 0.46 0.01 0.01 0.47 0.05 0.54 0.63 0.26 0.01 0.08 0.34
K2O 0.00 0.18 0.00 0.04 0.05 0.06 0.10 0.09 0.00 0.04 0.05 0.01
V2O5 0.00 0.00 0.14 0.00 0.02 0.00 0.03 0.02 0.00 0.00 0.00 0.00
SO3 0.00 0.14 0.00 0.00 0.04 0.00 0.08 0.08 0.00 0.06 0.04 0.06
NiO 0.00 0.00 0.00 0.00 0.04 0.00 0.00 0.00 0.00 0.00 0.00 0.00
CuO 0.00 0.00 0.00 0.00 0.02 0.00 0.00 0.02 0.00 0.00 0.00 0.01
La2O3 2.53 3.70 2.16 2.76 2.40 1.92 0.00 0.00 0.00 0.00 0.00 0.00
CeO2 0.50 1.52 1.15 2.41 1.34 1.10 0.00 0.00 0.00 0.00 0.00 0.00
PrO2 0.06 0.38 0.24 0.33 0.50 0.62 0.00 0.00 0.00 0.00 0.00 0.00
Nd2O3 0.06 0.32 0.04 0.16 0.49 0.42 0.00 0.00 0.00 0.00 0.00 0.00
Sm2O3 0.00 0.51 0.00 0.14 0.73 0.26 0.00 0.00 0.00 0.00 0.00 0.00
Eu2O3 0.00 0.24 0.00 0.14 0.25 0.14 0.00 0.00 0.00 0.00 0.00 0.00
Gd2O3 0.00 0.17 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00
H2O 3.67 3.55 4.83 3.62 3.94 4.33 2.61 4.32 4.15 3.09 3.65 4.12
Total 99.99 100.03 100.05 99.99 100.02 99.99 99.99 100.00 100.01 100.00 100.00 100.00
∑REE 3.15 6.84 3.59 5.94 5.71 4.46 0.00 0.00 0.00 0.00 0.00 0.00
Number of ions on the basis of 12.5 oxygen atoms
Si 3.139 3.237 3.017 3.025 3.054 3.101 3.109 3.119 3.117 3.198 3.122 3.115
Ti 0.004 0.000 0.000 0.002 0.002 0.002 0.001 0.007 0.007 0.005 0.003 0.003
Al 1.899 1.651 1.939 1.877 1.835 1.798 1.861 1.892 1.807 1.805 1.403 1.814
Cr 0.003 0.000 0.000 0.000 0.001 0.000 0.000 0.001 0.001 0.001 0.003 0.000
Fe3+ 0.984 0.909 0.958 1.013 0.880 0.902 0.978 0.931 0.990 0.954 1.107 0.964
Mn 0.008 0.000 0.012 0.016 0.010 0.013 0.009 0.012 0.011 0.007 0.052 0.003
Mg 0.012 0.024 0.004 0.019 0.024 0.000 0.008 0.008 0.070 0.088 0.048 0.058
Ca 1.850 1.831 1.943 1.858 1.931 2.032 1.929 1.910 1.935 1.926 2.078 1.983
P 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.018 0.003 0.161 0.000
Sr 0.002 0.002 0.004 0.004 0.003 0.002 0.004 0.004 0.003 0.003 0.002 0.002
Na 0.003 0.075 0.002 0.002 0.076 0.008 0.083 0.099 0.041 0.002 0.013 0.053
K 0.000 0.019 0.000 0.004 0.005 0.006 0.010 0.009 0.000 0.004 0.005 0.001
V 0.000 0.000 0.013 0.000 0.002 0.000 0.003 0.002 0.000 0.000 0.000 0.000
S 0.000 0.009 0.000 0.000 0.003 0.000 0.005 0.005 0.000 0.004 0.002 0.004
Ni 0.000 0.000 0.000 0.000 0.003 0.000 0.000 0.000 0.000 0.000 0.000 0.000
Cu 0.000 0.000 0.000 0.000 0.001 0.000 0.000 0.001 0.000 0.000 0.000 0.001
La 0.077 0.114 0.067 0.086 0.074 0.059 0.000 0.000 0.000 0.000 0.000 0.000
Ce 0.014 0.045 0.034 0.071 0.039 0.032 0.000 0.000 0.000 0.000 0.000 0.000
Pr 0.002 0.011 0.007 0.010 0.015 0.018 0.000 0.000 0.000 0.000 0.000 0.000
Nd 0.002 0.010 0.001 0.005 0.015 0.013 0.000 0.000 0.000 0.000 0.000 0.000
Sm 0.000 0.015 0.000 0.004 0.021 0.007 0.000 0.000 0.000 0.000 0.000 0.000
Eu 0.000 0.007 0.000 0.004 0.007 0.004 0.000 0.000 0.000 0.000 0.000 0.000
Gd 0.000 0.043 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000
Total 8.000 8.000 8.000 8.000 8.000 8.000 8.000 8.000 8.000 8.000 8.000 8.000
Fe/(Fe + Al) 0.34 0.36 0.33 0.35 0.32 0.33 0.34 0.33 0.35 0.35 0.44 0.35
a
