membuat ulasan tentang perkembangan teori atom
Setelah mempelajari bab ini, Anda harus mampu:
menganalisis berbagai besaran fisis pada gejala kuantum dan batas-batas berlakunya relativitas Einstein dalam paradigma Fisika Modern.
Hasil yang harus Anda capai:
Fisika Atom
A. Evolusi Model
Atom
B. Atom Berelektron
Banyak
>41@1CD9@5B>18=5>45>71BD5>D1>71D?=D?==5BE@1;1>2181> 41C1B@5>IECE>C5=E1J1DI1>7141491<1=9>9D5D1@91D?=2E;1>@1BD9;5< D5B;539< ;1B5>1 =1C98 141 @1BD9;5<@1BD9;5< CE21D?=9; *18E;18 >41 DE:E1> @1B1 9<=EG1> =5=@5<1:1B9 1D?= +>DE; =5>:1G12 @5BD1>I11> D5BC52ED >41 81BEC =5>:1G12 D5B<5298 418E<E @5BD1>I11>@5BD1>I11> 25B9;ED @1;18 >41 D18E D5>D1>7 C5B1D ?@D9; I1>7 21>I1; 497E>1;1> 41<1= 4E>91 D5<5;?=E>9;1C9 C5;1B1>7 9>9 @1;18 >41 D18E 2181> 1@1 I1>7 497E>1;1> C521719 B1>7;1 @5C1G1D E<1>71<9; 5>71> =5=@5<1:1B9 C5<E; 25<E; 1D?= @1B1 9<=EG1> 41@1D =5=?4969;1C9 E>CEB E>CEB C589>771 =5=9<9;9 C961D I1>7 4981B1@;1>
'1412129>9>411;1>=5=@5<1:1B95F?<EC9=?45<1D?=41>1D?= 25B5<5;DB?> 21>I1; $1D5B9 9>9 =5BE@1;1> 41C1B @5>75D18E1> E>DE; 25<1:1B 41> =5>71@<9;1C9;1> D5;>?<?79 D5>D1>7 69C9;1 =1D5B91<
Bab
8
A. Evolusi Model Atom
)5:1; J1=1> 4E<E =1>EC91 CE418 D5BD1B9; E>DE; =5>75D18E9 2181> 41C1B@5>IECE>C5<EBE8=1D5B91<41>J1DI1>7141491<1=9>9#1>41C1> 25B@9;9B>I1 C1>71D C545B81>1 =5B5;1 =5>71=29< 1>1<?79 C52E18 BE=18 I1>7 492E1D 41B9 21DE 41> ;1IE ;5=E491> 21DE D5BC52ED 49CECE> ?<58 @1BD9;5<@1BD9;5<21DEI1>7<5298;539<41>C5D5BEC>I1;89B>I1=5B5;1 C1=@19 @141 ;5C9=@E<1> 218G1 2181> C5=E1 =1D5B91< 41> J1D I1>7 141 49 1<1= 9DE 49CECE> ?<58 CE1DE 2181> 41C1B
1. Model Atom Demokritus
45 D5>D1>7 ;525B1411> 2181> 41C1B 9>9 ;1<9 @5BD1=1 49;5=E;1;1> ?<58,;*077;9<189BD18E>)$"5=E491>,46280:;9M
)$=5>75=21>7;1>@5>41@1D,;*077;945>71>=5=25B9>1=1,'%'+
E>DE;2181>41C1B9>9%1=19>9491=29<41B92181C1/E>1>9I1>725B1BD9 D941; 41@1D 492179 <179 !149,46280:;9 =5>I1D1;1> 218G1 C5=E1
=1D5B91< 41> J1D I1>7 141 49 1<1= 9>9 49CECE> ?<58 21791> D5B;539< I1>7 D941; 41@1D 492179 <179 I19DE 1D?=
'141 =1C1 D5BC52ED 21>I1; @1B1 69<CE6 I1>7 =5>75=E;1;1> @5>41@1D>I1 =5>75>19 1D?= B7E=5>D1C9 I1>7 =5B5;1 7E>1;1> 2E;1> 4941C1B;1>@14181<@5B3?211>=5<19>;1>25B41C1B;1><?79;1-1<1E@E> @5>5=E1>=5B5;19>9C521D1CD5?B9D5D1@981<9>9D5<18=5>:149D?>771; @5>D9>7 E>DE; @5;5=21>71> D5?B9 69C9;1 1D?= 41> 69C9;1 9>D9 )59B9>7 45>71> @5B;5=21>71> J1=1> 21>I1; 69C9;1G1> 41> ;9=91G1> I1>7 D5BD1B9; E>DE; =5=@5<1:1B9 D5?B9 1D?= 9>9 D5BEC =5=2E1D 5F?<EC9 =?45< 1D?= C589>771 4941@1D;1> =?45< 1D?= C5@5BD9 C5;1B1>7 9>9
2. Model Atom Dalton
6/5 (3:65M141<18C5?B1>79<=EG1> >77B9CI1>7;1<9
@5BD1=1 =5>I1D1;1> D5?B9 D5>D1>7 1D?= 25B41C1B;1> 81C9< 5;C@5B9=5> I1>7 49<1;E;1>69,7/ !86;9: C5?B1>7 ;9=91G1> 41B9 'B1>39C I1>7
=5>I1D1;1> 218G1 C5>I1G1 49CECE> ?<58 E>CEBE>CEB 45>71> @5B21>49>71> D5D1@ 1C9< 5;C@5B9=5>!86;9: 9>9 49C52ED E;E=
'5B21>49>71> *5D1@ '141 !86;9: =5<1;E;1> 5;C@5B9=5> 45>71>
=5B51;C9;1> E>CEB 894B?75> 41> E>CEB ?;C975> 1 =5>5=E;1> C5>I1G1 19B 45>71> @5B21>49>71> D5D1@ I19DE 71B >41 <5298 :5<1C @5B81D9;1> D125< 25B9;ED
#,),3;44,47,3(1(802659,70902(:642,81(2(53(/96(396(3),802;:+(3(4);2;3(:0/(5
Tes Kompetensi Awal
)52ED;1>41>:5<1C;1>=?45<=?45<1D?=I1>7@5B>18 141=E<1941B91G1<C1=@19=?45<I1>7D5B21BE
)52ED;1>>1=1>1=1;E<9DD5=@1D5<5;DB?>=5>7?B29D 41<1=1D?=41>25B1@1:E=<18=1;C9=E=5<5;DB?> I1>741@1D25B141@141D91@;E<9DD5BC52ED
?21>41@5B81D9;1>D125<@5B9?49;E>CEBE>CEB $5>EBED>4121719=1>1;18E;EB1>:1B9:1B91D?= 41<1=1C1DE@5B9?4541>2C1DE7?<?>71>
Tokoh
John Dalton (1766–1844)
John Dalton adalah seorang ilmuwan Inggris. Dia orang pertama yang mengemukakan tentang teori atom berdasarkan hasil eksperimen yang dilakukan Joseph Proust. Dalton menyatakan bahwa atom adalah bagian terkecil dari benda yang tidak dapat dibagi-bagi lagi.
Sumber: www.allbiographies.com
)5<19>81C9<5;C@5B9=5>'B?ECD5;C@5B9=5><19>I1>749:149;1>13E1> ?<58 1<D?> 41<1= =5=2E1D D5?B9 1D?=>I1 141<18 5;C@5B9=5> I1>7 49<1;E;1> ?<585:605, (<6090,8 41>!8,09:3,? '141 4E1 ?B1>7
;9=91G1>9DE=5<1;E;1>5;C@5B9=5>45>71>=5=1>1C;1>=5B;EB9?;C941 I1>7 498E2E>7;1> 45>71> E41B1 49 G1418 D5BDEDE@ 1C9< 5;C@5B9=5> =5>E:E;;1> =1CC1 C525<E= B51;C9 41> C5CE418 B51;C9 141<18 C1=1 '5B>I1D11> 9>9 49;5>1< C521719 E;E= #1F?9C95B
E1 81C9< 5;C@5B9=5> 9>9 =5=2E1D(3:65 =5=@5B31I19 D5>D1>7
;525B1411> 1D?= !9;1 CE1DE E>CEB D5B49B9 41B9 2181> 41C1B I1>7 D941; ;?>D9>E D941; =E>7;9> 141 @5B21>49>71> D5D1@ E>CEBE>CEB @5=25>DE; C5>I1G1 41> ;5;5;1<1> =1CC1 &<58 ;1B5>1 9DE @141 1<D?> =5>7ECE<;1> D5?B9 1D?= C521719 25B9;ED
1 D?= =5BE@1;1> 21791> D5B;539< CE1DE J1D I1>7 D941; 41@1D 492179 2179 <179
2 )5D91@ E>CEB =5=9<9;9 C961D E>9; I1>7 C5CE19 45>71> C961D 1D?= @5>IECE>>I1
3 E1 1D?= 1D1E <5298 I1>7 25B1C1< 41B9 E>CEBE>CEB 25B<19>1> 41@1D 25B712E>7 =5=25>DE; =?<5;E<
4 '141 CE1DE @5BC5>I1G11> E>DE; =5=25>DE; =?<5;E< :E=<18 =1CC1 C525<E= 41> C5CE418 @5BC5>I1G11> 141<18 C1=1
5 '141 CE1DE @5BC5>I1G11> E>CEBE>CEB @5=25>DE;>I1 C5<1<E 25B141 41<1= @5B21>49>71> D5D1@ 41> C545B81>1
3. Model Atom Thomson
'141 1;89B 1214 . .$/64(9 3<( +0965 25B81C9< =5>5=E;1>
65>?=5>1 5=9C9 D5B=1< 41<1= @5B3?211>>I1 E>DE; =5>39@D1;1> 2?<1 <1=@E5>?=5>19>9=5>4?B?>7@1B169C9;1G1>=5>5<9D9D5>D1>7D12E>7 C9>1B;1D?45*12E>7C9>1B;1D?45D5B49B91D1CC52E18D12E>781=@171C 4941<1=D12E>749@?=@1;5<E1BC1=@19D5;1>1>71CC5;9D1B==7 C1DE5<5;DB?45>571D96;1D?4541>C1DE5<5;DB?41@?C9D961>?45"5D9;1 5<5;DB?45498E2E>7;1>45>71>CE=25BD571>71>D9>779;,M;,1;1> 495=9C9;1> CE1DE @1BD9;5< I1>7 25B75B1; =5>E:E 1>?45 1B9 81C9< @5>5<9D91> 49;5D18E9 218G1 @1BD9;5< I1>7 495=9C9;1> ;1D?45 9>9 25B=E1D1> >571D96
'141C5?B1>769C9;1G1> >77B9C25B>1=169,7/6/5$/64965
=5<1;E;1> ;5=21<9 5;C@5B9=5> D5>D1>7 D12E>7 C9>1B ;1D?45 D5D1@9 45>71> 2525B1@1 =?4969;1C9 +>DE; <5298 :5<1C>I1 >41 29C1 =5<981D
(4)(8 25B9;ED
Tokoh
Sir J.J. Thomson (1856–1940)
Thomson adalah seorang ahli Fisika yang lahir di Checham Hill, Greater Manchester, Inggris. Dia belajar di Cambridge dan meraih gelar profesor pada 1884. Dia meraih hadiah Nobel Fisika tahun 1906. Eksperimennya yang terkenal tentang tabung sinar katode. Dari eksperimennya, dia menemukan nilai nisbah e/m.
