www.elsevier.com / locate / bres
Research report
Distinct signaling pathways involved in multiple effects of basic
fibroblast growth factor on cultured rat hippocampal neurons
a ,
*
b bHiroshi Katsuki
, Yuko Itsukaichi , Norio Matsuki
a
Department of Pharmacology, Graduate School of Pharmaceutical Sciences, Kyoto University, 46-29 Yoshida-shimoadachi-cho, Sakyo-ku, Kyoto606-8501, Japan
b
Laboratory of Chemical Pharmacology, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Bunkyo-ku, Tokyo 113-0033, Japan
Accepted 12 September 2000
Abstract
21
We investigated possible involvement of voltage-dependent Ca channels (VDCCs) and several intracellular signaling mechanisms in multiple actions of basic fibroblast growth factor (bFGF), such as survival promotion, induction of calbindin D28kexpression as well as acceleration of neuritic branch formation of cultured rat hippocampal neurons. Immunocytochemical staining with anti-g-aminobutyric acid (GABA) antibody showed that the promotion of neuron survival by bFGF in high cell-density cultures were exerted exclusively on GABA-negative neurons. Nicardipine (5mM) attenuated the effect of bFGF on neuronal survival and formation of neurite branches, suggesting that the activity of L-type VDCCs is required for these effects. In contrast, stimulation of calbindin expression by bFGF was not attenuated by nicardipine. A phospholipase C inhibitor U73122 (1mM) prevented the effect of bFGF on neurite branch formation, but not on neuronal survival or calbindin expression. On the other hand, chronic application of phorbol-12-myristate-13-acetate (1mM) inhibited the effect of bFGF on neuronal survival, without inhibiting the other bFGF actions. Forskolin (100mM) attenuated the effect of bFGF on neuronal survival and neurite branch formation, indicating that cyclic AMP plays negative regulatory roles in these actions of bFGF. Taken together, these results suggest that multiple biological actions of bFGF on hippocampal neurons are exerted through, and modulated by, distinct signaling pathways. 2000 Elsevier Science B.V. All rights reserved.
Theme: Development and regeneration
Topic: Neurotrophic factors: biological effects
Keywords: FGF-2; Hippocampus; Neuronal differentiation; Development; Calcium channel; Intracellular signaling
1. Introduction enhancement of neurite extension [39] and acceleration of neuritic branching [2]. bFGF also regulates the expression
Basic fibroblast growth factor (bFGF), originally iso- of enzymes and proteins with specific functions, such as
lated and identified from bovine brain and pituitary based calbindin [33]. Little information is available, however,
on the stimulatory activity on fibroblast proliferation, is concerning the cellular mechanisms leading to various
now considered as a potent neurotrophic factor in the consequences of biological actions of bFGF.
brain. bFGF and its high-affinity receptors are abundantly Manifestation of diverse biological actions of bFGF may
present in the embryonic as well as the adult central be attributable to the mobilization of distinct intracellular
nervous system [10,19,44], suggesting that they play a signaling pathways. Activation of membrane-associated
pivotal role in neuronal development and functions. In- high-affinity FGF receptors, possessing tyrosine kinase
deed, bFGF has been shown to exert various biological activity, is the initial trigger to exert the effects of bFGF
effects on neuronal cells, such as promotion of progenitor [11,13]. Activated receptor tyrosine kinases are known to
cell proliferation [25], support for cell survival [36], mobilize several downstream effectors. A small
GTP-binding protein Ras is converted to its active form in response to growth factor receptor stimulation [22,32].
*Corresponding author. Tel.: 181-75-753-4536; fax: 1
81-75-753-Activation of FGF receptors also leads to tyrosine
phos-4579.
E-mail address: [email protected] (H. Katsuki). phorylation of phospholipase C (PLC)-g to enhance its
enzymatic activity [3,23]. Moreover, phosphatidylinositol in the case of calbindin immunocytochemistry) was added
(PI) 3-kinase, which catalyzes phosphoinositide phos- for 1 h, then avidin–biotin peroxidase complex method
phorylation at the D-3 position of the myo-inositol ring, is was performed with Vectastain Elite ABC kit (Vector)
activated by growth factor receptor stimulation via interac- according to the manufacturer’s instructions.
tions of SH2 domains of the kinase regulatory subunit with Cell counting was performed as described previously
2
the receptor [24]. These signal transduction mechanisms [1]. Four areas of 1 mm , the total of which corresponds to
2
may be differentially involved in diverse biological conse- 4% of the whole area (1 cm ), were chosen at random
quences of receptor tyrosine kinase activation in various from each well and the number of neurons in the areas
cell types [12,18,43]. (400–1300 cells in each well) was counted under a
In the present study we set out for the experiments to microscope. Number of surviving neurons in each well
2
discriminate which signaling pathway(s) play an important was expressed as cells / cm and data from four culture
role in the bFGF-induced biological responses in fetal rat wells from the same sister culture were presented in figures
hippocampal neurons. We have previously demonstrated as mean6S.E.M. Reproducibility of results was confirmed
that increase in the amount of L-type voltage-dependent by using different sister cultures. In the present cultures,
21 21
Ca channels (VDCCs) and consequent increases in Ca more than 90% of the cells were labeled by antibodies to
influx may play a critical role in the acceleration of neurofilament and microtubule-associated protein-2 and
neuritic branch formation by bFGF [27]. Accordingly, the identified as neurons by their morphology. Therefore,
potential involvement of VDCCs in promotion of neuronal possible contaminating non-neuronal cells including
as-survival and enhancement of calbindin expression by troglia and fibroblast cells were estimated to be less than
bFGF was also investigated. 10%.
Cultures on 35-mm dishes were used for the evaluation of neurite branching, the methods essentially according to
2. Materials and methods those described previously [2,27]. Twenty-four hours after plating, the medium was changed to serum-free modified
Cultures of dissociated hippocampal neurons were pre- Eagle’s medium containing 2% B-27 supplement (Gibco).
pared as described previously [1,27]. Briefly, hippocampal As reported in a previous study [27], we found a difficulty
tissues were isolated from the brains of embryonic day 18 in examining the effect of nicardipine on low-density
Wistar rats. Dissociated cells were obtained by enzymatic culture maintained in N2-supplemented serum-free
digestion of minced tissues by 0.25% trypsin and 0.01% medium, because nicardipine showed potent cytotoxicity
deoxyribonuclease I, and subsequent mechanical dissocia- under these conditions by unidentified mechanisms. This
tion. The cell suspension was diluted in modified Eagle’s problem was resolved by usage of B-27 supplement.
medium containing 10% fetal bovine serum and plated on Therefore, we used B-27-supplemented medium in all
48-well plastic plates (Costar) coated with poly-L-lysine to experiments on low-density culture in the present study.
