The ability of pea transformation technology to transfer genes into
peas adapted to western Canadian growing conditions
P.L. Polowick *, J. Quandt
1, J.D. Mahon
Plant Biotechnology Institute,National Research Council of Canada,110Gymnasium Pl.,Saskatoon,Sask.,Canada S7N0W9
Received 9 September 1999; received in revised form 1 December 1999; accepted 3 December 1999
Abstract
Transgenic pea plants can be produced byAgrobacterium-mediated transformation of thin slices from developing embryo axes. To determine if the method is effective for different pea genotypes, seven pea breeding lines adapted to western Canadian growing conditions were tested, using three different Agrobacterium tumefaciens transformation vectors. All vectors contained the gus
(uidA) gene coding for the b-glucuronidase (GUS) protein, but with different chemical selection genes. In total, 323 transgenic plants were recovered from 39 independent transformation events. Transgenic plants were recovered from each genotype and each selection system, but not from all combinations. GUS-positive explants were obtained from seeds harvested between 24 and 31 days after flowering. The mean time fromAgrobacteriumtreatment to planting into soil averaged 186 days. Based on the initial number of seeds used, the transformation frequency was 0.6% (i.e. six independent transgenic events per 1000 axes sliced). The inserted genes were functional and inherited in a Mendelian fashion. Although more plants were recovered by selection on chlorsulfuron, GUS activity was generally greater in plants selected on kanamycin. GUS activity in the leaves of the original plants varied, but GUS activity in the second generation was correlated with that of the original transformants. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Plant transformation;Agrobacterium tumefaciens; Pea (Pisum sati6um); Genotype independence; GUS (b-glucuronidase)
www.elsevier.com/locate/plantsci
1. Introduction
Grain legumes are important world-wide, as a source of human and animal food and an impor-tant component in crop rotations. The demand for these crops is expanding but they are susceptible to weed competition and pathogen attack. There-fore, there is widespread interest in incorporating novel genetic traits, such as herbicide tolerance and disease and insect resistance, which can often be introduced only via genetic transformation technology. The application of plant biotechnol-ogy to crop improvement has developed rapidly
and transgenic (‘genetically engineered’) cultivars are available. However, the potential benefits of transgenics are not yet available for pulse crop improvement. Like many other grain legumes, peas are recalcitrant to regeneration in vitro. Moreover, regeneration of peas from transformed tissue culture explants has been reported to pro-duce such genetic abnormalities as polyploidy and loss of introduced traits [1], chimaeras and escapes [2] and infertility [3].
In 1993, scientists in the Division of Plant dustry, at the Commonwealth Scientific and In-dustrial Research Organization (CSIRO), Australia reported successful, reproducible trans-formation of peas usingAgrobacterium and devel-oping embryo axes of two pea genotypes, Greenfeast and Rondo [4]. Since 1995, the Legume Biotechnology Group of the Plant Biotechnology Institute has been producing transgenic pea plants
NRCC c43778.
* Corresponding author. Tel.: +1-306-9755584; fax: + 1-306-9754839.
E-mail address:[email protected] (P.L. Polowick) 1Present Address: Aventis Cropscience c203 – 407 Downey Road, Saskatoon, Sask., Canada S7N 4L8.
by inserting genes into Greenfeast, using a method modified from that used at CSIRO. The transgenic plants grow well and the inserted genes are func-tional and inherited [5]. However, Greenfeast is not suitable for western Canadian growing condi-tions or markets. Thus, an important question is: can we transform cultivars that are more suitable for the western Canadian industry, or is the ability to transform peas limited to a few genotypes?
To help answer this question, seven advanced breeding lines were obtained from three pea breed-ing programs and used for transformation experi-ments, with Greenfeast included for comparison. ThreeAgrobacteriumvectors with different chemi-cal selection systems were used in these transfor-mation experiments. The purpose of this study was to examine the effects of pea genotype, gene con-struct and developmental stage of pea seeds on the regeneration of transgenic pea.