Sumber: www.allbiographies.com
(99(0+86.,5 ?(5.08,(2902(5
.8(4
(99( 290.,5 ?(5.08,(2902(5
.8(4
(99(08 .8(4
#09((903",(290 .8(4
7B1=?;C975> 7B1=894B?75>
$(),3
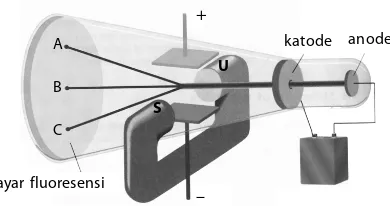
Gambar 8.1
Percobaan Thomson
(4)(8 =5>E>:E;;1> @141 21791> D5>718 D12E>7 C9>1B ;1D?45
4925B9 =541> <9CDB9; 41> =541> =17>5D I1>7 1B18>I1 C1<9>7 D571; <EBEC *'++ !$ I19DE =541> <9CDB9; 25B1B18 ;5 21G18 @141 71=21B 41>
=541>=17>5D25B1B18;541<1=;5BD1C@14171=21B*181@1>D181@1> 5;C@5B9=5> I1>7 49<1;E;1> ?<58$/64965 141<18 C521719 25B9;ED
"5D9;141> @1BD9;5<C9>1B;1D?4525B75B1;<EBEC
"5D9;1 =541> <9CDB9; 491;D96;1> C9>1B ;1D?45 25B75B1; ;5 1D1C 1< 9>9 49C5212;1> 71I1 5<5;DB?CD1D9C 1>D1B1 @1BD9;5< 25B=E1D1> >571D96 41> @5<1D ;EDE2 @?C9D96
5>71> D5D1@ =5>:171 25C1B =541> =17>5D 491;D96;1> )5CE19
45>71> ;19418 D1>71> ;1>1> 1B18 71I1 #?B5>DJ 141<18 ;5 21G18 5>71> =5>71DEB 25C1B>I1 C9>1B ;1D?45 D5BC52ED 1;1> 25B75B1; <EBEC ;5=21<9
1B9 5;C@5B9=5> D5BC52ED$/64965 =5>41@1D;1> @5B89DE>71>
=1D5=1D9C C521719 25B9;ED
"5<1:E1> @1BD9;5< 41<1= D12E>7 C9>1B ;1D?45 4941@1D;1> 41B9 @5BE2181> 5>5B79 @?D5>C91< <9CDB9; =5>:149 5>5B79 ;9>5D9; @1BD9;5< D5BC52ED
(<9CDB9; #@1BD9;5<
%.
%
. M
"5D5B1>71>
=E1D1> @1BD9;5< C9>1B ;1D?45 % =1CC1 @1BD9;5< C9>1B ;1D?45 . ;535@1D1> @1BD9;5< C9>1B ;1D?45 2541 @?D5>C91< I1>7 4925B9;1>
"5D9;1 @1BD9;5< C9>1B ;1D?45 D5D1@ 25B75B1; <EBEC G1<1E@E> 4925B9 =541> <9CDB9; 41> =5>41> =17>5D 25B1BD9
< 3
.
. M
5>71> =5>CE2CD9DEC9;1>!,89(4((5 @ ;5!,89(4((5 @ 1;1>
4941@1D;1>
%
M
katode anode
layar fluoresensi
+
–
U
S
A
B
C
B F
v
Jika ada elektron yang bergerak dengan kecepatan v dalam suatu medan magnet B, arah gaya Lorentznya mengikuti kaidah tangan kanan.
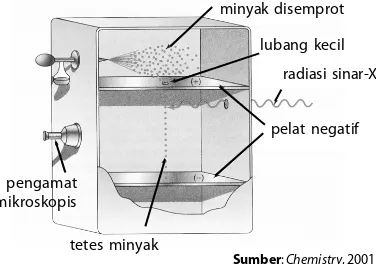
pengamat mikroskopis
minyak disemprot
lubang kecil
radiasi sinar-X
pelat negatif
tetes minyak
5C1B1>41> 41@1D49;5D18E941B9@5>7E;EB1>C589>771*8?=C?>
=5>41@1D;1>@5B21>49>71>=E1D1>41>=1CC1%@1BD9;5<C9>1B;1D?45
C521719 25B9;ED
%P ;7 M
$/64965 =5<1;E;1> 5;C@5B9=5> 9>9 25BE<1>7E<1>7 I19DE 45>71>
=5>771>D9 71C 41<1= @5<1D ;1D?45 45>71> E>CEB <19>>I1 41> =5>771>D9 71C41<1=C9>1B;1D?459>9;1>D5D1@981C9<I1>74941@1D;1>$/64965
D5D1@ C1=1 "5=E491>$/64965 =5>I9=@E<;1> 218G1 @1BD9;5< C9>1B
;1D?45 9>9 =5BE@1;1> 2181> I1>7 141 49 C5D91@ J1D$/64965 =5=25B9
>1=1@1BD9;5<9>9'*(-+$+D5D1@9@1BD9;5<9>9<529849;5>1<45>71>>1=1 $#,*'&
%18 1@1;18 >41 D18E =5>71@1$/64965 =5>I9=@E<;1> 218G1
5<5;DB?> 9>9 21791> 41B9 1D?= $5>71@1 91 D941; =5>I9=@E<;1> 218G1 5<5;DB?>9>9141<181D?=1D1E45>71>;1D1<19>1D?=25B=E1D1>>571D96 41 4E1 1<1C1> I1>7 =5>41C1B9 ;5C9=@E<1>$/64965 I19DE C521719
25B9;ED
"?>C5@41C1BD5>D1>71D?=I1>7=5>I1D1;1>218G11D?=9DE>5DB1< )11D9DE69C9;1G1>D5<18=5>75D18E9=1CC141B91D?=894B?75>I1>7 =5BE@1;1> 1D?= D5B9>71> 41B9 5;C@5B9=5> D5>D1>7 5<5;DB?<9C9C *5B>I1D1>9<19%:1E8<5298;539<C5;1B1>7D5<18D5B2E;D9B1C9?>I1
141<1841B9=1CC11D?=894B?75>
D1C 41C1B 9DE<18$/64965 =5>7ECE<;1> C52E18 =?45< 1D?= I1>7
49;5>1< 45>71> =?45< 1D?= ;E5 ;9C=9C I19DE C521719 25B9;ED
D?=25B25>DE;2E<1D@141D45>71>=E1D1>@?C9D96D5BC521B;5C5<EBE8 21791> 2?<1 D5BC52ED
$E1D1> @?C9D96 9>9 49>5DB1<;1> ?<58 =E1D1> >571D96 I1>7 =5<5;1D @141 @5B=E;11> 2?<1 D5BC52ED
"525B81C9<1>$/64965 =5>5=E;1> C1<18 C1DE ;5@9>71> @EJJ<5
1D?= 9>9 =5=25B9;1> 9>C@9B1C9 2179 69C9;1G1> <19>>I1 =5<1;E;1> 5;C@5B9=5>D5D5C=9>I1;I1>749<1;E;1>?<58"6),8:03302(5C5?B1>7
69C9;1G1> 41B9 =5B9;1 )5B9;1D ;C@5B9=5> D5D5C =9>I1; $9<<9;1> 9>9 25B81C9< =5>41@1D;1> 25C1B>I1 =E1D1> 41> =1CC1 41B9 5<5;DB?> +>DE; <5298 :5<1C>I1 C9<18;1> >41 @5B81D9;1>(4)(8 25B9;ED
)5@5BD9 49@5B<981D;1> @141(4)(8 03302(5 25B5;C@5B9=5>
45>71> =5>I5=@B?D;1> =9>I1; ;5 41<1= C52E18 D12E>7 1D1E ;?D1; "?D1; D5BC52ED =5=9<9;9 4E1 @5<1D 25B=E1D1> I19DE @5<1D @?C9D96 @141 21791> 1D1C 41> @5<1D >571D96 @141 21791> 21G18 $9>I1; I1>7 49C5=@B?D;1>D5BC52ED@14121791>1D1C@5<1D@?C9D96=5=25>DE;D5D5C1>
Sumber: Chemistry, 2001
Gambar 8.2
Model Atom Thomson
Gambar 8.3
Percobaan tetes minyak Millikan
– –
–
–
–
–
–
;1B5>1 @5>71BE8 71I1 25B1D *5D5C1> D5BC52ED :1DE8 ;5 21G18 =5<5G1D9 C52E18 <E21>7 ;539< @141 @5<1D @?C9D96 9 C9>9 D5D5C1> =9>I1; 49C9>1B9 45>71> C9>1B. CE@1I1 D5D5C1> D5BC52ED 25B=E1D1> >571D96 &<58 ;1B5>1 @5<1DD5BC52ED25<E=4925B9=E1D1>D5D5C1>=9>I1;D5BC52EDD5BEC25B75B1; ;5 21G18
)5<19> 71I1 25B1D @141 D5D5C1> =9>I1; D5BC52ED 25;5B:1 :E71 71I1 )D?;5C 41> 71I1 B389=545C 9>71D ;5=21<9 =1D5B9 D5>D1>7 6<E941 49 "5<1C . !149 C531B1 =1D5=1D9C 41@1D 49DE<9C;1> C521719 25B9;ED
%- * . M
&<58 ;1B5>1 D5D5C1> =9>I1; 491CE=C9;1> 25B25>DE; 2?<1!,89(4((5 @ 41@1D 49DE<9C;1> =5>:149
* % * - * . M
"5D5B1>71> %
=1CC1:5>9C=9>I1;
=1CC1 :5>9C E41B1
* :1B9:1B9 D5D5C1> =9>I1; . F?<E=5 D5D5C1> =9>I1; F9C;?C9D1C E41B1
-1<1E@E> 141 71I1 )D?;5C 41> 71I1 B389=545C D5D5C1> =9>I1; D5D1@25B75B1;;521G18;1B5>171I125B1D>I1<529825C1B)525<E=D5D5C1> =9>I1;D5BC52EDC1=@1949@5<1D>571D962541@?D5>C91<491;D96;1>C589>771 =E>3E<71I15<5;DB?CD1D9C;51D1C1>D1B1D5D5C1>=9>I1;41>@5<1D@?C9D96 !9;1 2541 @?D5>C91< 491DEB CE1DE C11D 1;1> D5B:149 ;5C5D9=21>71> 1>D1B1 71I1 25B1D 71I1 B389=545C 41> 71I1 5<5;DB?CD1D9C 141<18
%-) M
5>71>=5>75<9=9>1C9;1>!,89(4((5@41>!,89(4((5@1;1>
4941@1D;1>
)M * . M
) * .