4 2
obtain a final density of 5.0310 cells / cm , or on 35-mm The effect of bFGF on neurite morphogenesis was
similar-culture dishes (Iwaki Glass) coated with poly-L-lysine to ly observed in the presence of either N2 or B-27
supple-3 2
obtain a final density of 2.0310 cells / cm . They were ments. We selected neurons that have neurites longer than
cultured at 378C in a humidified 5% CO / 95% air2 their soma diameters and were free from contact with other
atmosphere. cells at 2 days in vitro (DIV), and recorded their locations
In the case of cultures on 48-well plates, the medium in the culture dish using an ACAS 470 work station
was changed to drug-containing, serum-free DMEM / F-12 (Meridian, Okemos, MI). These cells were photographed
medium supplemented with N2 hormones at 24 h after and then drugs were added to the cultures. The same cells
plating. After a certain period of culture (usually 3 days were again photographed 48 h later (at 4 DIV). In several
after the change of the medium), cells were fixed with 4% experiments, immunocytochemical staining was performed
paraformaldehyde for 30 min at 48C. Cultures were thereafter, the methods according to those described above.
subsequently processed for immunocytochemical examina- The number of branch points along the longest neurite was
tion. They were initially treated with 0.3% Triton X-100 counted on the photographs. If the selected neurons died
for 30 min, then treated with 0.3% H O in methanol for 12 2 within 48 h of drug treatment, data from these cells were
h after wash with phosphate-buffered saline (PBS). Fol- discarded.
lowing wash with PBS, 10% normal goat serum (10% Data are expressed as mean6S.E.M. Statistical
signifi-normal horse serum in the case of calbindin immuno- cance of difference between groups was evaluated by
cytochemistry) in PBS was added. After 1 h, dishes were two-way analysis of variance followed by Dunnett’s test. P
treated with either anti-g-aminobutyric acid (GABA) anti- values less than 0.05 were considered significant. bFGF
body (1:10 000, rabbit anti-GABA, Sigma) or anti-calbin- used in this study is CS23, a modified human bFGF in
din antibody (1:200, mouse anti-calbindin D28k, Sigma) in which serine is substituted for cysteine at amino acid
PBS for overnight at 48C. After wash with PBS, residues 70–88 to prevent conformational changes and
from Kyowa Hakko Kogyo Ltd. N-Acetyl-S-farnesyl-L
-cysteine (AFC) was from Calbiochem, U73122 was from Biomol Research Lab., and wortmannin and forskolin were from Sigma. Other drugs were purchased from Wako Chemicals.
3. Results
3.1. Effects of bFGF in relation to GABA and calbindin
immunoreactivity
Serum deprivation from the medium of hippocampal primary cultures, 24 h after plating at an initial density of
4 2
5310 cells / cm , resulted in a gradual but marked
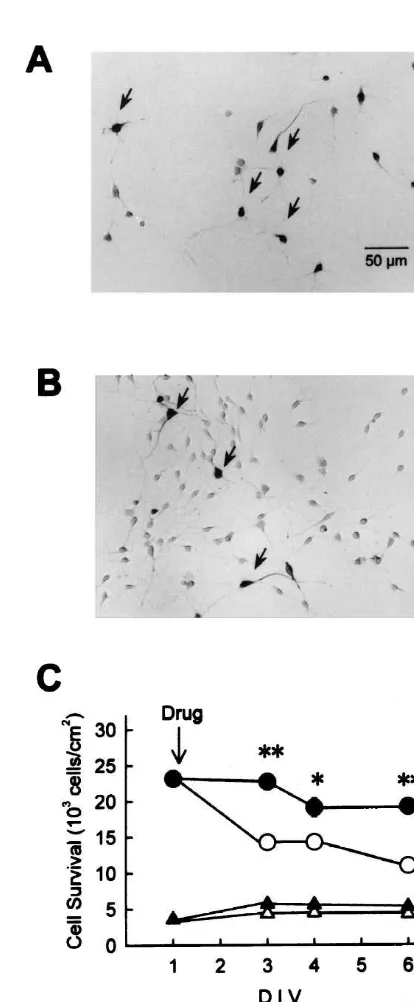
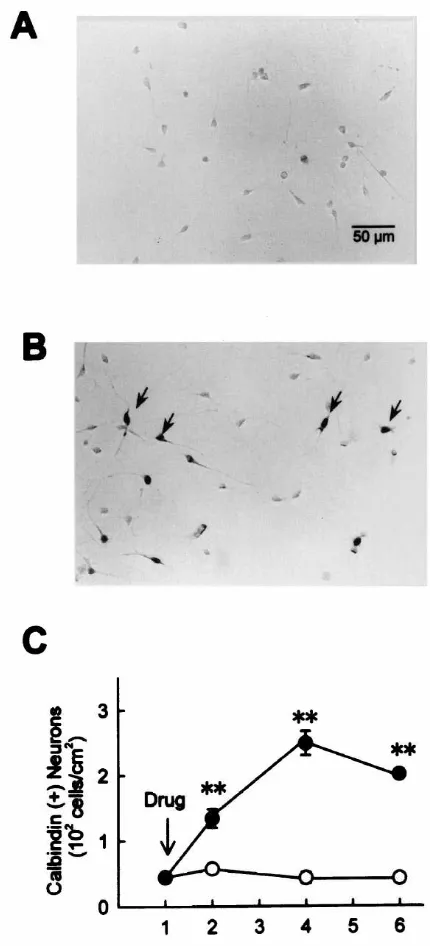
de-crease in the total number of surviving neurons. As previously demonstrated, addition of bFGF in the serum-free medium dramatically prevented the decrease in the number of hippocampal neurons. We performed immuno-cytochemical staining with anti-GABA antibody (Fig. 1A,B), and compared control and bFGF-treated cultures as to the number of surviving positive and GABA-negative neurons. As shown in Fig. 1C (triangles), while the total number of neurons gradually decreased after serum deprivation, the number of GABA-positive neurons remained constant. Moreover, addition of bFGF (5 ng / ml) markedly and significantly prevented the decrease in the total number of surviving neurons, whereas the number of GABA-positive neurons in bFGF-treated cultures showed no significant differences from that in control cultures (Fig. 1C). These results suggest that, under the present ex-perimental conditions, serum deprivation causes selective death of GABA-negative neurons that is reversed by bFGF. bFGF has also been shown to act as a differentia-tion factor for calbindin-expressing neurons [33]. Accord-ingly, we examined calbindin expression in high-density
4
cultures of hippocampal neurons (initial density of 5310
2
cells / cm ). In control cultures at 4 DIV, calbindin-positive
neurons represented a small population: only 2.460.37%
in number of total neurons was positively stained with anti-calbindin antibody. Consistent with previous reports
Fig. 1. The effect of bFGF on the number of GABA-positive and
[6,33], treatment with bFGF (5 ng / ml) markedly and
GABA-negative hippocampal neurons. (A) GABA-immunocytochemistry
significantly increased the number of calbindin-positive in control cultures at 4 DIV. Neurons immunoreactive for anti-GABA
neurons (Fig. 2). antibody are pointed by arrows. (B) GABA-immunocytochemistry in
bFGF (5 ng / ml)-treated cultures at 4 DIV. (C) Time-dependent changes
21 in the number of GABA-positive (triangles) and total (circles) neurons.