hedenbergite–johannsenite form a complete solid-solutionfield (Deer et al., 1992). However, because there is a very low abundance of Mg and Mn at the Astamal skarn, hedenbergite is the dominant clinopyroxene.
The clinopyroxene composition at the Astamal skarn is as follows:
Na(0–0.04)Ca(0.91–1.15)Mg(0–0.11)Zn(0–0.1)Fe2+(0.59–0.96)Fe3+(0–0.09)Al(0–0.01)
Si(1.96–2.04)O6.
Higher contents of Ca and Si in the clinopyroxenes rim is may be due to the intense retrograde alteration.Fig. 12shows similarities and differences between Astamal skarn clinopyroxenes and the clinopyroxenes from the Cananea, Yerington, Bingham and Twin Butte skarn deposits.
5.3. Epidote
Epidote group minerals associated with retrograde alteration are Fe-rich with high Fe/(Fe + Al) ratio (between 0.32 and 0.44). Representa-tive X-ray microanalysis data are presented inTable 3.
6. Discussion and interpretation
The Astamal skarn differs from the other skarn deposits associated with the Qara-Dagh batholith (except for Pahnavar Fe skarn;
Mokhtari, 2012) in that it is a distal skarn. The mineralogy and the chemical compositions of skarn minerals in distal skarns tend to be con-trolled by the chemical composition of the host rocks rather than the as-sociated intrusive body.
At the Astamal skarn retrograde alteration is well developed. This has caused most of the garnets to be extremely fractured and altered to secondary minerals, both in the rims and the cores. Similarly almost all of the clinopyroxene has been replaced by hydrous retrograde calc-silicate minerals. Brecciation of prograde mineral assemblages is com-mon in skarn deposits (e.g.,Einaudi et al., 1981; Meinert, 1992; Ciobanu and Cook, 2004) and is likely the mechanism responsible for initiation of pervasive infiltration offluids causing the retrograde alter-ation (Gaspar et al., 2008).
Meinert et al. (2005)suggested that garnet/pyroxene ratio will in-crease toward the causative intrusion and the appearance (color and texture) of garnet and pyroxene will also change, moreover, the distal zones will be more hedenbergitic and johannsenitic than the proximal zones. The Astamal clinopyroxene has a predominantly hedenbergite composition which supports the assertion that it is a distal skarn.
The Mn/Fe ratio and Zn content of pyroxene vary according type of metal concentrated in the deposit (Nakano et al., 1991). Most pyrox-enes of Cu–Fe skarn deposits are characterized by a low Mn/Fe ratio (b0.1) and a low Zn content (b200 ppm), whereas those of Pb–Zn
de-posits have a high Mn/Fe ratio and a high Zn content (N200 ppm) (Nakano et al., 1991). Clinopyroxene crystals of the Astamal skarn have a negligible Mn/Fe ratio (up to 0.05) but high Zn content (up to 1044 ppm). Large amounts of Fe2+are found in hedenbergite and diva-lent cations such as Zn2+substitute for Fe2+. The lack of any Zn
min-erals (such as sphalerite) in the deposit suggests that the Zn content of the hydrothermalfluids changed significantly between the time of clinopyroxene formation (prograde stage) and the time of sulfide depo-sition (retrograde stage).