M
"5D5B1>71>
) =E1D1> @1BD9;5<
F9C;?C9D1C
. ;535@1D1> D5D5C1> =9>I1; =541> <9CDB9;
1<1=5;C@5B9=5>>I103302(5=5>7E;EB<1>7CE>725C1B>I141> . D5D1@9 E>DE; =5>41@1D;1> >9<19 F9C;?C9D1C03302(5 =5<1;E;1>
5;C@5B9=5> D5B@9C18
$5>EBED @5>41@1D >41 1@1;18 =E1D1> @1BD9;5< ) 29C1 <1>7CE>7
4941@1D;1> 45>71> =5>77E>1;1>!,89(4((5 @ "5>41<1 I1>7 49
8141@9 E>DE; =5>77E>1;1>!,89(4((5@ 141<18 @1>:1>7 :1B9:1B9 *
41B9D5D5C1>=9>I1;D941;41@1D49E;EB<1>7CE>7'1>:1>7:1B9:1B9D5D5C1> =9>I1; 41@1D 49;5D18E9 45>71> =5>77E>1;1>!,89(4((5 @
FS FA
mg
Gambar 8.4
Gaya berat, gaya Archimedes, dan gaya stokes bekerja pada tetesan minyak pada percobaan Millikan.
Gambar 8.5
Tetesan minyak berada dalam kesetimbangan karena adanya gaya berat, gaya Archimedes, dan gaya elektrostatis.
Felektrostatis FA
mg
Tugas Anda 8.1
*
%-M * .
*
% -.
M
)E2CD9DEC9;1>!,89(4((5@ ;5!,89(4((5C589>7714941@1D;1>
)
%
-.
.
M
&<58 ;1B5>1- %!,89(4((5 @ 41@1D 49DE<9C;1> =5>:149
)
%
. .
M
)5D5<18 =5>7E<1>7 5;C@5B9=5> D5BC52ED 2525B1@1 ;1<903302(5
=5>41@1D;1> ;5C9=@E<1> C521719 25B9;ED
5C1B>I1 =E1D1> D5D5C1> =9>I1; D941; @5B>18 25B>9<19 <5298 ;539<
41B9PM
$E1D1>D5D5C1>>I1C5<1<E;5<9@1D1>2E<1D41B9PM
1B9 ;5C9=@E<1> 9>9 4941@1D;1> 81C9< 218G1 =E1D1> 5<5;DB?> 141<18
PMPM
41> =1CC1>I1 141<18
P ;7
%
%
P
P
M;7
4. Model Atom Rutherford
*5?B9 1D?= *8?=C?> @141 49E:9 ?<58 C5?B1>7 69C9;1G1> 1C1< >77B9C85,9:";:/,8-68+ 1=5>7E:9;525>1B1>D5?B9*8?=C?>45>71>
=5<1;E;1>@5B3?211>=5>77E>1;1>@1BD9;5<1<61I1>749D5=21;;1>@141 C52E18;5@9>7<?71=5=1CI1>7C1>71DD9@9C'1BD9;5<1<61141<18@1BD9;5< I1>7 49@1>31B;1> ?<58 E>CEB B149?1;D96 25B=E1D1> <9CDB9; @?C9D96 I1>7 25C1B>I14E1;1<9=E1D1>5<5;DB?>41>=1CC1>I15=@1D;1<9=1CC1@B?D?> );5=1 @5B3?211> (ED85B6?B441@1D >41 <981D @141(4)(8
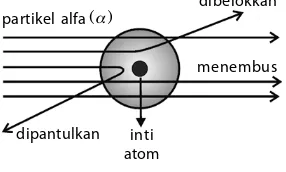
sumber partikel alfa
beberapa partikel alfa dipantulkan kembali
keping tipis emas
pancaran alfa
sebagian partikel alfa dibelokkan
sebagian partikel alfa menembus keping logam
layar detektor
Gambar 8.6
Percobaan Rutherford
Tokoh
Ernest Rutherford (1871–1937)
partikel alfa
inti atom
menembus dibelokkan
dipantulkan
";:/,8-68+ =5=9<9;9 1CE=C9 218G1 :9;1 D5?B9 1D?= *8?=C?> 25>1B
C5<EBE8 @1BD9;5< 1<61 45>71> 5>5B79 I1>7 25C1B 81BEC =5>5=2EC <EBEC ;5@9>7 D9@9C 5=1C D5BC52ED ;1B5>1 1D?=1D?= ;5@9>7 <?71= 5=1C >5DB1< D941; =5>781<1>79 @1BD9;5< 1<61 I1>7 25B=E1D1> <9CDB9; @?C9D96 )5D5<18 49<1;E;1> 2525B1@1 ;1<9 @5B3?211> 49C9=@E<;1> 218G1 C521791> 25C1B @1BD9;5< 1<61 =5>5=2EC ;5@9>7 <?71= D9@9C <EBEC =5>75>19 <1I1B ;1> D5D1@9 2525B1@1 @1BD9;5< 1<61 I1>7 <19>>I1 4925<?;;1> 218;1> 141 I1>7 49@1>DE<;1>)5D91@CE4ED@5=25<?;1>41>@5=1>DE<1>C9>1B1<61D5BC52ED 49E;EB 45>71> D5<9D9 ?<58";:/,8-68+
1;D1 9>9 ;5=E491> 491>1<9C9C ?<58";:/,8-68+ C521719 25B9;ED
5B41C1B;1>E;E=?E<?=2@1BD9;5<1<61I1>725B=E1D1>@?C9D9681>I1 1;1> 4925<?;;1> 1D1E 49@1>DE<;1> ?<58 CE1DE =E1D1> C5:5>9C I19DE =E1D1>@?C9D96BD9>I141<1=1D?=<?71=5=1C81BEC141=E1D1><9CDB9; @?C9D96 41> D941; D5BC521B 49 C5<EBE8 1D?= =5<19>;1> D5B@EC1D 49 CE1DE D5=@1D C589>771 =5>?<1; @1BD9;5< 1<61 D1C 41C1B 61;D1 9>9";:/,8-68+
25B;5C9=@E<1> C521719 25B9;ED
1 $E1D1><9CDB9;1D?=41>C521791>25C1B=1CC11D?=D5B@EC1D49CE1DE D9D9; I1>7 49C52ED ?<58";:/,8-68+ C521719 9>D9 1D?= >D9 1D?= 9>9
=5BE@1;1> CE1DE 415B18 I1>7 C1>71D ;539< 45>71> 491=5D5B C5;9D1B M=
2 '141:1B1;I1>7B5<1D96:1E841B99>D91D?=D5BC52ED@1BD9;5<25B=E1D1> >571D961D1E5<5;DB?>25B541B=5>75<9<9>799>D9$E1D1><9CDB9;@?C9D96 I1>725B@EC1D499>D91D?=>9<19>I1C1=125C1B45>71>:E=<18=E1D1> <9CDB9; >571D96 41B9 5<5;DB?>5<5;DB?> I1>7 =5>75<9<9>79>I1
$5C;9@E><5298219;41B9@141D5?B9!!*8?=C?>>1=E>D5?B91D?= (ED85B6?B4 =1C98 =5>I9=@1> ;5<5=181> "5<5=181> D5?B9 1D?= (ED85B6?B4 141<18 C521719 25B9;ED
*5?B9 1D?= (ED85B6?B4 D941; 41@1D =5>:5<1C;1> C@5;DBE= 3181I1 I1>7 49@1>31B;1> ?<58 1D?= 894B?75> ;5D9;1 71C 894B?75> D5BC52ED 49@1>1C;1> 1D1E 49=1CE;;1> ;5 41<1= D12E>7 41> 4925B9 2541 @?D5>C91< <9CDB9; I1>7 D9>779 1>D1B1 E:E>7E:E>7 D12E>7 D5BC52ED *5?B9 1D?= (ED85B6?B4 D941; 41@1D =5>:5<1C;1> ;5CD129<1> 1D?=
5B41C1B;1> E;E= ?E<?=2 5<5;DB?> I1>7 25B9>D5B1;C9 45>71> 9>D91D?=1;1>=5>71<1=971I1?E<?=2I1>7:E7125B6E>7C9C521719 71I1 C5>DB9@5D1< ;921D>I1 5<5;DB?> =5>71<1=9 @5B35@1D1> @5B35@1D1> C5>DB9@5D1< $5>EBED D5?B9 75<?=21>7 5<5;DB?=17>5D9; I1>749;5=E;1;1>?<58(>=,33:9;1=E1D1>5<5;DB?>=5>71<1=9
@5B35@1D1> =E1D1> D5BC52ED 1;1> =5=1>31B;1> 75<?=21>7 5<5;DB?=17>5D9; !9;1 45=9;91> 5>5B79 5<5;DB?> 25B;EB1>7 41> 1;89B>I1 1;1> :1DE8 D5BD1B9; ;5 9>D9 1D?=
5. Spektrum Atom Hidrogen
)E1DE71CI1>749=1CE;;1>;541<1=D12E>741>;54E1E:E>7D12E>7 D5BC52ED 4925B9 2541 @?D5>C91< <9CDB9; I1>7 D9>779 1;1> =5=1>31B;1> 3181I1 "5D9;1 71C 894B?75> 49=1CE;;1> 41<1= D12E>7 @5<E3ED1> 71C ;5=E491>49<1<E9?<58C9>1B;1D?45=1;171C894B?75>1;1>=5=1>31B;1> 3181I1 181I1 9>9 ;5=E491> 491>1<9C9C ?<58+(#,*'%,* I19DE C52E18
1<1D E>DE; =5<981D 41> =5=?DB5D C@5;DBE= 3181I1 C5;1<97EC =5>7E;EB @1>:1>775<?=21>7>I15B9;ED9>9141<18C@5;DBE=3181I1@141415B18 D1=@1; I1>7 49@1>31B;1> ?<58 71C 894B?75>
inti atom
elektron
Gambar 8.7
Skema penyimpangan sinar alfa.