3.2. Ca channels and the effects of bFGF
Open symbols, control cultures; closed symbols, bFGF (5 ng / ml)-treated cultures. bFGF inhibited the decrease in the number of total neurons,
We have previously demonstrated that an L-type VDCC whereas it did not show significant effect on the number of
GABA-blocker nicardipine at a concentration of 5mM suppresses positive neurons. *P,0.05; **P,0.01 vs. control at each corresponding time point.
the promoting effect of bFGF on the formation of neurite branches [27]. Here we questioned if L-type VDCCs are also involved in the actions of bFGF on neuronal survival
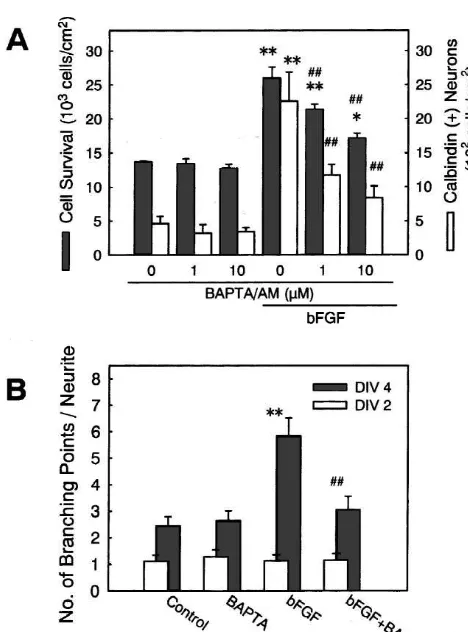
and calbindin expression. Nicardipine (1–5 mM) co-ap- concentrations of nicardipine had no significant effect on
plied with bFGF (5 ng / ml) counteracted the effect of the increase in the number of calbindin-expressing neurons
bFGF on neuronal survival in a concentration-dependent by bFGF (Fig. 3A, open bars). Consistent with previous
Fig. 3. The effect of nicardipine on the actions of bFGF. (A) bFGF (5 ng / ml) and nicardipine at indicated concentrations were simultaneously applied to high-density hippocampal cell cultures at 1 DIV. At 4 DIV, the numbers of total neurons (hatched bars) and calbindin-positive neurons (open bars) were determined. Nicardipine inhibited the effect of bFGF on survival of total neurons, but had no significant effect on the increase in the number of calbindin-expressing neurons. **P,0.01 vs. no drug
[[
treatment (0mM nicardipine); P,0.01 vs. bFGF alone. (B) bFGF (1 ng / ml) and nicardipine (5 mM) were simultaneously applied to low-density cell cultures at 2 DIV. At 2 DIV (open bars) and 4 DIV (hatched bars), the number of branch points along the longest neurite was counted. Nicardipine inhibited the increase in neuritic branches by bFGF. **P,
[[
0.01 vs. control; P,0.01 vs. bFGF alone.
neuronal survival, enhancement of calbindin expression, or
Fig. 2. The effect of bFGF on the expression of calbindin D28k in acceleration of neuritic branch formation (data not shown).
hippocampal neurons. (A) Calbindin-immunocytochemistry in control The effective blockade by nicardipine of bFGF actions
21
cultures at 4 DIV. (B) Calbindin-immunocytochemistry in bFGF (5
implies that Ca influx through L-type VDCCs is
in-ng / ml)-treated cultures at 4 DIV. Neurons immunoreactive for
anti-volved in the effect of bFGF, especially on neuronal
calbindin D28k antibody are pointed by arrows. (C) Time-dependent
survival and neurite branch formation. Therefore, we
changes in the number of calbindin-positive neurons. Open symbols,
control cultures; closed symbols, bFGF (5 ng / ml)-treated cultures. performed next set of experiments to verify if the elevated
21
Treatment with bFGF resulted in a marked increase in the number of cytosolic Ca is required for the actions of bFGF. Cells calbindin-expressing neurons. **P,0.01 vs. control at each
corre-were treated with
1,2-bis(2-aminophenoxy)-ethane-sponding time point.
N,N,N9,N9-tetraacetic acid tetrakis(acetoxymethyl ester)
(BAPTA /AM; 1–10 mM), a membrane-permeable
deriva-21
ng / ml) was markedly inhibited by concurrent application tive of a potent Ca chelator BAPTA, which is expected
21
of 5mM nicardipine (Fig. 3B). We also usedv-conotoxin to diminish increases in cytoplasmic free Ca . A previous
GVIA, a selective N-type VDCC blocker [37], to examine study by others [16] have demonstrated that treatment with
if N-type VDCC activity is involved in the actions of BAPTA /AM at 5mM effectively prevents PC12 cell death
21
bFGF. v-Conotoxin GVIA (1 mM) had no significant caused by disruption of intracellular Ca homeostasis. We
attenuated the effects of bFGF on neuronal survival (Fig. 4A) and neurite branch formation (Fig. 4B). Interestingly, BAPTA /AM was also effective in attenuating the increase in the number of calbindin-expressing cells by bFGF (Fig. 4A).
21
We next questioned whether Ca influx via L-type
VDCCs is sufficient for the promotion of neuronal survival and formation of neurite branches. To activate L-type VDCCs, we utilized culture medium containing a high
1
level of K (24.2 mM instead of 4.2 mM in normal
medium), and Bay K 8644 (5 mM), an L-type VDCC
agonist. Bay K8644 at 1mM has been shown to mimic the
1
effect of high K depolarization in olfactory neurons [5].