The garnets are predominantly an andradite–grossular solid solu-tion. The growth is characterized by a continuous outward decrease of XAdrand XFe[= Fe/(Fe + Mg)] and an attendant increase of XGrsand
Fig. 11.Al vs. (Ti + Cr + Na) displaying metamorphic genesis for clinopyroxene crystals from the Astamal skarn (Berger et al., 2005).
Fig. 12.Comparison of the Fe/(Fe + Al) mole fraction of clinopyroxene from the Astamal skarn and some other skarn deposits. Adopted fromEinaudi (1982).
Fig. 13.Bivariate diagram showing positive correlation between almandine and grossular values in the Astamal skarn garnets.
XAlm.Einaudi and Burt (1982) suggested that almandine content
increases with increasing substitution of Al for Fe3 +(increasing Grs/
Adr;Fig. 13). The observed increase in almandine content toward the edge of the crystals could also be due to an increase in the Fe2+activity
and a decrease inƒO2during the garnet growth. Similar to Zn, Cu and Ni
are considerably enriched in the garnets. However, chalcopyrite and massive Ni-containing magnetite are also present in the Astamal skarn; therefore, it is concluded that Cu and Ni were presented in both of the early stage garnet-forming and the late stage ore-bearing hydro-thermalfluids.
Garnet growth reflects the interplay of heating andfluid infiltration (e.g.Jamtveit, 1997; Meinert et al., 2005) of the host rocks. Coarse-grained garnets in skarn deposits are usually formed in the periphery of the associated intrusive bodies. However,Einaudi et al. (1981) sug-gested that dimensions of garnet grains are more associated withfluid flow rate and equilibrium condition betweenfluidflow and wall rock. Relatively high rates offluidflow result in supersaturation of elements such as Fe, Mg, Al and Ca. In the magmatic hydrothermal environments situated distal to the intrusive bodies (such as the Astamal skarn),fluid movement is relatively limited and consequently the degree of super-saturation is limited. In such conditions, crystals grow slowly and form very coarse-grained crystals.
Most of the garnets in the Astamal skarn are isotropic and there is no specific zoning along the growth lines. The formation of the hornfelsic cap rock (Fig. 4c) has probably prevented the penetration cold meteoricfluids into the system which may have resulted in formation of homogenous garnets. This is because the hornfelsic cap may have acted as a blanket that prevented the loss of heat from the system. Alternatively, the lack of significant zoning in the garnets can be explained by an increased dif-fusion rate within the mineral at high temperatures (Dietvorst, 1982). The presence of high temperature minerals such as wollastonite and cor-dierite in the study area confirm that the garnets were formed at temper-atures greater than 550 °C, making this a viable reason for the observed homogeneity.
The stability field of the andradite-grossular solid solution at XCO2= 0 to 1 is shown inFig. 14. In most skarns, XCO2vary from initial
values of 0.2 to later values of 0.05 (Einaudi et al., 1981). However, these values can be slightly higher when wollastonite is present. Andradite is stable in relatively low XCO2conditions, but its decomposition to
hedenbergite, calcite and magnetite and the later decomposition of
hedenbergite to actinolite, calcite and quartz is concomitant with in-creasing XCO2(Uchida, 1983). Therefore, XCO2values are relatively
high in both of prograde and retrograde alteration stages. Microprobe analyses show the compositional variation of Astamal garnets are Adr94.3–65.4Grs21.9–2.7Alm11.1–0.2. Thus, based on T-XCO2 diagram
(Fig. 14), the garnets are believed to have been formed at temperatures between 500 and 560 °C.