2. Materials and methods
2.1. Construction of binary Agrobacterium
transformation 6ectors for pea transformation
2.1.1. LBG66
A bi-functional fusion gene (uidA::nptII) confer-ring both b-glucuronidase (GUS) and neomycin
phosphotransferase (NPTII) activities [6], with a 35S35SAMV promoter [7], a NOS terminator and an intron [8] was cloned as a HindIII – EcoR1 fragment into pPBI3008 (Fig. 1) to produce the binary plasmid pPBI3010.
2.1.2. LBG71
An intron-containing uidA gene with a 35S35SAMV promoter and a NOS terminator was introduced into the pBIN19 binary transformation vector [11]. A phosphinothricin N -acetyltrans-ferase (pat) gene [12] with a 35S35SAMV pro-moter and a 35S terminator was inserted 5% to the uidA gene, in the same transcriptional orientation, to produce the binary vector pPBI3011.
2.1.3. LBG75
A mutant acetohydroxy acid synthase (ahas3r) gene [13] with a 35S35SAMV promoter and its own terminator sequence, was introduced into pPBI3010, 3% to the uidA::nptII gene and in the opposite transcriptional orientation, to produce the binary vector pPBI3020.
Each of these binary plasmids was electropo-rated into the EHA105 disarmed Agrobacterium strain [14]. The Agrobacterium cultures were grown in darkness at 28°C on a rotary shaker in 2YT medium containing (l−1): yeast extract (10 g),
tryptone (1 g) and NaCl (5 g), pH 7.0 with appro-priate antibiotics. Overnight cultures were cen-trifuged and resuspended in 2YT to a final concentration of 108bacteria cells ml−1of culture
(A660=0.06).
2.2. Plant germplasm used for transformation and
plant culture
Eight pea (Pisum sati6um L.) genotypes were used in the transformation experiments (Table 1). A line selected from the cultivar Greenfeast, from which the recovery of transgenic peas has already been demonstrated [4,5], was used as the control genotype.
Pea seeds were planted in 15 cm plastic pots filled with a 1:1 mixture of commercial potting soil (Sunshine Mix c1) and vermiculite. Plants were grown in a controlled environment chamber at 20/15°C with a 16 h photoperiod at 200 mmol quanta m−2s−1supplied by mixed
fluorescent-in-candescent lamps. At the 4 – 6 leaf stage, Nutricote Type 100 (14 – 14 – 14) slow release fertilizer was
Fig. 1. Construction of binary plasmid pPBI3008. The binary plasmid pPBI3008 was constructed from: a 2332 bpHindIII –
Table 1
Pea genotypes used in pea transformation experiments: descriptions and sources
Label Genotype Description Source
Selected line, wrinkled green seed, normal leaf
Greenfeast CSIROa
GF
Austrian winter pea cultivar, speckled seed, semi-leafless
VI CDC Vienna CDCb
Breeding line, green seed, normal leaf, high yield, early maturing
S2-90-25E CDC
25E
93-4-18G
18G Breeding line, green seed, semi-leafless CDC Breeding line, yellow seed, normal leaf, high yield
MP1338 AA-FCc
M38
Breeding line, yellow seed, semi-leafless
M82 MP1382 AA-FC
Breeding line, green seed, semi-leafless
AWPNZ66 AWPd
NZ66
AWP1512
1512 Breeding line, green seed, semi-leafless AWP
aH.E. Schroeder, Plant Industry Division, CSIRO, Australia.
bA.E. Slinkard, Crop Development Centre, University of Saskatchewan, Saskatoon, Sask., Canada. cT.D. Warkentin, Agriculture and Agri-Food Canada, Morden Man., Canada.
dT. Ferguson, Alberta Wheat Pool, Calgary, Alta, Canada.
added to each pot. The closed flower of pea makes it difficult to determine anthesis without poten-tially causing damage to the flower. Therefore, three times per week, all newly opened flowers were tagged with coloured tape so that stages of seed development could be identified, based on the number of days after flowering (DAF).