1
Treatment with high K medium alone, or in combination
with Bay K8644, had no significant effect on neuronal 1
survival (Fig. 5A, hatched bars). The effect of high K and
Bay K8644 was also examined on the number of
calbin-Fig. 5. The effect of VDCC activation on neuronal survival, calbindin expression and neurite branching. (A) Bay K8644 (5mM) was simul-1 taneously applied with medium containing 4.2 (normal) or 24.2 mM K to high-density hippocampal cell cultures at 1 DIV. At 4 DIV, the numbers of total neurons (hatched bars) and calbindin-positive neurons (open bars) were determined. Neither of these treatments had significant effects in the number of total neurons and calbindin-expressing neurons. (B) Bay K8644 (5mM) was simultaneously applied with medium containing 4.2
1
or 24.2 mM K to low-density cell cultures at 2 DIV. At 2 DIV (open Fig. 4. The effect of BAPTA /AM on the actions of bFGF. (A) bFGF (5 bars) and 4 DIV (hatched bars), the number of branch points along the ng / ml) and BAPTA /AM at indicated concentrations were simultaneously longest neurite was counted. Treatment with Bay K8644 plus high K applied to high-density hippocampal cell cultures at 1 DIV. At 4 DIV, the resulted in a significant increase in the number of neuritic branches. numbers of total neurons (hatched bars) and calbindin-positive neurons *P,0.05 vs. control. (C) The effects of drugs on neurite branch (open bars) were determined. Increases in total and calbindin-expressing formation stimulated by direct activation of VDCCs. Nicardipine (nicar.; neurons by bFGF were significantly attenuated by BAPTA /AM. **P, 5mM), U73122 (U; 100 nM) or forskolin (fors.; 100mM) was applied to
[[ 1
0.01 vs. no drug treatment (0mM BAPTA /AM); P,0.01 vs. bFGF low-density cell cultures at 2 DIV, simultaneously with high K (24.2 alone. (B) bFGF (1 ng / ml) and BAPTA /AM (1mM) were simultaneouly mM) plus Bay K8644 (5mM). At 2 DIV (open bars) and 4 DIV (hatched applied to low-density cell cultures at 2 DIV. At 2 DIV (open bars) and 4 bars), the number of branch points along the longest neurite was counted.
1 DIV (hatched bars), the number of branch points along the longest neurite Only nicardipine was effective in blocking the effect of high K plus Bay
[[ 1
was counted. BAPTA /AM blocked the effect of bFGF. **P,0.01 vs. K8644. **P,0.01 vs. control (cont.); P,0.01 vs. high K plus Bay
[[
1
din-expressing neurons. High K treatment marginally
increased the number of calbindin-expressing neurons, but its effect was not altered by co-application of Bay K8644 (Fig. 5A, open bars). On the other hand, Bay K8644 or
1
high K alone induced a modest increase in the number of
branches of the longest neurite, and the combination of these treatments significantly increased the number of neurite branches (Fig. 5B). Increase in neurite branching
1
by high K plus Bay K8644 was almost entirely blocked
by co-application of 5 mM nicardipine (Fig. 5C),
indicat-ing that the activation of L-type VDCCs is crucial for this
21 effect. Taken together, these results suggest that Ca influx via L-type VDCCs is sufficient for the acceleration of neurite branching but not for the promotion of neuronal survival or the increase in the number of calbindin-ex-pressing cells.
3.3. Signaling mechanisms in the effects of bFGF
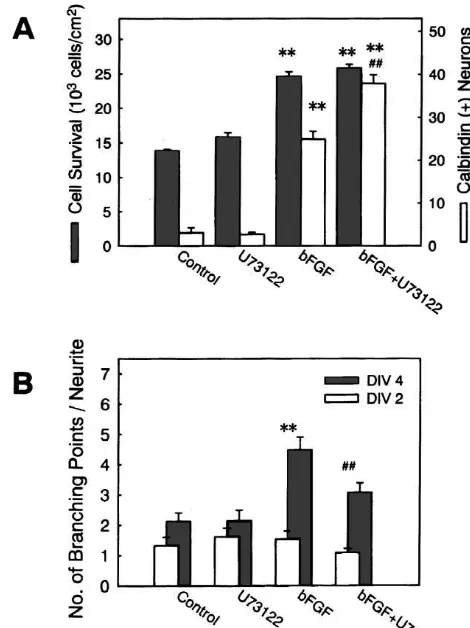
We next examined potential involvement of various intracellular signal transduction pathways in the effects of bFGF. U73122, a broad spectrum inhibitor of PLC, was used for possible counteraction against the effect of bFGF. U73122 effectively inhibits PLC activity at concentrations
of 0.1–1 mM [30]. When U73122 (1 mM) was tested on
the survival of hippocampal neurons in high density cultures, the effect of bFGF (5 ng / ml) was not blocked by this agent (Fig. 6A, hatched bars). In the same cultures,
Fig. 6. The effect of U73122 on the actions of bFGF. (A) bFGF (5
increase in calbindin-expressing neurons by bFGF (5 ng / ng / ml) and U73122 (1mM) were simultaneously applied to high-density
ml) was not inhibited by U73122 (1 mM) either. Rather, hippocampal cell cultures at 1 DIV. At 4 DIV, the numbers of total
neurons (hatched bars) and calbindin-positive neurons (open bars) were
the increase in the number of calbindin-expressing neurons
determined. Increase in total surviving neurons was not affected by
was significantly potentiated (Fig. 6A, open bars). In
U73122, whereas the increase in calbindin-expressing cells was
sig-contrast, promoting effect of bFGF (1 ng / ml) on neurite [[
nificantly augmented by U73122. **P,0.01 vs. control; P,0.01 vs.
branch formation was markedly attenuated when U73122 bFGF alone. (B) bFGF (1 ng / ml) and U73122 (100 nM) were
simul-(100 nM) was applied concurrently with bFGF (Fig. 6B), taneously applied to low-density cell cultures at 2 DIV. At 2 DIV (open bars) and 4 DIV (hatched bars), the number of branch points along the
while U73122 alone had no significant influences on the
longest neurite was counted. U73122 significantly attenuated the effect of
number of branches of the longest neurite. The same [[
bFGF. **P,0.01 vs. control; P,0.01 vs. bFGF alone.
concentration (100 nM) of U73122 did not inhibit the formation of neurite branches stimulated by co-application
1
of high K (24.2 mM) and 5 mM Bay K8644 (Fig. 5C). Several lines of evidence suggest that protein kinase C
We also tested possible counteraction by wortmannin plays a role in receptor tyrosine kinase signaling [4].