Gambar 8.8
)5?B1>7 7EBE C5;?<18 =5>5>718 25B;521>7C11> )G54916/(55,9 (34,8 =5>31D1D 218G1 ;5<?=@?; @1>:1>7 75<?=21>7 C@5;DBE= 71B9C
@1>31B 894B?75> 41<1= 415B18 D1=@1; 41@1D 493?3?;;1> C531B1 D5@1D 45>71> BE=EC C521719 25B9;ED
&
# &
M
Keterangan:
& 29<1>71>I1>725B>9<19=E<1941B9 # ;?>CD1>D1>=
@1>:1>7 75<?=21>7 >=
!,89(4((5 @ 49;5>1< C521719 BE=EC 1<=5B 41> 45B5D1> 71B9C
C@5;DBE= I1>7 3?3?; 45>71> @5BC1=11> D5BC52ED 49>1=1;1> 45B5D 1<=5B"?+),8. ;5=E491> =5>5=E;1> BE=EC C5BE@1 @141 E>CEB 1<;1<9
#9%1"41>C5B5D1<=5BE>DE;C@5;DBE=894B?75>49DE<9C41<1= 29<1>71> 75<?=21>7. 9<1>71> 75<?=21>7 E>DE; 45B5D 1<=5B
45B5D 3181I1 D1=@1;141<18
45>71>
&
&
M
Keterangan:
;?>CD1>D1(I425B7P=
'1>:1>775<?=21>7@14145B5DC@5;DBE=1D?=894B?75>49D1=@9<;1> 45>71> @5BC1=11> C521719 25B9;ED
5B5D #I=1> 45B5D E<DB1 E>7E
45>71>
&
&
5B5D 1<=5B 45B5D 3181I1 D1=@1;
45>71>
&
&
5B5D '1C385> 45B5D 9>6B1 =5B18
45>71>
&
&
5B5D B13;5DD 45B5D 9>6B1 =5B18
45>71>
&
&
5B5D '6E>4 45B5D 9>6B1 =5B18
45>71>
&
&
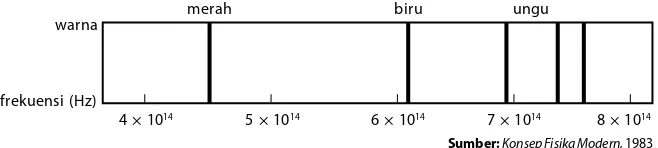
warna
frekuensi (Hz)
merah biru ungu
4 × 1014 5 × 1014 6 × 1014 7 × 1014 8 × 1014
Gambar 8.9
Spektrum hidrogen pada daerah tampak
Tantangan
untuk Anda
Diskusikanlah bersama teman Anda, apakah Anda dan teman Anda dapat
menduga mengapa dalam eksperimennya Rutherford
menggunakan sinar dan lempeng emas?
Tantangan
untuk Anda
Hitunglah panjang gelombang terpendek dan terpanjang untuk deret Balmer, deret Lyman, deret Pascha, deret Bracket, dan deret Pfund.
'14145B5D#I=1>:9;1;?>CD1>D1(I425B7P =89DE>7@1>:1>7
75<?=21>71D5B@1>:1>741>2D5B@5>45; !1G12
+>DE;45B5D#I=1>25B<1;E@5BC1=11>C52171925B9;ED
45>71>
&
&
1 '1>:1>775<?=21>7D5B@1>:1>74981C9<;1>E>DE;&
= =
PM=
2 '1>:1>775<?=21>7D5B@5>45;4981C9<;1>E>DE;&K
= =
K
=
Contoh
8.1
6. Model Atom Bohr
)5D5<18";:/,8-68+=5>75=E;1;1>218G1=1CC141>=E1D1>@?C9D96
1D?= D5B89=@E> @141 CE1DE 415B18 ;539< 49 @EC1D 1D?= 69C9;1G1> 1C1< 5>=1B;0,39 6/8 @141 =5>75=E;1;1> 218G1 CDBE;DEB 1D?=
=9B9@ 45>71> C9CD5= D1D1 CEBI1 >D9 1D?= 25B141 49 @EC1D C9CD5= 1D?= 41>5<5;DB?>25B141@141<9>D1C1>D5BD5>DEI1>749C52ED;E<9D1D?=+>DE; =5>7E1D;1>D5?B91D?=I1>749ECE<;1>>I16/8=5>IECE>4E1@?CDE<1D
41<9< D5>D1>7 1D?= '?CDE<1D @5BD1=1
1<1=CE1DEC9CD5=1D?=5<5;DB?>D941;=5=1>31B;1>B1491C9;5D9;1 91 =5>7?B29D 9>D9 1D?= #9>D1C1><9>D1C1> 5<5;DB?> =5>7?B29D D1>@1 =5=1>31B;1> B1491C9 49>1=1;1> <9>D1C1> CD1C9?>5B 1D1E ?B29D CD1C9?>5B '?CDE<1D 9>9 25B<1G1>1> 45>71> D5?B9 5<5;DB?=17>5D9; ;<1C9;
'?CDE<1D ;54E1
'5=1>31B1>41>@5>I5B1@1>75<?=21>75<5;DB?=17>5D9;41<1=CE1DE 1D?= 25B8E2E>71> 45>71> DB1>C9C9 5<5;DB?> 41B9 4E1 <9>D1C1> CD1C9?>5B
+>DE; C545B81>1>I1 @5B81D9;1>(4)(8 $?45< 1D?= ?8B
E>DE; 1D?= 894B?75> =5=@5B<981D;1> 218G1 C52E18 5<5;DB?> =5>79D1B9 C52E18 9>D9 1D?= 25B=E1D1> @?C9D96 !1B9:1B9 ?B29D <9>7;1B1>>I1* 41>
5<5;DB?> 25B=1CC1% 25B75B1; 45>71> <1:E <9>51B D5D1@. 1I1 D1B9;
?E<?=2 25B@5B1> =5=25B9;1> @5B35@1D1> C5>DB9@5D1< C589>771
3?E<?=2C5>DB9@5D1<
) ) %. %.
* *
* *
&<58 ;1B5>1 E>DE; 1D?= 894B?75> @5BC1=11> D5BC52ED =5>:149
* %.* %. *
>5B79;9>5D9;5<5;DB?>141<18 &<58;1B5>1
#
%. %. #
*
=1;1
#
* M
Gambar 8.10
Model atom Bohr (Z = 1) untuk hidrogen m
v
F
r
inti
elektron
41@E>5>5B79@?D5>C91<5<5;DB?>141<185>5B79@?D5>C91<?E<?=2I1>7 25C1B>I1 C521719 25B9;ED
( * M
C589>771 5>5B79 D?D1< I1>7 49=9<9;9 ?<58 5<5;DB?> 141<18
# ( * * * M "5D5B1>71>
PM %=
=E1D1> <9CDB9; * :1B9:1B9 <9>D1C1> =
a. Jari-Jari Lintasan Stasioner
9C9;1 ;<1C9; =5B1=1<;1> 218G1 C52E18 =E1D1> 5<5;DB9; I1>7 =5>71<1=9@5B35@1D1>C5@5BD95<5;DB?>I1>7=5>7?B29D41<1==?45<1D?= 81BEC=5B1491C9;1>5>5B795<5;DB?=17>5D9;C531B1;?>D9>E"5D9;15>5B79 9>9 49@1>31B;1> 5>5B79 D?D1< 5<5;DB?> =5>EBE> 41> 5<5;DB?> 1;1> 25B75B1; C@9B1< =5>E:E 9>D9 1D?= C589>771 1D?= 1;89B>I1 :1DE8 ;5 9>D9 1D?= +>DE; =5>71D1C9 ;5CE<9D1> 9>96/8 =5>7ECE<;1> 7171C1> ?B29D
CD1C9?>5B@?CDE<1D@5BD1=11B9C9>96/8=5>I9=@E<;1>218G141<1=
;51411> 9>9 =?=5>DE= CE4ED ?B29D1< 5<5;DB?> 25B>9<19 ;5<9@1D1> 41B9 81B71 1D1E 49DE<9C 45>71>
M
,5;D?B =?=5>DE= CE4ED 41<1= 69C9;1 ;<1C9; 494569>9C9;1> C521719
+>DE;=?=5>DE=CE4ED5<5;DB?>I1>725B541B=5>75<9<9>79
9>D9 1D?=* D571; <EBEC( C589>771 @5BC1=11> =?=5>DE= CE4ED 41@1D
49C545B81>1;1> =5>:149 *( %.* !149 =5>EBED @?CDE<1D ?8B
=?=5>DE= CE4ED 141<18
% . *& M
"5D5B1>71>
&29<1>71>2E<1D&
5>71> =5=1CE;;1> 81B71. @141!,89(4((5 @ ;5 41<1=
!,89(4((5 @ 49@5B?<58
# * %. *
% &%* *
%*& *
* % M !9;1 %
4941@1D;1>*&
Tokoh
Niels Bohr (1885–1962)
Pada awal 1900-an, struktur atom menjadi lebih jelas, namun hukum Fisika pada saat itu tidak dapat menjelaskan mengapa elektron tidak cepat menghasilkan spiral menuju inti. Niels Bohr, murid
Rutherford, membantu memecah-kan misteri itu dengan menunjukmemecah-kan bahwa elektron-elektron hanya diberi energi tertentu. Ia menemukan bahwa elektron-elektron yang memiliki energi terendah mengorbit paling dekat ke inti, dan elektron dengan energi tinggi mengorbit paling jauh. Pada 1913, Niels Bohr juga menjelaskan hubungan antara materi dan cahaya. Ia menunjukkan bahwa jika elektron berpindah dari satu tingkat energi ke tingkat energi lainnya, elektron-elektron itu mengeluarkan atau menyerap paket radiasi dalam bentuk cahaya. Paket ini dinamakan foton.
1B9!,89(4((5 @ 41@1D 49@5B?<58 :1B9:1B9 5<5;DB?> I1>7
49@5B;5>1>;1> E>DE; C5D91@ <9>D1C1> 5C1B1>? 494569>9C9;1> C521719
:1B9:1B9?8B+>DE;1D?=894B?75>6/8=5>41@1D;1>81B71?
>= 1D1E 1>7CDB?= 5B41C1B;1> D5?B9 1D?= ?8B 9>9 :1B9:1B9 ?B29D 5<5;DB?>81>I141@1D25B>9<19?????41>C5D5BEC>I1!1B9
:1B91D?==5>EBEDD5?B99>9D941;@5B>1825B>9<19?1D1E? b. Energi Elektron Berdasarkan Teori Bohr
5C1B 5>5B79 5<5;DB?> 41@1D 49@5B?<58 45>71> =5>7712E>7;1>
!,89(4((5 @ 41>!,89(4((5 @ I19DE
& %
&
M
'5BC1=11> 9>9 =5>I1D1;1> 218G1 5>5B79 5<5;DB?> D5B;E1>D9C1C9 BD9>I1 81>I1 >9<19>9<19 5>5B79 D5BD5>DE I1>7 49@5B;5>1>;1> !9;1 41B9 @5BC1=11> D5BC52ED C5=E1 >9<19 D5D1@>I1 4989DE>7 4941@1D @5BC1=11> I1>7 <5298 C545B81>1 C5@5BD9 25B9;ED
5, &
&
M
'141D9>7;1DD5B5>418>9<19&5>5B795<5;DB?>C525C1BM5,
41> 25B541B @141 :1B9:1B9 ?8B L *9>7;1D 5>5B79 5<5;DB?> @141 ;51411> I1>7 <5298 D9>779 49DE>:E;;1> @141(4)(8
'1415<5;DB?>41>9>D91D?=I1>7D5B@9C18C1>71D:1E8I19DEE>DE;
&49@5B?<58!9;15<5;DB?>9DE25BDB1>C9C91D1E25B@9>41841B9
<9>D1C1> <E1B ;5 <9>D1C1> 41<1= C589>771 91 25B141 @141 ?B29D D5BD5>DE 41<1= <9>D1C1>& D5BD5>DE 5<5;DB?> 1;1> =5=9<9;9 81B71 5>5B79 <5298
;539< 41B9@141 ;51411> C5=E<1 '141 ;51411> D5BC52ED 1;1>
49@1>31B;1> 5>5B79 C525C1B> )521<9;>I1 :9;1 5<5;DB?> @141 ;51411> & 5<5;DB?>>I1 41@1D 492521C;1> 41B9 9;1D1> 9>D9 45>71> =5=25B9>I1
5>5B79 C525C1B> >5B79 9>9 49;5>1< 45>71> 5>5B79 9;1D ;51411>& !9;1
5>5B79 I1>7 492521C;1> =5<52989 5>5B79 9;1D ;5<52981> 5>5B79 41@1D =E>3E< C521719 5>5B79 ;9>5D9; 5<5;DB?> I1>7 ;9>9 D5<18 2521C 41> D941; D5B9;1D <179 @141 9>D9 1D?=
?8B=5=@?CDE<1D;1>218G1=5C;9@E>5<5;DB?>D941;=5=1>31B;1> B1491C9 5<5;DB?=17>5D9; ;5D9;1 25B541B @141 CE1DE D9>7;1D D5BD5>DE 5<5;DB?>41@1D25B@9>41841B9CE1DE;51411>5>5B79D5BD5>DE;5;51411> 5>5B79 I1>7 <19> )5<9C98 5>5B79 1>D1B1 4E1 ;51411> D5BC52ED =E>3E< C521719 C52E18 B1491C9 75<?=21>7 5<5;DB?=17>5D9; 25B5>5B79 BD9>I1
:9;15<5;DB?>25B@9>41841B9;51411>1G1<;5;51411>1;89B1;1>D5B:149 @1>31B1> 1D1E @5>I5B1@1> B1491C9 5<5;DB?=17>5D9; B5;E5>C9 75<?=21>7 5<5;DB?=17>5D9; I1>7 49C5B1@ 1D1E 49@1>31B;1> 41B9 4E1 ;51411> CD1C9?>5B 141<18
!