Garnet coexists with clinopyroxene in most of skarn deposits (Rose and Burt, 1979). Due to a lack of replacement textures between garnet and clinopyroxene (also, garnet and actinolite which is formed by alter-ation of clinopyroxene), particularly low Mg/Mg + Fe ratios, low con-tent of TiO2, MnO and Cr2O3and enrichment of Fe and Ca content in
Fig. 14.T–XCO2diagram in Pfluid= 1000 bars (Adapted fromSweeney (1980)). Gr100is pure Ca–Al garnet (grossular), Ad is pure Ca–Fe garnet (andradite) and Gr20–Gr80are andradite– grossular solid solution.
garnet and clinopyroxene in the Astamel skarn we conclude that these two minerals have grown simultaneously. This suggests thatƒO2was
moderate and temperature was relatively high (Burt, 1972). Based on these data, the formation temperature of andradite can also be consid-ered as the same for hedenbergite.Zhang and Saxena (1991)suggested that the oxidation capacity of skarns where an andradite and hedenbergite assemblage is present decrease in the order Cu, Pb–Zn, Fe, Mo and W (Sn) ore deposits (Fig. 15). Therefore, iron skarns are formed at moderate and/or relatively low oxidation conditions. Accord-ing toRose and Burt (1979), the andradite and hedenbergite end-members coexist over only a limited range of oxygen fugacities, but substitutions of Al3+for Fe3+and Mg2+and Mn2+for Fe2+allow the
two minerals to exist over a wide range of oxygen fugacities in natural systems. As mentioned above, Al3+/Fe3+substitutions occurred in the
Astamal skarn. The stabilityfields of these two minerals are illustrated inFig. 16. This diagram shows the andradite and the hedenbergite can be stable and coexist at temperatures between 490 and 560 °C and LogƒO2=−16 to−31.
Compared with other skarn deposits in the Qara-Dagh area (and also many skarn deposits in the world), the Astamal skarn is unique in its high level of LREE enrichment. This type of mineralization is found as LREE-bearing epidote and allanite which are known to be major hosts for trace and rare earth elements.Jansson and Allen (2013)also attrib-uted high values of LREEs in the Smaltarmossen iron skarn to the pres-ence of allanite. The iron ore also displays LREE-enrichment. Whole-rock analyses show (Table 4) that La, Ce and Pr contents are enriched in the southern iron ore body, with values up to 676 ppm, 566 ppm and 175 ppm, respectively. The other iron ore bodies have lower LREE concentrations in comparison.
7. Conclusions
(1) The Astamal Fe-LREE skarn is a distal skarn deposit. It was emplaced in the Upper Cretaceous volcano-sedimentary se-quences at a distance of 600 m from the Oligo-Miocene Qara-Dagh granodioritic batholith by utilizing a set of NE–SW fractures and faults asfluid pathways.
(2) The Astamal skarn has higher Fe grade and higher LREEs concen-tration than other skarns in the Qara-Dagh area (and many skarn deposits in the world). The LREE mineralization is contained within LREE-bearing epidote and allanite.
(3) The Astamal skarn has been the intensely replaced by retrograde alteration. Most of the garnet has been extremely altered to a
secondary mineral assemblage, such as epidote, calcite and quartz. The majority of the clinopyroxene has been replaced by hydrous retrograde calc-silicate minerals such as tremolite, ac-tinolite and chlorite.
(4) The garnets are generally isotropic with a narrow range of varia-tion in composivaria-tion along the growth lines and are in andradite– grossular solid-solution; containing less than 15 mol% (alman-dine + spessartine + pyrope) with high Fe/(Fe + Al) ratios. The almandine content increases with increasing Grs/Adr ratio (substitution of Al for Fe3 +) in these garnets. Relatively high amounts of Cu and Ni within the garnets together with the pres-ence of chalcopyrite and Ni-bearing magnetite in the deposit suggest that Cu and Ni were presented in both of prograde and retrograde hydrothermalfluids. These minerals are generally coarse to very coarse-grained, which suggests a low ratefluid flow and non-supersaturation of Fe, Mg, Al and Ca in the Astamal skarn.