2.3. Preparation of embryo explants
Seeds, aged between 23 and 34 DAF, were collected in groups of the same age. For the purpose of reporting, the explants were combined into three age groups of: 23 – 26, 27 – 30 and 31 – 34 DAF. The seeds were removed from the pods and surface sterilized for 1 min in 70% ethanol and 8 min in 1% (w/v) NaClO, and then rinsed thor-oughly with sterile distilled water. The seed coat and one cotyledon were removed from each seed and the radicle was excised. The remaining em-bryo axis tissue was sliced longitudinally into five slices, with a blade dipped in the Agrobacterium, as described by Schroeder et al. [4]. The slices were placed on a co-cultivation medium which was modified from Brown and Atanassov [15] and contained (l−1): KNO
3 (3 g), CaCl2 (770 mg),
L-glutamine (800 mg), MgSO4·7H2O (500 mg),
L-serine (100 mg), glutathione (10 mg), adenine (1 mg), sucrose (30 g), 2,4-D (1 mg), kinetin (0.2 mg) and agar (0.8%). In addition, acetosyringone was added to the co-cultivation medium after autoclav-ing, to a final concentration of 100 mM. A 5 ml droplet of Agrobacterium suspension culture was added to each of 35 thin slices in a 60×15 mm plastic Petri dish.
2.4. Co-culti6ation of explants and reco6ery of
transgenic plants
The explants were incubated at 25°C with a 16 h photoperiod of fluorescent light at 35 – 55 mmol quanta m−2 s−1 for 4 days and were then rinsed
in 300 mg l−1Timentin®, a mixture of the
antibi-otic ticarcillin and clavulanic acid [16]. The ex-plants were transferred to a callusing medium, P1 [4], with 150 mg l−1Timentin®, and the
appropri-ate selection chemical (L-phosphinothricin (L-PPT), 10 mg l−1; kanamycin, 40 mg l−1; or
chlorsulfuron, 10 mg l−1). The explants were
re-turned to the same incubation conditions for 14 days. For an early indication of cell transforma-tion, random samples of five slices per Petri dish were collected for a quantitative determination of GUS activity.
The remaining explants were transferred to a shoot induction P2 medium [4], also with Ti-mentin® and the appropriate selection chemical.
Any shoots that developed were excised and dis-carded as they probably arose from pre-existing meristems. Surviving explants were transferred to fresh P2 medium every 3 weeks. Any shoots, or clusters of shoots produced thereafter were excised from the explants and transferred to 100×25 mm plastic Petri dishes with MS7T medium, a MS [17] medium with B5 vitamins [18], 3% sucrose, 150 mg l−1Timentin®and benzylaminopurine (BAP; 1 mg
l−1), to enhance shoot elongation. The
concentra-tion of selecconcentra-tion chemicals was increased (L-PPT, 15 mg l−1; kanamycin, 50 mg l−1; chlorsulfuron,
50 mg l−1). Elongated shoots (1.5 – 2 cm) were
B5/2T rooting medium, with half-strength B5 salts and vitamins, 3% sucrose, 150 mg l−1 Timentin®,
and NAA (0.185 mg l−1). The selection chemicals
remained at the same level as in the shoot elonga-tion (MS7T) medium. Once roots were estab-lished, the young transformants were transferred to pots of soil and grown as described for the original plants.
2.5. Analysis of putati6e transgenic plants for
presence and expression of inserted genes
2.5.1. b-Glucuronidase assays
A b-glucuronidase (X-Gluc) histochemical reac-tion [19] was used on small amounts of tissue (leaf, stipule, tendrils) for visual identification of GUS activity in young regenerated plants. For a quanti-tative determination of GUS activity, assays using 4-methyl umbelliferyl b-D-glucuronide (MUG) as a substrate [20] were performed. Tissue equivalent to one axis (five slices) was homogenized in 500ml of GUS extraction buffer [20]. The samples were centrifuged at 5°C (14 000 rpm) for 2 min and a 100 ml sample was removed for measurement of protein concentration using a dye-binding Brad-ford assay [21] (BioRad Richmond, CA). This volume was replaced with 100 ml of a 5 mM solution of MUG and the samples were incubated at 37°C. After 15 min and again after a further 4 h, the samples were vortexed and centrifuged as above. A 100 ml sample of the supernatant was added to tubes containing 900 ml of stop buffer (0.2 M Na2CO3). The 4-methyl umbelliferone
(MU) concentration was determined with a Perkin Elmer LS50 fluorometer and GUS activity was expressed as pmol MU axis−1 min−1. The same
procedure was used with two leaf discs (8 mm) from putative transformants, once established in the soil.