M
"5D5B1>71>
! 5>5B79 =E<1=E<1 !
;?>CD1>D1'<1>3;PM!C
5>5B79 1;89B !
6B5;E5>C9 B1491C9 75<?=21>7 5<5;DB?=17>5D9; J Gambar 8.11
Tingkat-tingkat energi atom hidrogen.
n n = 4
n = 3
n = 2
E = –0,8 eV
E = –1,5 eV
E = –3,4 eV
n = 1 E = –13,6 eV
E = 0
Pembahasan Soal
Dalam model atom Bohr, energi yang dibutuhkan oleh elektron hidrogen untuk pindah dari orbit dengan bilangan kuantum 1 ke 3 adalah ....
a. 1,50 eV b. 1,90 eV c. 2,35 eV d. 12,10 eV e. 13,60 eV
UMPTN 1992 Pembahasan:
Energi yang dibutuhkan E = E3 – E1
Diketahui bahwa energi dasar = E1
= –13,6 eV Maks,
E3 = 1 2
E n
= 2
13,6 eV 3
Jadi,
E= 13,6 eV
9 – (– 13,6 eV)
= 12,10 eV
9DE>7<18;535@1D1>5>5B79;9>5D9;5>5B79@?D5>C91<41>5>5B79D?D1<5<5;DB?>@141 29<1>71>;E1>DE=&25B41C1B;1>D5?B9?8BE>DE;1D?=894B?75>
(=()
9;5D18E9 & % PM;7
PM!C PM
*PM= # P%=
1 ;535@1D1>5<5;DB?>4989DE>745>71>=5>5>DE;1>:1B9:1B9?B29D&D5B<5298
418E<EI19DE
* &PM=PM==1;1
.
!C
& %* ;7 =
. P= C
2 >5B79;9>5D9;5<5;DB?>#4981C9<;1>=5<1<E9@5BC1=11>
;7 = C
#
% .
#PM!
3 >5B79@?D5>C91<5<5;DB?>(4981C9<;1>=5<1<E9@5BC1=11>
%= ! =
(
#
*
4 >5B79D?D1<5<5;DB?>4981C9<;1>=5<1<E9@5BC1=11>
#(
PM!MPM!
MPM!
Contoh
8.2
Kata Kunci
• model atom Demokritus • model atom Dalton • Hukum Perbandingan Tetap • Hukum Lavoisier
• model atom Thomson • tetes minyak Millikan • model atom Rutherford • model atom Bohr • jari-jari Bohr • orbit stasioner
• energi elektron terkuantisasi
Tantangan
untuk Anda
Jika sebuah atom terdiri atas beberapa atom, apakah ada gaya elektrostatik antarelektron tersebut? Kemudian, bagaimana lintasan orbit elektron tersebut?
!9;1:1B9:1B95<5;DB?>I1>725B75B1;@141<9>D1C1>CD1C9?>5B*L25B1@1;18
;5<1:E1>5<5;DB?>D5BC52ED
9;5D18E9=1CC141>=E1D1>>I1%PM;741>PM
!1G12
"5<1:E1>5<5;DB?>.4981C9<;1>45>71>=5>77E>1;1>@5BC1=11>
#
# % . #
* *
%=
= C ;7 =
. #
%*
!149;5<1:E1>5<5;DB?>D5BC52ED141<18P= C
Contoh
8.3
)52E185<5;DB?>@1411D?=894B?75>25B@9>41841B95>5B795;C9D1C9&;5&
5B1@1;186B5;E5>C9@1>31B1>6?D?>>I1
(=()
9;5D18E9 5,
&
&
5, PM! &
PM!C
B. Atom Berelektron Banyak
1. Kelemahan Teori Atom Bohr
"5B1>7;1 41C1B D5?B9 1D?= ?8B C525>1B>I1 =5>713E ;5@141 =?45< 1D?= (ED85B6?B4
525B1@1 ;5<5=181> D5?B9 1D?= ?8B I1>7 41@1D 49C5=@EB>1;1> C589>77141@1D=5>I9>7;1@=9CD5B9D5>D1>71D?=141<18C52171925B9;ED *5?B9 1D?= ?8B D941; 41@1D =5>:5<1C;1> C531B1 B9>39 D5>D1>7 71B9C C@5;DBE= *5BED1=1 @5B25411> @1>:1>7 75<?=21>7 41> 9>D5>C9D1C 71B9C C@5;DBE= D5BC52ED
!E=<18 5<5;DB?> I1>7 D5B<921D 41<1= 1D?= 81>I1 C1DE 5<5;DB?> C589>771 E>DE; 1D?= 25B5<5;DB?> 21>I1; D941; 41@1D 497E>1;1> <179 ?>D?8>I1 1D?= 894B?75> =5=9<9;9 C1DE 5<5;DB?> 41> 71I1 5<5;DB?CD1D9C I1>7 141 81>I1 71I1 D1B9;=5>1B9; 1>D1B1 9>D9 1D?= 41> 5<5;DB?> 41@E> E>DE; 1D?= I1>7 =5=9<9;9 <5298 41B9 C1DE 5<5;DB?> C5<19> 71I1 D1B9;=5>1B9; =1C98 141 71I1 D?<1; 1>D1B1 5<5;DB?> I1>7 C1DE 41> 5<5;DB?> I1>7 <19>6/8=5>71219;1> 71I1
D?<1; 9>9
D?=1D?= 25B712E>7 =5=25>DE; J1D 1D1E =1D5B9 >D5B1;C9 1>D1B 1D?= =5>I5212;1> C961DC961D I1>7 25B2541 1>D1B1 E>CEB I1>7 C1DE 41>E>CEBI1>7<19>'5>I5212@5B25411>C961DC961DD5BC52EDD5B>I1D1 D941; =1=@E 49D5B1>7;1> ?<58 D5?B9 1D?= ?8B
B5;E5>C9@1>31B1>6?D?>4981C9<;1>45>71>=5>789DE>741>D5B<5298418E<E
5, 5, 5, !
&
5, 5, 5, !
&
1
! !
!C
J
$5>71@1 $/6496529C1=5>I9=@E<;1>218G1
5<5;DB?>=5BE@1;1>21791>41B91D?=
!5<1C;1> ;5<5=181>;5<5=181> 41<1= D5?B9 1D?= (ED85B6?B4
9DE>7 @1>:1>7 75<?=21>7 6?D?> D5B@5>45; 41> D5B@1>:1>741B945B5D1<=5B1D?=894B?75>
9DE>7@1>:1>775<?=21>76?D?>I1>749@1>31B;1> 1;921D DB1>C9C9 41B9& ;5& DB1>C9C9 ;5
=5BE@1;1>45B5D'1C385>
1B9 29<1>71> ;E1>DE= ;5 25B1@1;18 DB1>C9C9 I1>7 =5>781C9<;1>@1>:1>775<?=21>7L41B945B5D #I=1>
1B929<1>71>;E1>DE=;525B1@1;18DB1>C9C9I1>7=5>7 81C9<;1>@1>:1>775<?=21>7L41B945B5D1<=5B
9DE>7;535@1D1>5>5B79;9>5D9;5>5B79@?D5>C91<41> 5>5B79D?D1<5<5;DB?>@141?B29D;5?8BE>DE;1D?= 894B?75>
*5>DE;1><18@1>:1>775<?=21>76?D?>I1>749C5B1@ 1D?=894B?75>:9;15<5;DB?>25B5;C9D1C941B9&;5
D9>7;1D&
<5;DB?>1D?=894B?75>41@1D25B5;C9D1C9;5D9>7;1D 5>5B79I1>7<5298D9>7791;921D@5>5=21;1>*5>DE;1> 6B5;E5>C96?D?>3181I1I1>749C5B1@?<581D?=894B?75> 171B5<5;DB?>25B5;C9D1C941B9&;5D9>7;1D&
D?= 894B?75> 49D5=21; 45>71> 5<5;DB?> I1>7 =5=9<9;95>5B795,*5>DE;1>@1>:1>775<?=21>7 B1491C9I1>749@1>31B;1>71C894B?75>
Tes Kompetensi
Subbab
A
$?45<1D?=?8B=5<1>771B@B9>C9@;5D941;@1CD91>I1>749;5=E;1;1> ?<58,09,5),8.
1<1= =5>71D1C9 ;5;EB1>71> D5BC52ED 2525B1@1 69C9;1G1> C5@5BD9
6;09 +, 86.30,&63-.(5. !(;308=05 #*/86+05.,8 41>&(85,8
,09,5),8. =5=@5B;5>1<;1> =5;1>9;1 75<?=21>7 1D1E =5;1>9;1
;E1>DE= E>DE; =5>771=21B;1> ;51411> 5>5B79 5<5;DB?> 41<1= 1D?=
2. Bilangan Kuantum
<5;DB?>5<5;DB?> 25B75B1; =5>75<9<9>79 9>D9 41> 49;5<?=@?;;1> ;5 41<1= ;E<9D;E<9D $5>EBED D5?B9 1D?= ?8B E>DE; =5>I1D1;1> @?C9C9 5<5;DB?>81>I149@5B<E;1>C1DE!$&&#-&,-%I19DE29<1>71>;E1>DE=
ED1=1 5B2541 45>71> D5?B9 1D?= =5;1>9;1 ;E1>DE= @141 D5?B9 9>9 5<5;DB?>41<1=CE1DE1D?=4971=21B;1>41<1=5=@1D29<1>71>;E1>DE= I19DE
29<1>71> ;E1>DE= ED1=1 C9=2?<&
29<1>71> ;E1>DE= ?B29D1< C9=2?< 29<1>71> ;E1>DE= =17>5D9; C9=2?< %
29<1>71> ;E1>DE= C@9> C9=2?<%C a. Bilangan Kuantum Utama (n)
9<1>71> ;E1>DE= ED1=1 & =5>I1D1;1> 25C1B 5>5B79 D?D1< 41<1=
;E<9D 1D?= 1< 9>9 25B1BD9 E>DE; =5>75<E1B;1> 5<5;DB?> 41B9 ?B29D>I1 49@5B<E;1> C5:E=<18 5>5B79 >5B79 D5B5>418 49=E<19 41B9 ;E<9D " ;5=E491> ;E<9D # $ % & 41> C5D5BEC>I1 D?= 894B?75> =5=9<9;9 5>5B79 D?D1< C5CE19 45>71> @5BC1=11>
5, &
&
M
+>DE; 9?> 5 I1>7 81>I1 =5=9<9;9 C52E18 5<5;DB?> 45>71>0
25C1B 5>5B79 D?D1< 5<5;DB?>>I1 141<18
5, 5,
&
0
& &
M
>5B79 D?D1< 5<5;DB?> 41<1= 1D?= 141<18 ;?>CD1> 41>,*#-&,!++!