(5) Similar to the garnets, the homogenous, hedenbergitic clinopyroxene has high Fe/Fe + Mg ratios and is poor in TiO2,
MnO and Cr2O3. The clinopyroxene also has elevated Zn content
(up to 1044 ppm). This is in agreement with the structure of hedenbergite (with high Fe2 +), which can substitute divalent
cations such as Zn2 +for Fe2 +. A lack of Zn minerals (such as
sphalerite) in the Astamal skarn, suggests the Zn content of hy-drothermal fluids decreased significantly from the time of clinopyroxene formation to the later sulfide deposition phase. These high Zn in clinopyroxene concentrations are characteris-tics of pyroxenes from Pb–Zn distal skarn deposits.
(6) The garnet and the clinopyroxene coexist and have grown simul-taneously. This is suggested by their compositional similarities and lack of any replacement texture between them. These min-erals can be stable and coexist at temperatures between 490 and 560 °C and LogƒO2=−16 to−31, suggesting that these
were the conditions present during formation of the Astamal skarn.
Acknowledgments
This research forms part of M.Sc. thesis undertaken by the corre-sponding author at Tabriz University. We thank Masoud Baghban and Vahideh Mohammadi-Pour for their fruitful discussion. We are in in-debted to Reza Hosseinzadeh for hisfield assistance. Sincere gratitude Fig. 16.LogƒO2–T diagram for andradite + quartz bulk composition + excess H2O at (a) Pfluid= 2000 bars and (b) Pfluid= 500 bars (Liou, 1974).
goes to Dr. Vartan Simmonds, Dr. Parinesa Moshefi, Prof. Cristiana Ciobanu and Prof. Pavel Kartashov, for their assistance and constructive comments on the paper.
References
Akbarpour, A., 2005. Economic geology studies of Kiamaki region with special reference to copper and gold mineralization in Masjed-Daghi area, Jolfa, Eastern Azerbaijan province, Iran. Unpublished Ph.D. thesis, Science and Research branch, Islamic Azad University, Iran, 241 pp. (in Persian with English abstract).
Armbruster, T., Birrer, J., Libowitzky, E., Beran, A., 1998.Crystal chemistry of Ti-bearing an-dradites. Eur. J. Mineral. 5, 907–921.
Ashrafi, N., Ameri, A., Jahangiri, A., Hasebe, N., Eby, G.N., 2009.Mineral chemistry of gar-nets in the Kaleybar alkaline igneous intrusion, NW Iran. Iran. J. Crystallogr. Mineral. 17, 357–368 (in Persian with English abstract).
Baghban, S., 2013. Genesis, mineralogy, and geochemistry of Astamal Iron skarn, NE of Kharvana, Eastern Azerbaijan province, Iran. Unpublished M.Sc. thesis, Tabriz Univer-sity, 185 pp. (in Persian with English abstract).
Baniadam, F., 2003. Geology and genesis of gold–copper mineralization in Nabijan area. Unpublished M.Sc. thesis, Institute of Geoscience, Geological Survey of Iran. 167 pp. (in Persian with English abstract).
Berger, J., Femenias, O., Mercier, J.C.C., Demaiffe, D., 2005.Oceanfloor hydrothermal meta-morphism in Limousin ophiolites (Western French Massif Central): evidence of a rare preserved Variscan oceanic marker. J. Metamorph. Geol. 23, 795–812.
Burt, D.M., 1972. Mineralogy and geochemistry of Ca–Fe–Si skarn deposits. Unpublished PhD thesis, Harvard University, 256 pp.
Calagari, A.A., 2003.Fluid inclusion studies in quartz veinlets in the porphyry copper de-posit at Sungun, East-Azarbaijan, Iran. J. Asian Earth Sci. 23, 179–189.
Calagari, A.A., 2004.Stable isotope (S, O, H and C) studies of phyllic and potassic–phyllic alteration zones of the porphyry copper deposit at Sungun, East-Azarbaijan, Iran. J. Asian Earth Sci. 21, 767–780.