2.5.2. Southern hybridization analysis
To confirm the incorporation of inserted genes, determine the number of inserts, and identify inde-pendent transgenic events, Southern blot analysis was performed on each putative transgenic plant. Genomic DNA was extracted from four leaf discs of each plant using a CTAB method modified from Murray and Thompson [22]. The DNA was digested with restriction enzymes as follows: LBG66, HindIII forgus; LBG71 HindIII for gus, EcoRI for pat; LBG 75,EcoRI for both gus and
ahas. The gus probe was an 1800bp BamH1/Sst1 fragment from pBI121 [19]. The pat probe was a 550 bp SalI fragment of the plasmid pPAT2, acquired from AgrEvo Canada. The ahas probe was a mixture of 2 – 300 bp HindIII fragments from ahas3r [23]. The probes were labelled with [32P]dCTP using random primers. Hybridization
was done with the QuikHyb system (Stratagene).
2.5.3. Segregation analysis
To examine the inheritance of thegus gene, the T1 progeny from different plant genotype/
Agrobacterium vector combinations were planted
under the same conditions as the donor plants. Leaf discs (8 mm) were collected for analysis by both X-Gluc and MUG assays.
3. Results and discussion
Due to genotypic differences in flowering and seed production, the number of explants prepared on any given day was variable. Nevertheless, over the course of the experimentation, explants from all combinations of Agrobacterium construct (3), genotype (8) and seed age group (3) were co-culti-vated. This produced a total of 72 treatments and the mean number of plates (seven seeds per plate) for each treatment was 4296 (S.E.M.).
Because of the long culture time required for the recovery of transgenic pea plants, a quantitative MUG determination of GUS activity at the end of the P1 culture stage was used as an interim check on the proper functioning of both the gene con-structs and their insertion into cells of the ex-plants. GUS activity is an indicator of the extent to which the T-DNA was incorporated into plant cells. Mean GUS activities from each treatment (Fig. 2) indicated that pea genotype had no effect on GUS activity. There were obvious differences in GUS activity produced by the three Agrobac
-terium vectors used to transform the plants, even
meth-Fig. 2. Meanb-glucuronidase (GUS) activity per mg protein in embryo axis explants co-cultivated with differentAgrobacterium
vectors, after 14 days on P1 callusing medium. The capped bars indicate one-half of the 95% confidence intervals for the individual genotypes or seed age (days after flowering, DAF) classes.
ods, the LBG71-type of construct produced only 20 – 50% of GUS activity produced by a similar construct lacking only the pat gene (unpublished results).
The original description of this method empha-sized the importance of using seeds at a stage of maturation representing 2 – 5 days beyond maxi-mum fresh weight [4]. In the present study, seeds ranging in age from 23 to 34 days after flowering had similar GUS activities at the end of the P1 stage.
GUS activity in explants co-cultivated with dif-ferent Agrobacterium vectors could also be influ-enced by the chemical selection systems used. Selection chemicals are used to inhibit (or kill) plant cells that are not protected by the presence of the corresponding resistance gene — i.e. un-transformed cells. Thus, culture in the presence of the selection chemical should increase the propor-tion of transformed to untransformed cells and increase GUS activity in surviving tissues. Three different selection systems were used with these constructs and they may have differed in their ability to enrich the proportion of transformed cells. Differences in selection pressure can affect the GUS activity of explants as seen from a pre-liminary experiment in which an increase in chlor-sulfuron in the P1 medium (from 5 to 10 mg l−1)
raised the GUS activity from 14 to 700 pmol mg protein−1 min−1.