?<58 29<1>71> ;E1>DE=& 9<1>71> ;E1>DE= ED1=1 =5=9<9;9 >9<19
C541>7;1> >1=1 ;E<9D C5CE19 45>71> 25C1B >9<19& I19DE
E>DE;&>1=1;E<9D"
E>DE;&>1=1;E<9D#
E>DE;&>1=1;E<9D$
E>DE;&>1=1;E<9D%41>C5D5BEC>I1
1>I1;>I1 5<5;DB?> =1;C9=1< I1>7 41@1D =5>79C9 C5D91@ ;E<9D 41@1D 49DE<9C;1> 45>71> BE=EC
&45>71>& M
$9C1<>I1E>DE;;E<9D#29<1>71>;E1>DE=ED1=1>I1&!14921>I1;>I1
Gambar 8.12
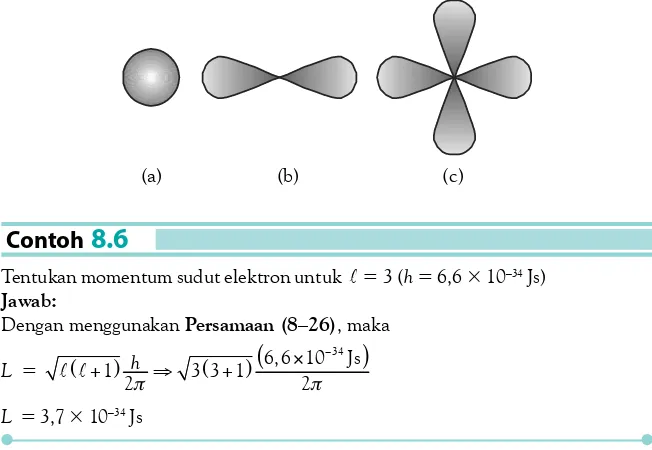
Bentuk-bentuk orbital (a) Orbital s merupakan sebuah bola; (b) Orbital p memiliki bentuk seperti balon yang terpilin dua; (c) Orbital d memiliki bentuk seperti empat buah balon yang terpilin.
D?==5=9<9;95<5;DB?>*5>DE;1>14125B1@1;E<9D41>25B1@15<5;DB?>@141
C5D91@;E<9D
(=()
5B41C1B;1>;?>697EB1C95<5;DB?>BE=EC&4941@1D;1>;E<9D"=5=9<9;95<5;DB?>
;E<9D#=5=9<9;95<5;DB?>41>5<5;DB?>=5>5=@1D9;E<9DC589>771'
!149@1411D?='D5B41@1D;E<9D"M#M$
Contoh
8.5
b. Bilangan Kuantum Orbital ()
>41D5<18=5>75D18E9218G15<5;DB?>25B?D1C9=5>79D1B99>D91D?= @141 ?B29D>I1 9<1>71> ;E1>DE= ?B29D1< =5>I1D1;1> =?=5>DE= CE4ED 5<5;DB?> I1>7 25B?D1C9 D5B8141@ @?B?C 9>D9 1D?= $5<1<E9 @5BC1=11>
*'!&* 25C1B =?=5>DE= CE4ED 5<5;DB?> 141<18
M
45>71>
PM!C
%9<19 25B71>DE>7 @141 >9<19 29<1>71> ;E1>DE= ED1=1& I19DE =E<19 41B9 >?< C1=@19 45>71> & M 1D1E 49>I1D1;1> C521719
&M
)5D91@ ?B29D1< =5=9<9;9 >1=1 41> 25>DE; D5BD5>DE %1=1 ?B29D1< I1>7 49>I1D1;1> ?<58 >9<19 141<18
49>1=1;1> CE2;E<9D++ *( 49>1=1;1> CE2;E<9D((*!&!($ 49>1=1;1>CE2;E<9D!-+ 49>1=1;1> CE2;E<9D -&%&,$
(4)(8 =5=@5B<981D;1> 25>DE; C5D91@ ?B29D1< 9<1>71> ;E1>DE=
?B29D1< :E71 C5B9>7 49C52ED 45>71> 29<1>71> ;E1>DE= 1J9=ED
3 2
1
*5>DE;1>=?=5>DE=CE4ED5<5;DB?>E>DE; PM!C
(=()
5>71>=5>77E>1;1>!,89(4((5@=1;1
!C
PM!C
Contoh
8.6
Atom adalah partikel yang sangatkecil sehingga tidak dapat diamati dengan cahaya tampak.
Menggambarkan struktur atom merupakan pekerjaan yang sangat menarik. Untuk mempelajari atom, para ahli mempelajarinya dari cahaya yang diesensikan oleh atom-atom dari unsur murni. Spektrum cahaya tersebut mengandung informasi tentang struktur, atom, energi ionisasi, dan lain-lain.
Atom are tiny, and not observable using visible light. Trying to figure out the structure of atoms is a facinating task. A major source of information about atoms comes from a study of the light emitted by the atoms of a pure material. The spectrum of the light give information about atom structure, ionization energy, etc.
Informasi
untuk Anda
$(),3
$?=5>DE=)E4ED&B29D1<<5;DB?>
%1=1
+ ( !
CE2;E<9D
"51411>=?=5>DE=CE4ED?B29D5<5;DB?>49>1=1;1>45>71>CE2;E<9D I19DE CE2;E<9D+ E>DE; ;51411> CE2;E<9D( E>DE; ;51411> CE2;E<9DE>DE;;51411>41>C5D5BEC>I1'5B81D9;1>$(),3
5C1B>I1=?=5>DE=CE4ED?B29D1<5<5;DB?>E>DE;45B5D@5BD1=1I1>7 25B1C1< 41B9 C@5;DBE= I19DE 45B5D + + *(( (*!&!($ !-+ 41> -&%&,$ 141<18
)E2;E<9D+25C1B=?=5>DE=CE4ED>I1
)E2;E<9D(25C1B=?=5>DE=CE4ED>I1
)E2;E<9D25C1B=?=5>DE=CE4ED>I1
)E2;E<9D25C1B=?=5>DE=CE4ED>I1
12E>71> 29<1>71> ;E1>DE= ED1=1 & 45>71> 29<1>71> ;E1>DE=
?B29D1< 291C1>I1 497E>1;1> E>DE; =5>I1D1;1> ;51411> 5<5;DB?> 41<1= CE1DE 1D?= $9C1<>I1 + =5>I1D1;1> ;51411> 5<5;DB?> 45>71> &C541>7;1>=5>I1D1;1>;51411>5<5;DB?>45>71>& 41>45=9;91>C5D5BEC>I1
1>I1;>I1 5<5;DB?> =1;C9=E= 41<1= CE2;E<9D+ 141<18 5<5;DB?>
41<1=CE2;E<9D(141<185<5;DB?>CE2;E<9D141<185<5;DB?>CE2;E<9D 141<185<5;DB?>41>C5D5BEC>I1C5@5BD9$(),3
c. Bilangan Kuantum Magnetik (m
)
&<58 ;1B5>1 5<5;DB?> 25B=E1D1> <9CDB9; 75B1; 5<5;DB?> 41@1D 49@5>71BE89 ?<58 =541> =17>5D9; ;921D>I1 5<5;DB?> I1>7 25B75B1; 49 41<1= ?B29D>I1 =5=9<9;9 =?=5> =17>5D9; $?=5> =17>5D9; 41B9 5<5;DB?> 9>9 49>I1D1;1> 45>71> 29<1>71> ;E1>DE= =17>5D9; =
9<1>71> ;E1>DE= =17>5D9; =5>I1D1;1> 1B18 =?=5>DE= CE4ED 5<5;DB?>!9;149D5D1@;1>1B18=541>=17>5D9;<E1BC5:1:1B45>71>CE=2E
0 ;5=E>7;9>1> 41<1= 1B180 49>I1D1;1> 45>71> @5BC1=11>
0
%
M
9<1>71> ;E1>DE= =17>5D9; = =5=9<9;9 >9<19 1>D1B1 M41>
$9C1<>I1
E>DE;81B71=1D1ED5B41@1D?B29D1< E>DE;81B71=
M41>1D1ED5B41@1D?B29D1<
E>DE;81B71=
MM1D1ED5B41@1D?B29D1<
5C1BJ41@1D 49<981D @141(4)(8
$(),3 1>I1;<5;DB?>@141
)5D91@)E2;E<9D
)E2;E<9D + (
1>I1;%5
1>I1;5<5;DB?>
P21>I1;=
2
0
–2
–
m= 2
m= 1
m= 0
m= 1
m= 2
= 2
Lz
Gambar 8.13
Arah dan besar momentum sudut
'141 (4)(8 49@5B<981D;1> F5;D?BF5;D?B =?=5>DE= CE4ED
45>71> 29<1>71> ;E1>DE= ?B29D1< D5BD5>DE 9<1>71> ;E1>DE= =17>5D9; :E71 =5>771=21B;1> ;535>45BE>71> ;54E4E;1> ?B29D1< 49 41<1= BE1>7 C5@5BD9 D5B<981D @141(4)(8
3 2
1
Gambar 8.14
Arah momentum sudut-sudut orbital s, p, dan d
(a) orbital hanya memiliki 1 kemungkinan arah; (b) orbital p memiliki 3 kemungkinan; dan (c) orbital d memiliki 5 kemungkinan.
z z z
Z 0
0
Z
2
h
Z 2
h
1 2 Z
h
Z
2
h
Z 0 Z0
Z
2
h
Z h
Gambar 8.15
Elektron dalam subkulit s, p, dan d.