Calagari, A.A., Hosseinzadeh, G., 2006.The mineralogy of copper-bearing skarn to the east of the Sungun-Chay river, Eastern Azarbaijan, Iran. J. Asian Earth Sci. 28, 423–438. Ciobanu, C.L., Cook, N.J., 2004.Skarn textures and a case study: the Ocna de Fier-Dognecea
orefield, Banat, Romania. Ore Geol. Rev. 24, 315–370.
Deer, W.A., Howie, R.A., Zussman, J., 1978.Rock-forming minerals. 2nd edition. Single-chain Silicates vol. 2A. Longman (668 pp.).
Deer, W.A., Howie, R.A., Zussman, J., 1992.An Introduction to the Rock-forming Minerals. 2nd edition. Longman (696 pp.).
Dietvorst, E.J.L., 1982.Retrograde garnet zoning at low pressure in metapelitic rocks from Kemio, SW Finland. Contrib. Mineral. Petrol. 79, 37–45.
Dingwell, D.B., Brearley, M., 1985.Mineral chemistry of igneous melanite garnets from analcite-bearing volcanic rocks, Alberta, Canada. Contrib. Mineral. Petrol. 90, 29–35. Einaudi, M.T., 1982.General Features and Origin of Skarns Associated With Porphyry
Cop-per Plutons, Advances in Geology of Porphyry CopCop-per Deposits, Southwestern North America, Tucson. University of Arizona Press, pp. 185–209.
Einaudi, M.T., Burt, D.M., 1982.Introduction terminology, classification and composition of skarn deposits. Econ. Geol. 77, 745–754.
Einaudi, M.T., Meinert, L.D., Nwberry, R.J., 1981.Skarn Deposits. Econ. Geol. 75th Anniver-sary, volume pp. 317–391.
Gaspar, M., Knaach, C., Meinert, L.D., Moretti, R., 2008.REE in skarn systems: a LA-ICP-MS study of garnets from the Crown Jewel gold deposit. Geochim. Cosmochim. Acta 72, 185–205.
Hassanpour, Sh., Rasa, I., Heydari, M., Motakan, A.A., Moayyed, M., 2011.Geology, alter-ation, and mineralization in Haft-Cheshmeh copper–molybdenum porphyry deposit. Q. J. Iran. Geol. 15, 15–28 (in Persian with English abstract).
Heydarzadeh, R., 2007. Mineralization, alteration, and genesis of gold mineralization in Zaglik-Sarilar area. Unpublished M.Sc. thesis, Institute of Earth Science, Geological Survey of Iran. 223 pp. (in Persian with English abstract).
Hosseinzadeh, M.R., 1999. Studies of Anjerd copper skarn, NW of Ahar, Eastern Azerbaijan province, Iran. Unpublished M.Sc. thesis, Tabriz University, 118 pp. (in Persian with English abstract).
Hosseinzadeh, M.R., 2008. Geology, geochemistry,fluid inclusion, alteration, and genesis of Saunajil porphyry copper deposits, East of Heris, Eastern Azerbaijan province, Iran. Unpublished Ph.D. thesis, Tabriz University, 230 pp. (in Persian with English abstract).
Huggins, F.E., Virgo, D., Huckenholz, H.G., 1977.Titanium containing silicate garnets II, the crystal chemistry of melanites and schorlomites. Am. Mineral. 62, 646–665. Hwang, S.L., Shen, P., Yui, T.F., Chu, H.T., 2003.On the mechanism of resorption zoning in
metamorphic garnet. J. Metamorph. Geol. 21, 761–769.
Jafari, F., Esmailzadeh, A., 2011.The Iron exploration reported by magnetometers in the Astamal area, Eastern Azarbaijan province, Iran. Geological Survey of Iran (32 pp.). Jamali, H., Yagobpour, M., Mehrabi, B., 2010.Geology, geochemistry, and possible origins
of multi-metal mineralization in Mivehrood, northwestern Iran. Q. J. Geosci. Geol. Surv. Iran. 71, 53–62 (in Persian with English abstract).