(100 nM) and AFC (20 mM) of the effect of bFGF. Accordingly, we examined the effects of agents
conven-Wortmannin is a potent and selective inhibitor of PI tionally used for manipulation of protein kinase C
ac-3-kinase [42], which is reported to inhibit the survival- tivities. Co-application of 30–100 nM calphostin C, a
promoting effect of insulin-like growth factor on cerebellar potent inhibitor of protein kinase C with an IC50 of 50 nM
granule neurons at 10–100 nM, and almost completely [17], had no significant influences on the effect of bFGF on
block PI 3-kinase activity in the same preparation at 100 neuronal survival, calbindin expression and neurite branch
nM [7]. AFC inhibits Ras-mediated signal transduction formation (data not shown). On the other hand, application
through the inhibition of carboxyl methylation of the of 1mM phorbol-12-myristate-13-acetate (PMA), a potent
C-terminal cysteine residue of Ras proteins [34], and is activator of protein kinase C, largely attenuated the effect
previously demonstrated to inhibit the enhancement by of 5 ng / ml bFGF on neuronal survival (Fig. 8A).
Accele-21
bFGF of VDCC-mediated Ca responses in hippocampal ration of neurite branching and stimulation of calbindin
neurons at a concentration of 20 mM [15]. However, expression by bFGF were not significantly inhibited by
neither AFC (Fig. 7) nor wortmannin (not shown) at- co-application of PMA (Fig. 8A,B).
tenuated the effect of bFGF on neuronal survival, calbindin Intracellular cyclic AMP-mediated events, including
Fig. 7. The effect of AFC on the actions of bFGF. (A) bFGF (5 ng / ml) Fig. 8. The effect of PMA on the actions of bFGF. (A) bFGF (5 ng / ml) and AFC (20 mM) were simultaneously applied to high-density hip- and PMA (1mM) were simultaneously applied to high-density hippocam-pocampal cell cultures at 1 DIV. At 4 DIV, the numbers of total neurons pal cell cultures at 1 DIV. At 4 DIV, the numbers of total neurons (hatched bars) and calbindin-positive neurons (open bars) were deter- (hatched bars) and calbindin-positive neurons (open bars) were
deter-[[
mined. *P,0.05; **P,0.01 vs. control. (B) bFGF (1 ng / ml) and AFC mined. **P,0.01 vs. control; P,0.01 vs. bFGF alone. PMA blocked (20 mM) were simultaneouly applied to low-density cell cultures at 2 the effect of bFGF on total neuron number, whereas it did not show DIV. At 2 DIV (open bars) and 4 DIV (hatched bars), the number of significant effect on calbindin expression. (B) bFGF (1 ng / ml) and PMA branch points along the longest neurite was counted. **P,0.01 vs. (1mM) were simultaneouly applied to low-density cell cultures at 2 DIV. control. AFC had no significant influences on the effects of bFGF. At 2 DIV (open bars) and 4 DIV (hatched bars), the number of branch points along the longest neurite was counted. **P,0.01 vs. control. PMA had no significant influence on the effect of bFGF on neurite branch
or inhibit the effects of growth factor receptor activation formation.
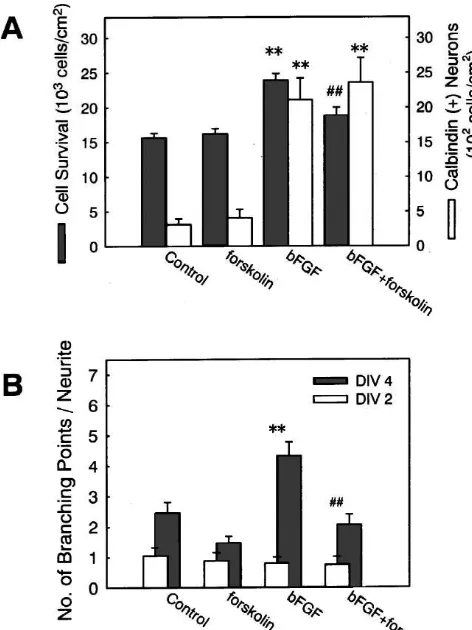
[35,41]. Therefore, we tested the effect of an adenylyl cyclase activator, forskolin, either alone or on the effect of
bFGF. Our previous study has demonstrated that forskolin give insight into the mechanisms involved in multiple
at 50 mM markedly attenuates the effect of bFGF on cellular consequences such as promotion of survival,
VDCC responses [15]. Forskolin (100 mM) alone had no enhancement of calbindin expression and acceleration of
significant influences on neuronal survival, calbindin ex- neuritic branching. Our results indicate differential
in-pression or neurite branch formation. Application of volvement of VDCC activities in different bFGF actions.
forskolin, however, blocked the effect of bFGF on neuro- Three kinds of bFGF actions also differ from each other
nal survival and neurite branch formation (Fig. 9). In with respect to the intracellular signaling pathways
in-contrast, forskolin (100mM) did not attenuate the effect of volved.
1
high K (24.2 mM) plus Bay K8644 (5 mM) on neurite
branch formation (Fig. 5C). The increase in calbindin- 4.1. Multiple biological effects of bFGF
expressing cells by bFGF was not affected by
co-applica-tion of forskolin (Fig. 9A, open bars). Examination with anti-GABA antibody revealed that
serum deprivation caused selective decrease of GABA-negative neurons in high cell density cultures, since the
4. Discussion number of GABA-positive neurons did not decline during the entire observation period. Several lines of evidence
The present study has investigated the characteristics of have shown that GABAergic neurons in vivo are relatively
proliferation in bFGF-treated cultures. Therefore, we used the term ‘promotion of survival’ to refer to the effect of bFGF, although we cannot entirely exclude the possibility that the contribution of cell proliferation might be masked by continual cell loss that was a dominant feature of our hippocampal cells at later stage of development (em-bryonic day 18) than those used in other studies.
Calbindin D28k is a member of the EF-hand family of
21
high-affinity Ca binding proteins that is distributed in
pyramidal neurons as well as interneurons of the hip-pocampus [29]. bFGF has previously been shown to
promote calbindin D28kexpression in hippocampal neurons
[33], and the present study confirmed their results. A marked increase in the number of calbindin-expressing neurons after application of bFGF is consistent with the view that bFGF induced the expression of calbindin in otherwise negative cells, rather than promoted the survival of calbindin-containing neurons selectively [33].