3
2 1
Gambar 8.16
Arah perputaran dan vektor momentum sudut S.
dxy dxz dyz dx2y2dx2y2
s
px py pz
1
2
1 2
Z 4h
S p
Z 4h
S p
S
$(),3
"5C9=@E<1>"55=@1D9<1>71>"E1>DE=
9<1>71>;E1>DE=ED1=1 &
9<1>71>;E1>DE=?B29D1< >M
9<1>71>;E1>DE==17>5D9; % MMM
9<1>71>;E1>DE=C@9> %C
03(0?(5.07,82,5(52(5
(4( 6:(90
*E>:E;;1><18218G1;E<9D=1;C9=E=49D5=@1D9?<585<5;DB?> (=()
"E<9D=5=9<9;929<1>71>;E1>DE=&
+>DE;&>9<1929<1>71>;E1>DE=?B29D1<>I141>
+>DE;D5B41@1DP2E18>9<19%
+>DE;D5B41@1DP2E18>9<19%
!E=<18 2E18>9<19%
Contoh
8.7
d. Bilangan Kuantum Spin (m s)
9<1>71> ;E1>DE= C@9> %C =5>I1D1;1> 1B18 @5B@ED1B1> 5<5;DB?>
D5B8141@ CE=2E>I1 C@9> I1>7 41@1D =5>9=2E<;1> =?=5> =17>5D9; %9<1929<1>71>;E1>DE=C@9>1414E1I19DE%C E>DE;1B18@ED1B1> 5<5;DB?> ;5 ;1>1> 41>%C E>DE; 1B18 @ED1B1> 5<5;DB?> ;5 ;9B9
$1C9>7=1C9>7 49DE<9C 45>71> >?D1C9 1B18 @1>18 ;5 1D1C 41> ;5 21G18 )E2;E<9D + =5=9<9;9 C52E18 ?B29D1< 41> @1<9>7 21>I1; 499C9 4E1 5<5;DB?>
)E2;E<9D(=5=9<9;9 D971 ?B29D1< (H(I(J 41> @1<9>7 21>I1; 499C9 ?<58
P5<5;DB?><5;DB?>41<1=CE2;E<9D+(41>41@1D4971=21B;1>
45>71> 4917B1= ?B29D1< C5@5BD9(4)(8
5>41I1>725B@ED1B=5=9<9;9=?=5>DE=CE4ED,5;D?B=?=5>DE= CE4ED I1>7 25B;19D1> 45>71> 29<1>71> ;E1>DE=+ 9>9 141<18 I1>7
@1>:1>7>I1
M
B18=?=5>DE=CE4ED49D5>DE;1>?<58;?=@?>5>F5;D?B@141CE=2E0
I19DE CE=2E D571; @141 C9CD5= ;??B49>1D D971 49=5>C9
C
45>71>%C 1D1E M
+>DE;2E18%49D5=@1D9?<582E185<5;DB?>!149E>DE;2E18%49D5=@1D9
?<58P2E185<5;DB?>
(:(:(5
M E>DE;&29<1>71>;E1>DE=?B29D1<>I141>
M E>DE;&29<1>71>;E1>DE=?B29D1<>I141>41>C5D5BEC>I1
*5<18 >41 ;5D18E9 218G1 29<1>71> ;E1>DE= =17>5D9; 5B1D 8E2E>71>>I1 45>71> =541> =17>5D9; I1>7 49D9=2E<;1> ?<58 75B1; 5<5;DB?> 21719=1>1 :9;1 1D?= 894B?75> 49D5=@1D;1> 41<1= =541> =17>5D 8?=?75> '5B81D9;1> 75B1; 5<5;DB?> 41<1= =541> =17>5D
@141(4)(8 !9;1 C52E18 5<5;DB?> 25B=1CC1% 25B75B1; 41<1=
?B29DI1>725B:1B9:1B9*45>71>6B5;E5>C9=?=5>DE=CE4EDI1>749=9<9;9
5<5;DB?> 141<18 %5.* %5* C541>7;1> 1B18 =?=5>DE=>I1
25BCE4ED D5B8141@CE=2E05C1B>I11BECI1>7D9=2E<1;921D75B1;1>
5<5;DB?> 494569>9C9;1> C521719 21>I1;>I1 =E1D1> I1>7 =5>71<9B D91@
45D9; !149 1BEC I1>7 49D9=2E<;1> 75B1;1> 5<5;DB?> ) )
>41:E71=5=181=9218G175B1;1>5<5;DB?>:E71=5>9=2E<;1>=541> =17>5D 9>71D =541> =17>5D 49 C5;9D1B 1BEC <9CDB9; "5D9;1 49 D5=@1D 5<5;DB?>21>I1;4925B9;1>=541>=17>5DI19DE=541>=17>5D41B9<E1B =1;11;1>D5B:1499>D5B1;C91>D1B14E1=17>5D49D5=@1D9>9 =@<9;1C9>I1 141<18 41@1D =5=25B9;1> D?BC9 I1>7 =5>I5212;1> 75B1;1> 5<5;DB?>
25BE218 5C1B>I1 D?BC9 1;921D =541> =17>5D 141<18
P M
45>71>
M
"5D5B1>71>
=?=5>=17>5D9;
<E1C41B92941>75<5;DB?>
&<58;1B5>1M41> B>4141@1D=5>E<9C;1>
*M
*
%
% *
M
*1>41>571D96=5>E>:E;;1>1B18%25B<1G1>1>45>71>1B18=?=5>DE=CE4ED !14925C1B>I1D?BC9I1>749C5212;1>?<58=541>=17>5D 141<18
%
C9> M
*?BC9 9>9<18 I1>7 =5=ED1B ?B29D 5<5;DB?> C589>771 25BE218 1B18 '5B81D9;1>(4)(8 I1>7 =5<E;9C;1> ?B29D?B29D 5<5;DB?> 41>
@5B81D9;1> @5BE2181> 1B18 @ED1B1>>I1 E>DE; =5>75D18E9 25B1@1 ;5=E>7;9>1>@5BE2181>75B1;1>5<5;DB?>41@1D;9D1<981D=5<1<E95>5B79 =17>5D9; 41B9 @5>71BE8 75B1;1> 5<5;DB?> 41B9 =541> =17>5D <E1B
5C1B 5>5B79 =17>5D =5=5>E89 @5BC1=11>
3?C 3?C
%
Gambar 8.17
Gerak elektron dalam medan magnet B.
Tantangan
untuk Anda
Tentukan besar momentum sudut untuk subkulit:
a. f; b. g; c h.
L Z
Gambar 8.18
Arah momentum sudut elektron.
Lz = me
Z
Gambar 8.19
Terpecahnya garis spektrum akibat medan magnet.
5C1B1> % 49>1=1;1> @5=21>49>7 79B?=17>5D9; /*'%&,! *,!'
'5B81D9;1>(4)(8 1=21B D5BC52ED =5>E>:E;;1> 218G1
3?C %
C589>771;9D1@5B?<58
%
3?C
%
%
%
%
M
&<58 ;1B5>1%5 =5=9<9;9 2525B1@1 >9<19 41> 25BC961D 49C;B9D 5>5B79
9>D5B1;C9 =541> =17>5D9; 9>9 :E71 =5=9<9;9 2525B1@1 >9<19
%
%
)5217193?>D?8:9;129<1>71>;E1>DE=5>5B795<5;DB?>1;1> =5>71<1=9 @5BE2181> C521719 25B9;ED
"5D9;1%55>5B795<5;DB?>=5>:149D?D5<5;DB?>1G1<M
%
"5D9;1%5 5>5B79 5<5;DB?> D5D1@ D941; 25BE218
"5D9;1%5M5>5B795<5;DB?>=5>:149D?D5<5;DB?>1G1<
%
!149D9>7;1D5>5B795<5;DB?>@5318=5>:14921791>+>DE; 5>5B79 5<5;DB?> 1;1> @5318 =5>:149 21791> C5CE19 45>71> >9<19%
9>9<18 @5>D9>7>I1 29<1>71> ;E1>DE= =17>5D9; 41<1= =5>5>DE;1> 75B1; ?B29D 5<5;DB?>
'5318>I1 D9>7;1DD9>7;1D 5>5B79 9>9 D5<18 491=1D9 ?<58',,4(5
=5<1<E9 5;C@5B9=5>>I1 1 =5>71=1D9 C@5;DBE= 1D?= 894B?75> ;5D9;1 4925B9 =541> =17>5D9; ;E1D D5B>I1D1 71B9C71B9C C@5;DBE= I1>7 491=1D9 @5318 =5>:149 2525B1@1 21791>
'5318>I171B9CC@5;DBE=9>9D5B>I1D11;921DDB1>C9C941B9D9>7;1D5>5B79 I1>7 D5B@5318 9DE 41@1D >41 @181=9 @141(4)(8 '5BE2181>
;539<5>5B79=5=5>E89@5BC1=11> C589>77141@1D=5=5>71BE89
@1>:1>775<?=21>7=5<1<E9=5D?454965B5>C91C9 1;1>49@5B?<58
D1E 45>71> =5>71=29< >9<19 =ED<1;>I1 49@5B?<58
9DE>7<18@5BE2181>@1>:1>775<?=21>76?D?>I1>7D5B:1491;921DDB1>C9C941B9(;5
+1@129<11D?=894B?75>49D5=@1D;1>41<1==541>=17>5DC525C1B* (=()
M
& &
M
!149@1>:1>775<?=21>7I1>749@1>31B;1>141<18
P !C P = C
5, P ! 5,
P=
'5BE2181>5>5B79 PM! **PM!
!14925B41C1B;1>!,89(4((5@49@5B?<58
P = P !C P = C
PM!PM=
3. Sifat Atom
)961DC961D CE1DE E>CEB 41@1D 491=1D9 45>71> =5<981D CDBE;DEB 1D?=>I1)ECE>1>5<5;DB?>41<1=CE1DE1D?=41@1D49@1;19C52171941C1B E>DE; =5>75D18E9 C961DC961D 1D?= D5BD5>DE '141 C521791> E>CEB ;E<9D ;E<9D 1D?= 141 I1>7 D5B9C9 @5>E8 5<5;DB?> 41> 141 I1>7 ;?C?>7 "E<9D I1>7 D941; @5>E8 D5B9C9 5<5;DB?> 25B141 @141 ;E<9D @1<9>7 <E1B <5;DB?> @141 ;E<9D D5B<E1B 9>9 49>1=1;1>$#,*'& .$&+!