Jamtveit, B., 1997.Crystal growth and intercrystalline zonation patterns in hydrothermal system. In: Jamtveit, B., Meakin, P. (Eds.), Growth and Dissolution and Pattern Forma-tion in Geosystems, Netherland. Am. Mineral. 76. Kluwer Academic, pp. 65–82. Jansson, N.F., Allen, R.L., 2013.Timing and setting of skarn and iron oxide formation at the
Smältarmossen calcic iron skarn deposit, Bergslagen, Sweden. Miner. Deposita 48, 313–339.
Konstantinov, M.M., Kryazhev, S.G., Ustinov, V.I., 2010.Characteristics of the ore-forming system of the zod gold–tellurium deposit (Armenia) according to isotopic data. Geochem. Int. 48, 946–949.
Kozerenko, S.V., 2004.Hydrothermal system of the Zod gold sulfide deposit, Armenia: ore sources and formation conditions. Geochem. Int. 42, 188–190.
Liou, J.G., 1974.Stability relation of andradite-quartz in the system Ca–Fe–Si–O–H. Am. Mineral. 59, 1016–1025.
Mahmoudinia, H., 2013. Study of petrology and petrography of Gavdel intrusive rocks with special emphasize to skarnization in Ahar region, Eastern Azarbaijan, Iran. Un-published M.Sc. thesis, Tabriz University, 143 pp. (in Persian with English abstract). Mederer, J., Moritz, R., Zohrabyan, S., Vardanyan, A., Melkonyan, R., Ulianov, A., 2014.Base and precious metal mineralization in Middle Jurassic rocks of the Lesser Caucasus: a review of geology and metallogeny and new data from the Kapan, Alaverdi and Mehmana districts. Ore Geol. Rev. 58, 185–207.
Mehrpartou, M., 1997. Geological map of Siahrood (1:100000). Geological Survey of Iran. Table 4
Geochemical data of the samples selected from southern iron ore body.
Sample A 16 A 17 A 18 A 19 AS.38 A 39 A 42
Major oxides, weight percent
SiO2 13.62 13.92 12.40 14.78 7.37 9.00 12.50
Al2O3 1.37 1.52 2.34 2.59 1.28 1.55 2.55
Fe2O3 49.93 47.26 50.99 48.89 55.89 52.84 50.91 FeO 23.66 26.03 24.82 20.99 28.97 27.57 25.87
CaO 4.13 3.93 2.96 6.88 1.62 3.49 4.53
MgO 1.56 1.70 1.12 1.65 1.35 1.94 2.39
Na2O 0.08 0.08 0.11 0.08 0.08 0.08 0.08
K2O 0.08 0.08 0.08 0.08 0.08 0.08 0.08
P2O5 0.10 0.08 0.08 0.14 0.08 0.08 0.08
TiO2 0.15 0.08 0.14 0.13 0.08 0.08 0.13
MnO 0.15 0.16 0.14 0.20 0.11 0.15 0.16
SO3 2.15 1.37 1.89 1.00 1.93 2.45 0.08
LOI 2.97 3.80 3.03 2.40 0.80 0.60 0.50
Total 99.94 99.98 100.08 99.80 99.62 99.89 99.84
Trace elements, parts per million
These samples correspond toBaghban (2013).
Meinert, L.D., 1992.Skarn and skarn deposits. Geosci. Can. 19, 145–162.
Meinert, L.D., Dipple, G.M., Nicdescu, S., 2005.World skarn deposits. Econ. Geol. 100th An-niversary, vol. pp. 299–336.
Micko, J., Tosdal, R.M., Bissig, T., Chamberlain, C.M., Simpson, K.A., 2014.Hydrothermal al-teration and mineralization of the Galore Creek alkalic Cu–Au porphyry deposit, Northwestern British Columbia, Canada. Econ. Geol. 109 (4), 891–914.