We also observed acceleration of neurite branch forma-tion by bFGF. In preliminary experiments we found that the acceleration of neurite branch formation was robustly induced by 1 ng / ml bFGF. On the other hand, higher concentrations of bFGF were required to ensure consistent and robust effect on neuronal survival, although 1 ng / ml bFGF was indeed effective in supporting the survival. Therefore, in the present study we used 1 ng / ml bFGF in experiments on neurite morphology, and 5 ng / ml bFGF in experiments on neuronal survival and calbindin expression.
Fig. 9. The effect of forskolin on the actions of bFGF. (A) bFGF (5
It is possible that a subtle difference in
concentration-ng / ml) and forskolin (100mM) were simultaneously applied to
high-dependence of different biological effects of bFGF is
density hippocampal cell cultures at 1 DIV. At 4 DIV, the numbers of total
related to the involvement of different signal transduction
neurons (hatched bars) and calbindin-positive neurons (open bars) were
determined. Forskolin blocked the effect of bFGF on neuronal survival, mechanisms. whereas it did not show significant effect on calbindin expression.
[[
**P,0.01 vs. control; P,0.01 vs. bFGF alone. (B) bFGF (1 ng / ml)
and forskolin (100mM) were simultaneouly applied to low-density cell 21
4.2. Ca channels in relation to multiple effects of
cultures at 2 DIV. At 2 DIV (open bars) and 4 DIV (hatched bars), the
bFGF
number of branch points along the longest neurite was counted. Forskolin blocked the effect of bFGF on neurite branch formation. **P,0.01 vs.
[[
control; P,0.01 vs. bFGF alone. Our present results suggest that L-type VDCCs play
important roles in the effect of bFGF on neuronal survival.
21
epilepsy [14,28], and that GABA-containing neurons in Indeed, Ca influx through L-type VDCCs is shown to be
dissociated cerebrocortical cultures are spared from beneficial for the survival of neuronal cells. That is,
1
NMDA-induced cell death [31]. In our high-density cul- elevated levels of K can support the survival of various
tures, about 15% of hippocampal neurons at 1 DIV were types of cells including cerebellar granule cells and
immunoreactive for anti-GABA antibody, the value resem- cerebral cortical neurons, and these effects are attenuated
bling those in the intact hippocampus where GABA-con- by L-type VDCC blockers [9]. In contrast, under our
taining neurons represent about 11% of total neurons [40]. experimental conditions, the survival of dissociated
hip-1
bFGF was effective in maintaining the number of GABA- pocampal neurons was not supported by elevated K ,
negative neurons in high-density culture. Ray et al. [25] whereas the effect of bFGF on neuronal survival was
have shown that bFGF can promote survival (50 pg–1 blocked by an L-type VDCC blocker nicardipine. These
ng / ml) and proliferation (10–20 ng / ml) of hippocampal results indicate that the increased activity of L-type
progenitor neurons from embryonic day 16 rats. Vicario- VDCCs is necessary, but not sufficient for supporting the
Abejon et al. [33] have also demonstrated that bFGF survival of hippocampal neurons. Namely, additional
21
promotes proliferation of primary hippocampal cells de- factors mobilized by bFGF may act in concert with Ca
rived from embryonic day 16 rats. In the present study, we influx through L-type VDCCs, leading to promotion of
did not observe an apparent increase in absolute number of neuronal survival. Alternatively, the conditions required
21
cells during the course of experiments shown in Fig. 1, and for Ca to support the survival of hippocampal neurons
of other types of neurons. In this case, while the effect of for the apparent discrepancy is that VDCC responses
21
bFGF is exerted entirely through enhanced Ca influx, examined in the previous study [15] were those observed
1 1
chronic presence of high K or high K plus Bay K8644 on neuronal cell bodies, whereas VDCCs involved in the
may not meet the criteria that is effective in supporting formation of neuritic branches are expected to be located
hippocampal neurons, and therefore may not mimic the along the neurites. Thus, it is possible that VDCCs on cell
effect of bFGF on neuronal survival. bodies and those on neurites undergo different regulation.
21
Nicardipine was also effective in attenuating the effect Measurement of Ca levels on neurites may help
eluci-of bFGF on the formation eluci-of neurite branches, which is date these alternatives.
consistent with our previous findings [27]. A marked We tested the effects of agents that interrupt signal
difference of the results on neurite morphology from those transduction through Ras, PLC and PI 3-kinase, all of
on neuronal survival is that the effect of bFGF was which are presumed to be coupled directly with FGF
1
mimicked by co-application of high K and Bay K8644. receptors. However, none of these agents inhibited the
This result means that the increased activities of L-type effects of bFGF on neuronal survival and calbindin
expres-VDCCs are sufficient for promoting the formation of sion. Therefore, signaling mechanisms other than those
21
neurite branches. In other words, elevated Ca influx via tested in the present study are involved as immediate
L-type VDCCs can solely account for accelerated forma- downstream effectors of FGF receptors in the regulation of
21
tion of neurite branches by bFGF. Ca is generally survival and calbindin expression. Src tyrosine kinase is a
considered to play a critical role in the establishment of potential candidate. This non-receptor protein tyrosine
neurite morphology [20]. The importance of VDCC-me- kinase is able to associate with activated FGF receptors
21
diated Ca influx in neurite morphogenesis has been [45]. Moreover, Kuo et al. [18] have reported that
bFGF-21
demonstrated in cerebellar granule cells, where Ca influx induced morphological differentiation of an immortalized
through N- and L-type VDCCs is both necessary and hippocampal cell line requires the activation of Src.
sufficient for the promotion of neurite elongation [38]. Whether or not Src is involved in the signaling pathway
21
Ca influx through L-type VDCCs does not seem to be leading to various biological effects of bFGF on fetal
involved in the increased number of calbindin-expressing hippocampal neurons is an interesting issue to be
ex-cells by bFGF, because calbindin expression was not amined.