'5>79C91> 5<5;DB?> 49=E<19 41B9 D9>7;1D 5>5B79 D5B5>418 "?>697EB1C9 I1>7=1>D1@D5B41@1D@141CE2;E<9DI1>7D5B9C9@5>E8!9;1CE2;E<9DD5<18 D5B9C9 @5>E8 C9C1 5<5;DB?> 1;1> =5>79C9 CE2;E<9D C5<1>:ED>I1 $9C1<>I1 141 C1DE 5<5;DB?> I1>7 D5B9C9 @141 CE2;E<9D D5B<E1B =1;1 5<5;DB?> 9>9 35>45BE>7 =E418 <5@1C CE@1I1 1D?= =5>:149 CD129< '5<5@1C1> 5<5;DB?> 9>9 41@1D D5B:149 45>71> 31B1 25B9;1D1> 45>71> 1D?= <19>
)521719 3?>D?8 1D?= %1 I1>7 =5=9<9;9 ;?>697EB1C9
+ (
+ +
"E<9D D5B49B9 1D1C CE2;E<9D + (41> 1B9 ;?>697EB1C9 1D?= %1 @141 (4)(8 D5B<981D 81>I1 141 C1DE 5<5;DB?> I1>7 =5>79C9 ;E<9D
I19DE@141?B29D++>DE;=5>:149CD129<1D?=%135>45BE>7=5<5@1C;1>
C1DE 5<5;DB?> D5B<E1B>I1 I1>7 D5B41@1D @141 ;E<9D )521<9;>I1 :9;1
@141 CE2;E<9D D5B<E1B ;5;EB1>71> C1DE 5<5;DB?> E>DE; =5>:149 CD129< 1D?= 9>9 35>45BE>7 =E418 =5>5B9=1 C1DE 5<5;DB?> 41B9 <E1B
'5B81D9;1>:E71(4)(8 '1411D?=$>;E<9DD5B<E1B>I1I19DE
;E<9D D5B9C9 5<5;DB?> "E<9D D5B9C9 =1;C9=1< 5<5;DB?> 25B1BD9
D5B41@1D;5;EB1>71>5<5;DB?>C589>7711D?=$>35>45BE>7=5>5B9=1 C1DE 5<5;DB?> 41B9 1D?= <19> CE@1I1 =5>:149 CD129< D?= %1 41> $> 41@1D 25B712E>7 =5>:149 =?<5;E< I1>7 CD129< ;1B5>1 @141 C5D91@ 1D?= 1;1> =5=9<9;9 ;E<9D 1D1E CE2;E<9D I1>7 D5B9C9 @5>E8 ?<58 5<5;DB?>
Contoh
8.8
Gambar 8.21
(a) Atom Mn menerima elektron dari atom Na.
(b) Atom Na yang tidak stabil.
2 1
elektron dilepas
Na Na
Gambar 8.20
(a) Atom Na, kulit terluarnya terisi satu elektron.
(b) Atom Na yang stabil setelah melepas elektron terluarnya.
2 1
e
1B9 EB191> D5BC52ED 41@1D 491=29< ;5C9=@E<1> 218G1 1D?=1D?= 35>45BE>7 E>DE; =5=9<9;9 ;?>697EB1C9 C5@5BD9 71C =E<91 I19DE =5=9<9;9 ;51411> I1>7 CD129< 45>71> 31B1 =5<5@1C 1D1E =5>5B9=1 5<5;DB?>
4. Sistem Periodik
)9CD5= @5B9?49; E>CEBE>CEB 49CECE> 25B41C1B;1> ;5>19;1> >?=?B 1D?= 1D1E 25B41C1B;1> :E=<18 5<5;DB?> )9CD5= @5B9?49; 25BDE:E1> =5>:5<1C;1>C961DE>CEBE>CEB@141@5B9?49;41>7?<?>71>D5BD5>DEC5BD1 =5>:5<1C;1>=5>71@1E>CEBE>CEB41<1=C1DE@5B9?45=5=9<9;9C961DI1>7 25B2541 1>D1B1 7?<?>71> I1>7 C1DE 41> 7?<?>71> I1>7 <19>>I1
+>CEBE>CEB 7?<?>71> 1<;1<9 41> 1<;1<9 D1>18 ;54E1>I1 D5B=1CE; 7?<?>71>+ ;1B5>1 41<1= ;?>697EB1C9 5<5;DB?> 491;89B9 45>71> CE2;E<9D+
!1B9:1B91D?=49C525<18;9B9B5<1D96<529825C1B4921>49>7;1>45>71>E>CEB E>CEB C525<18 ;1>1>>I1 41>I1 5<5;DB?> D5B<E1B 9>9 =5=25B9;1> C961D ;?>4E;D?B <9CDB9; I1>7 219; "5B51;D961>>I1 3E;E@ D9>779 E>DE; 25B51;C9 45>71>E>CEB<19>D5BED1=145>71>E>CEB7?<?>71>81<?75>;1B5>1I1>7 C1DE =E418 =5<5@1C;1> 5<5;DB?> 41> I1>7 <19> =5=9<9;9 ;535>45BE>71> 25C1B E>DE; =5>5B9=1 5<5;DB?>
+>CEBE>CEB7?<?>71>(I1>7D941;D5B9C9@5>E8?<585<5;DB?>41<1=
;?>697EB1C9>I1 D5B<981D ;5;EB1>71> 5<5;DB?> E>DE; =5=9<9;9 ;?>697EB1C9 C5@5BD9 71C =E<91 !9;1 >41 @5B81D9;1> 7?<?>71> 81<?75> E>CEBE>CEB 9>9 35>45BE>7 =E418 =5>1>7;1@ 5<5;DB?> E>DE; =5=25>DE; C5>I1G1 9;1D1;1> E>CEBE>CEB D5BC52ED =5=9<9;9 ;5B51;D961> I1>7 D9>779 4921>49>7;1> 45>71> E>CEB 7?<?>71>( <19>>I1
?<?>71>71C=E<91=5=9<9;9;?>697EB1C95<5;DB?>I1>7CE418@5>E8 @141 ;E<9D D5B<E1B>I1 "535>45BE>71> =5>1>7;1@ 1D1E =5>5B9=1 5<5;DB?> C1>71D ;539< 1< 9>9 =5>I5212;1> E>CEB 71C =E<91 D941; =5=25>DE; C5>I1G1 45>71> E>CEB <19>
'B9>C9@ '1E<9 1DEB1> E621E 41> ;19418 E>4 C1>71D =5=21>DE E>DE; =5=181=9 ;5D5B1DEB1> CECE>1> 5<5;DB?> 1D1E CE2;E<9D 41<1= 416D1B 25B;1<1 "?>697EB1C9 5<5;DB?> 41<1= 1D?= 141<18 CECE>1> 5<5;DB?> 41<1= 1D?= C5CE19 45>71> D9>7;1D 5>5B79>I1 1<1= @5=2181C1> 9>9 1;1> >41 D9>:1E ;?>697EB1C9 5<5;DB?> @5B CE2;E<9D 5>71>;?>697EB1C95<5;DB?>D5BC52ED41@1D49;5D18E929<1>71>;E1>DE= I1>7 25B;19D1> 41> C961DC961D>I1 41@1D 49;5D18E9 41B9 :E=<18 5<5;DB?> D5B<E1B DEB1> @5>E<9C1> ;?>697EB1C9 5<5;DB?> 25B41C1B;1> 81<81< 25B9;ED 9>9
a. Aturan Aufbau
$5>EBED 1DEB1> E621E 5<5;DB?> 41<1= CE1DE 1D?= 1;1> =E<19 =5>79C9 CE1DE ?B29D1< 41B9 D9>7;1D I1>7 5>5B79>I1 @1<9>7 B5>418 C1=@19 I1>7@1<9>7D9>779*91@D9>7;1D1>5>5B79499C95<5;DB?>C521>I1;=E>7;9> C1=@19@5>E8*91@;1<9;51411>&
@5>E8;51411>25B9;ED>I1=E<19499C945=9;91>C5D5BEC>I1'B9>C9@@5>79C91>9>941@1D4971=21B;1>41<1= 25>DE; 4917B1= @141 (4)(8
'5B81D9;1> 8E2E>71> 29<1>71> ;E1>DE= ED1=1 & 41> 29<1>71>
;E1>DE= ?B29D1< 1D1E 1J9=ED C521719 25B9;ED +BED1> D9>7;1D 5>5B79 C5CE19 45>71> EBED1> 1B18 @1>18 I19DE
++(+(+(+(+45>71>
+
5<5;DB?>(5<5;DB?>5<5;DB?>5<5;DB?>Tantangan
untuk Anda
Jelaskan sifat-sifat yang dimiliki atom berikut ini.
1. 40 20Ca 2. 30
31Ga 3. 80
35Br
Gambar 8.22
Pengisian elektron menurut aturan Aufbau. 1s
2s
3s
4s
5s
6s
7s 2p
3p
4p
5p
6p 3d
4d
5d
)521719 3?>D?8 @5B81D9;1> 31B1 @5>E<9C1> ;?>697EB1C9 5<5;DB?> 25B9;ED
D?==5=9<9;9>?=?B1D?=25B1BD941<1=@141C5D91@1D?=>I1 D5B41@1D5>1=5<5;DB?>C589>771;?>697EB1C9>I1++(
D?= " =5=9<9;9 >?=?B 1D?= 25B1BD9 @141 C5D91@ 1D?=>I1 D5B41@1D 5<5;DB?> C589>771 ;?>697EB1C9 5<5;DB?>>I1" ++
(+(+
D?= ? =5=9<9;9 >?=?B 1D?= 25B1BD9 @141 C5D91@ 1D?=>I1 D5B41@1D 5<5;DB?>C589>771;?>697EB1C95<5;DB?>>I1?++
(+(+
1B1 @5>E<9C1> D5BC52ED 3E;E@ @1>:1>7 C589>771 81BEC 49<1;E;1> @5>I9>7;1D1> 25B41C1B;1> ;?>697EB1C9 71C =E<91 I19DE
5 +
%5 ++( B ++(+(
"B ++(+(+(
5>71> 45=9;91> ;?>697EB1C9 5<5;DB?> @141 3?>D?8 D5BC52ED 49C9>7;1D C521719 25B9;ED
++( 49C9>7;1D 5 + ( " ++(+(+ 49C9>7;1D B + ? ++(+(+ 49C9>7;1D B +
c. Aturan Hund
$5>EBED;5+41<1=?B29D1<I1>7C5D9>7;1D5<5;DB?>5<5;DB?>D941;
2?<58 25B@1C1>71> C525<E= C5<EBE8 ?B29D1< C5D9>7;1D D5B9C9 ?<58 C52E18 5<5;DB?>'5>5=@1D1>5<5;DB?>@141?B29D1<( I1>7=5=9<9;9D9>7;1D
5>5B79 I1>7 C1=1 @141 CE2;E<9D I1>7 C1=1 =1C9>7=1C9>7 499C9 45>71> C1DE5<5;DB?>D5B<5298418E<E45>71>1B18C@9>I1>7C1=121BE;5=E491> 499C9 45>71> 5<5;DB?> 25B9;ED>I1 45>71> 1B18 I1>7 25B<1G1>1>
1< 9>9 25B1BD9 C5=E1 5<5;DB?> 25B=E1D1> C1=1 C589>771 5<5;DB?> 1;1> =5>5=@1D9 ?B29D1< I1>7 =1C98 ;?C?>7 C525<E= 25B@1C1>71> $9C1<;1> 1D?= 45>71> >?=?B 1D?= 41> ;?>697EB1C9 5<5;DB?>>I1 ++( 4917B1= ;?>697EB1C9 5<5;DB?>>I1 141<18
2E;1>
c. Asas Larangan Pauli
$5>EBED&63-.(5.!(;3041<1=CE1DE1D?=D941;2?<581415<5;DB?>
I1>7=5=9<9;9;55=@1D29<1>71>?B29D1<41>=17>5D9;I1>7C1=19<1>71> ;E1>DE= C@9>>I1 81BEC 25B<1G1>1> &%
41>%C
2E;