Mohammadi, B., 2004.A brief report of exploration of porphyry Au–Cu–Mo in Masjed-Daghi area. Geological Survey of Iran (26 pp., in Persian with English abstract). Mokhtari, M.A.A., 2009. Petrology, geochemistry, and petrogenesis of Qara-Dagh batholith
(east of Siahrood, Eastern Azerbeijan) and associated skarn deposits with considering on mineralization. Unpublished Ph.D. thesis, Tarbiat Modares University, 303 pp. (in Persian with English abstract).
Mokhtari, M.A.A., 2012.The mineralogy and petrography of the Pahnavar Fe skarn, In the Eastern Azarbaijan, NW Iran. Cent. Eur. J. Geosci. 4, 578–591.
Mokhtari, M.A.A., Hosseinzadeh, R., 2013.General exploration of Astamal Iron mineraliza-tion, NE of Kharvana. Geological Survey of Iran (86 pp., in Persian).
Mokhtari, M.A.A., Moein-Vaziri, H., Gorbani, M.R., Mehrpartou, M., 2012.Introduction of mineralizations associated with Qara-Dagh batholith, Eastern Azarbaijan, NW of Iran. J. Iran. Min. Eng. Organ. 15, 3–15 (in Persian).
Mokhtari, M.A.A., Moein-Vaziri, H., Gorbani, M.R., Mehrpartou, M., Hosseinzadeh, M.R., 2013.Mineralogy and petrology of Kamtal skarn, east of Kharvana, Eastern Azarbaijan. Q. J. Geosci. Geol. Surv. Iran. 86, 213–220 (in Persian with English abstract).
Mokhtari, M.A.A., Moein-Vaziri, H., Gorbani, M.R., Mehrpartou, M., 2014.Geology and geochemistry of Aniq-Qarachilar Au–Cu–Mo mineralization (NE of Kharvana, Eastern Azarbaijan). Q. J. Geosci. Geol. Surv. Iran. 90, 135–150 (in Persian with English abstract).
Mollai, H., 1993. Petrochemistry and genesis of granodiorite and associated iron–copper skarn deposit of Mazraeh, Ahar, East Azarbaijan, Iran. Unpublished Ph.D. thesis, Uni-versity of Roorkee, India, 256 pp.
Nakano, T., Yoshino, T., Shimazaki, H., Shimizu, M., 1991.Pyroxene composition as an in-dicator in the classification of skarn deposits. Econ. Geol. 89, 1567–1580. Papike, J.J., Shearer, C.K., Ryder, G., 1998.Lunar samples. In: Papike, J.J. (Ed.), Planetary
Ma-terials. Rev. Mineral. 36, pp. 5-1–5-234.
Rose, A.W., Burt, D.M., 1979.Hydrothermal alteration. In: Barnes, H.L. (Ed.), Geochemistry of Hydrothermal Ore Deposits: New York. John Wiley and Sons, United States, pp. 173–235.
Russell, J.K., Dipple, G.M., Lang, J.R., Lueck, B., 1999.Major element discrimination of titanian andradites from magmatic and hydrothermal environments: an example from the Canadian Cordillera. Eur. J. Mineral. 6, 919–935.
Sweeney, M.1., 1980. Geochemistry of garnets from the North Ore shoot, Bingham dis-trict, Utah. Unpublished M.Sc. thesis, University of Utah, 154 pp.
Uchida, E., 1983.Grunerite from the Shinyama ore deposit, Kamaishi mine, Japan. Can. Mineral. 21, 517–528.
Whitney, D.L., Evans, B.W., 2010.Abbreviations for names of rock-forming minerals. Am. Mineral. 95, 185–187.
Zhang, Zh., Saxena, S.K., 1991.Thermodynamic properties of andradite and application to skarn with coexisting andradite and hedenbergite. Contrib. Mineral. Petrol. 107, 255–263.
Zvezdov, V.S., Migachev, I.F., Girfanov, M.M., 1993.Porphyry copper deposits of the CIS and the models of their formation. Ore Geol. Rev. 7, 511–549.