1
significantly altered either by nicardipine or by high K Adenylyl cyclase activator forskolin was able to inhibit
21
plus Bay K8644. Nevertheless, the results with a Ca the effect of bFGF on neuronal survival and neurite branch
21
chelator BAPTA /AM suggest that intracellular Ca plays formation. The effect of forskolin, at least on the neurite
important roles in enhanced expression of calbindin stimu- branch formation, should be exerted through the
mecha-21
lated by bFGF. The sources of Ca involved in the nisms lying upstream of L-type VDCC activation since the
1
enhancement of calbindin expression remain elusive: high acceleration of neurite branching by high K plus Bay
1
K medium could marginally increase the number of K8644 was not blocked by forskolin. Additionally, protein
calbindin-expressing neurons, implying that VDCCs other kinase C activator PMA selectively inhibited the effect of
than L- and N-type could be involved. bFGF on neuronal survival. Prolonged application of PMA
is known to cause downregulation of PKC [8]. Therefore,
4.3. Signal transduction mechanisms involved in the it is possible that down-regulation, rather than activation of
effects of bFGF PKC is responsible for the effect of PMA. Although elucidation of the precise mechanisms of actions of cyclic
U73122 effectively blocked the acceleration by bFGF of AMP and protein kinase C requires further investigation,
neurite branch formation, suggesting that PLC plays an these results again suggest that multiple actions of bFGF
important role in this effect of bFGF. U73122 had no are modulated by distinct intracellular signaling
mecha-significant effect on the neurite branch formation stimu- nisms.
lated by direct activation of L-type VDCCs, which means Mattson et al. [21] have previously reported that PMA
that PLC activation lies upstream of VDCC activation. The (100 nM–1 mM) by itself causes cell death, and that
involvement of PLC in the establishment of neurite forskolin (10–100 mM) by itself promotes neurite
out-morphology again resembles the findings on cerebellar growth in cultured rat hippocampal neurons. Neither of
granule neurons [12]. these actions was prominent in the present study. The
We have previously demonstrated that Ras, but not PLC, discrepancies between their study and the present study
is involved in bFGF-induced enhancement of VDCC may be attributable to the differences in experimental
responses in hippocampal neurons [15]. The present results conditions: particularly, a major difference is that they
that PLC but not Ras is involved in bFGF-stimulated routinely used culture medium with an elevated level of
1
formation of neurite branches seem to contradict with the K (20 mM), and therefore, performed all experiments
previous results, because acceleration of neurite branch under depolarizing conditions. In that case, homeostatic
formation by bFGF is considered to be causally related to control and stimulation-induced changes in intracellular
21
Doherty, Inhibition of FGF-stimulated phosphatidylinositol
hydrol-different characteristics from those in the present
ex-ysis and neurite outgrowth by a cell-membrane permeable
phos-perimental conditions, thus yielding different results of
phopeptide, Curr. Biol. 6 (1996) 580–587.
drug actions. [13] M. Jaye, J. Schlessinger, C.A. Dionne, Fibroblast growth factor
In conclusion, the present study revealed a part of receptor tyrosine kinases: molecular analysis and signal
transduc-different signaling mechanisms that are involved in, or tion, Biochim. Biophys. Acta 1135 (1992) 185–199.
[14] F.F. Johansen, Interneurons in rat hippocampus after cerebral
have influences on, divergent cellular consequences
in-ischemia. Morphometric, functional, and therapeutic investigations,
duced by bFGF in fetal rat hippocampal neurons. The
Acta Neurol. Scand. Suppl. 150 (1993) 1–32.
present results may also provide a clue to elucidate precise [15] H. Katsuki, Y. Shitaka, H. Saito, N. Matsuki, A potential role of
cellular mechanisms of bFGF actions on hippocampal Ras-mediated signal transduction for the enhancement of
de-21
neurons as well as other types of neuronal cells responsive polarization-induced Ca responses in hippocampal neurons by
basic fibroblast growth factor, Dev. Brain Res. 111 (1998) 169–176.
to bFGF.
[16] J.N. Keller, Q. Guo, F.W. Holtsberg, A.J. Bruce-Keller, M.P. Mattson, Increased sensitivity to mitochondrial toxin-induced apop-tosis in neural cells expressing mutant presenilin-1 is linked to
Acknowledgements perturbed calcium homeostasis and enhanced oxyradical production, J. Neurosci. 18 (1998) 4439–4450.
[17] E. Kobayashi, H. Nakano, M. Morimoto, T. Tamaoki, Calphostin C
This work was supported in part by a Grant-in-Aid for
(UCN-1028C), novel microbial compound, is a highly potent and
Scientific Research from Japan Society for the Promotion specific inhibitor of protein kinase C, Biochem. Biophys. Res.
of Science. Commun. 159 (1989) 548–553.
[18] W.-L. Kuo, K.-C. Chung, M.R. Rosner, Differentiation of central nervous system neuronal cells by fibroblast-derived growth factor requires at least two signaling pathways: roles for Ras and Src, Mol.
References Cell. Biol. 17 (1997) 4633–4643.
[19] A. Matsuyama, H. Iwata, N. Okumura, S. Yoshida, K. Imaizumi, Y. [1] K. Abe, M. Takayanagi, H. Saito, Effects of recombinant human Lee, S. Shiraishi, S. Shiosaka, Localization of basic fibroblast basic fibroblast growth factor and its modified protein CS23 on growth factor-like immunoreactivity in the rat brain, Brain Res. 587 survival of primary cultured neurons from various regions of fetal (1992) 49–65.
rat brain, Jpn. J. Pharmacol. 53 (1990) 221–227. [20] M.P. Mattson, S.B. Kater, Calcium regulation of neurite elongation [2] A. Aoyagi, K. Nishikawa, H. Saito, K. Abe, Characterization of and growth cone motility, J. Neurosci. 7 (1987) 4034–4043.
basic fibroblast growth factor-mediated acceleration of axonal [21] M.P. Mattson, P.B. Guthrie, S.B. Kater, Intracellular messengers in branching in cultured rat hippocampal neurons, Brain Res. 661 the generation and degeneration of hippocampal neuroarchitecture,
(1994) 117–126. J. Neurosci. Res. 21 (1988) 447–464.
[3] W.H. Burgess, C.A. Dionne, J. Kaplow, R. Mudd, R. Friesel, A. [22] M. Nakafuku, T. Satoh, Y. Kajiro, Differentiation factors, including Zilberstein, J. Schlessinger, M. Jaye, Characterization and cDNA nerve growth factor, fibroblast growth factor, and interleukin-6, cloning of phospholipase C-g, a major substrate for heparin-binding induce an accumulation of an active Ras–GTP complex in rat growth factor 1 (acidic fibroblast growth factor)-activated tyrosine pheochromocytoma PC12 cells, J. Biol. Chem. 267 (1992) 19448– kinase, Mol. Cell. Biol. 10 (1990) 4770–4777. 19454.
[4] L.C. Cantley, K.R. Auger, C. Carpenter, B. Duckworth, A. Graziani, [23] S. Nishibe, M.I. Wahl, S.M.T. Hernandez-Sotomayor, N.K. Tonks, R. Kapeller, S. Soltoff, Oncogenes and signal transduction, Cell 64 S.G. Rhee, G. Carpenter, Increase of the catalytic activity of (1991) 281–302. phospholipase C-g1 by tyrosine phosphorylation, Science 250 [5] E. Cigola, B.T. Volpe, J.W. Lee, L. Franzen, H. Baker, Tyrosine (1992) 1253–1256.
hydroxylase expression in primary cultures of olfactory bulb: role of [24] S. Raffioni, R.A. Bradshaw, Activation of phosphatidylinositol 3-L-type calcium channels, J. Neurosci. 18 (1998) 7638–7649. kinase by epidermal growth factor, basic fibroblast growth factor, [6] D. Collazo, H. Takahashi, R.D.G. McKay, Cellular targets and and nerve growth factor in PC12 pheochromocytoma cells, Proc.
trophic functions of neurotrophin-3 in the developing rat hippocam- Natl. Acad. Sci. USA 89 (1992) 9121–9125.
pus, Neuron 9 (1992) 643–656. [25] J. Ray, D.A. Peterson, M. Schinstine, F.H. Gage, Proliferation, [7] S.R. D’Mello, K. Borodezt, S.P. Soltoff, Insulin-like growth factor differentiation, and long-term culture of primary hippocampal
and potassium depolarization maintain neuronal survival by distinct neurons, Proc. Natl. Acad. Sci. USA 90 (1993) 3602–3606. pathways: possible involvement of PI3-kinase in IGF-1 signaling, J. [26] M. Seno, R. Sasada, M. Iwane, K. Sudo, T. Kutokawa, K. Ito, K. Neurosci. 17 (1997) 1548–1560. Igarashi, Stabilizing basic fibroblast growth factor using protein [8] M. Favaron, H. Manev, R. Siman, M. Bertolino, A.M. Szekely, G. engineering, Biochem. Biophys. Res. Commun. 151 (1988) 701–
DeErausquin, A. Guidotti, E. Costa, Down-regulation of protein 708.
kinase C protects cerebellar granule neurons in primary culture from [27] Y. Shitaka, N. Matsuki, H. Saito, H. Katsuki, Basic fibroblast growth 21
glutamate-induced neuronal death, Proc. Natl. Acad. Sci. USA 87 factor increases functional L-type Ca channels in fetal rat (1990) 1983–1987. hippocampal neurons: implications for neurite morphogenesis in [9] J.L. Franklin, E.M. Johnson Jr., Suppression of programmed neuro- vitro, J. Neurosci. 16 (1996) 6476–6489.
poly-morphonuclear neutrophils: effects of a novel inhibitor of phos- F.S. Walsh, Calcium influx into neurons can solely account for cell pholipase C-dependent processes on cell responsiveness, J. Phar- contact-dependent neurite outgrowth stimulated by transfected L1, J. macol. Exp. Ther. 253 (1990) 688–697. Cell Biol. 199 (1992) 883–892.
[31] E.S. Tecoma, D.W. Choi, GABAergic neocortical neurons are [39] E.J. Williams, J. Furness, F.S. Walsh, P. Doherty, Characterisation of resistant to NMDA receptor-mediated injury, Neurology 39 (1989) the second messenger pathway underlying neurite outgrowth
stimu-676–682. lated by FGF, Development 120 (1994) 1685–1693.
[32] P. van der Geer, T. Hunter, R.A. Lindberg, Receptor protein-tyrosine [40] W. Woodson, L. Nitecka, Y. Ben-Ari, Organization of the GABAer-kinases and their signal transduction pathways, Annu. Rev. Cell gic system in the rat hippocampal formation: A quantitative im-Biol. 10 (1994) 251–337. munocytochemical study, J. Comp. Neurol. 280 (1989) 254–271. [33] C. Vicario-Abejon, K.K. Johe, T.G. Hazel, D. Collazo, R.D.G. [41] J. Wu, P. Dent, T. Jelinek, A. Wolfman, M.J. Weber, T.W. Sturgill,
McKay, Functions of basic fibroblast growth factor and neuro- Inhibition of the EGF-activated MAP kinase signaling pathway by trophins in the differentiation of hippocampal neurons, Neuron 15 adenosine 39,59-monophosphate, Science 262 (1993) 1065–1069. (1995) 105–114. [42] H. Yano, S. Nakanishi, K. Kimura, N. Hanai, Y. Saitoh, Y. Fukui, Y. [34] C. Volker, R.A. Miller, W.R. McCleary, A. Rao, M. Poenie, J.M. Nonomura, Y. Matsuda, Inhibition of histamine secretion by wor-Backer, J.B. Stock, Effects of farnesylcysteine analogs on protein tmannin through the blockade of phosphatidylinositol 3-kinase in carboxyl methylation and signal transduction, J. Biol. Chem. 266 RBL-2H3 cells, J. Biol. Chem. 268 (1993) 25846–25856. (1991) 21515–21522. [43] R. Yao, G.M. Cooper, Requirement for phosphatidylinositol-3 kinase [35] M.R. Vossler, H. Yao, R.D. York, M.-G. Pan, C.S. Rim, P.J.S. Stork, in the prevention of apoptosis by nerve growth factor, Science 267
cAMP activates MAP kinase and Elk-1 through a B-Raf- and (1995) 2003–2006.
Rap1-dependent pathway, Cell 89 (1997) 73–82. [44] N. Yazaki, Y. Hosoi, K. Kawabata, A. Miyake, M. Minami, M. [36] P. Walicke, W.M. Cowan, N. Ueno, A. Baird, R. Guillemin, Satoh, M. Ohta, T. Kawasaki, N. Itoh, Differential expression Fibroblast growth factor promotes survival of dissociated hippocam- patterns of mRNAs for members of the fibroblast growth factor pal neurons and enhances neurite extension, Proc. Natl. Acad. Sci. receptor family, FGFR-1-FGFR-4, in rat brain, J. Neurosci. Res. 37
USA 83 (1986) 3012–3016. (1994) 445–452.
[37] D.B. Wheeler, A. Randall, R.W. Tsien, Roles of N-type and Q-type [45] X. Zhan, C. Plourde, X. Hu, R. Friesel, T. Maciag, Association of 21