3.1. Reco6ery of transgenic plants
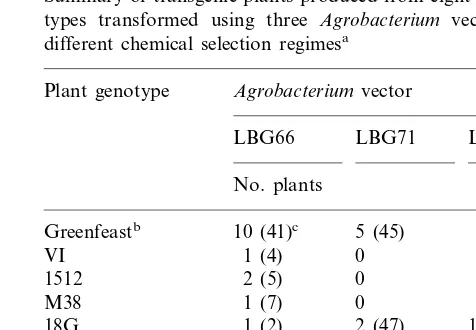
During the course of this project, 323 transgenic pea plants were recovered from the seven new genotypes tested (Table 2). A single explant may over time produce a large number of shoots. Such shoots were considered as clones as many of these would be from a common transformation event.
Table 2
Summary of transgenic plants produced from eight pea geno-types transformed using three Agrobacterium vectors with different chemical selection regimesa
Plant genotype Agrobacterium vector
LBG66 LBG71 LBG75
No. plants
10 (41)c 5 (45) 3 (12)
Greenfeastb
6 (119) 0
VI 1 (4)
4 (22)
1512 2 (5) 0
M38 1 (7) 0 2 (23)
2 (47) 15 (60) 1 (2)
18G
1 (1)
NZ66 0 1 (1)
0
25E 0 2 (29)
M82 1 (3) 0 0
aTotal number of independent events (including Greenfeast
control)=57; total number of independent events with new genotypes=39.
bGreenfeast data includes plants from previous
experi-ments.
cNumber in parentheses=total number of plants
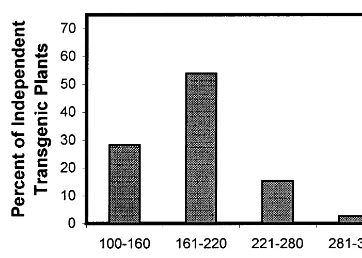
Fig. 3. Time required from co-cultivation of embryo explants to the planting of rooted shoots into soil for 39 independent transgenic pea plants.
plants and transplanting into soil of the first rooted plant from each transgenic event was 186 days (6 months). The greatest number of inde-pendent transgenic plants (54%) was recovered between 161 and 220 days, with 28% between 100 and 160 days, and 18% requiring more than 220 days (Fig. 3). Different regeneration systems for pea tissue have been reported to require 4 [2], 7 [24], 9 months [4] or longer [1].
A second problem is the low frequency of trans-genic plant recovery. In the present study, the overall mean percent of embryo axes that pro-duced transgenic pea plants was 0.6% (Table 3). There was variability among treatments, ranging from 0 to 6.4%; however, with the small sample size, ANOVA analysis showed no significant dif-ference between plant genotypes or Agrobacterium vectors. This value of 0.6% indicates that we might reasonably expect to recover six genetically differ-ent transgenic plants per 1000 seeds (5000 axis slices). Considering this low frequency of success, it is not surprising that transgenic plants did not arise from all treatment combinations over the 1-year term of the experiment. With other re-ported methods of pea transformation, the success rate ranged from 1.1 to 2.5% [2,4,24]. In all cases, the rate was much lower than that of efficiently transformed species such as Brassica carinata(30 – 50%) [26] and Arabidopsis (\50%) [26]. In to-bacco, multiple independent transgenic plants routinely develop from a single leaf disc [27].
The chemical used for selection can also influ-ence transformant recovery. Babic et al. [25] recov-ered fewer B. carinata plants using L-PPT selection (1 – 2% success) as compared to kanamycin (30 – 50%). In the present study, there were more plants regenerated on chlorsulfuron than on either kanamycin or L-PPT (Tables 2 and 4). In other pea transformation systems, the choice of selection chemical was important for the suc-cessful recovery of transformed plants. Several groups reported that the nptIIgene for kanamycin resistance was ineffective in transgenic pea selec-tion [1,4], although it was reported as effective in a method utilizing immature pea cotyledons [28]. In the present study, as well as in preliminary work with the cultivar Greenfeast, kanamycin was effec-tive in producing transgenic plants with high ex-pression of the gus gene. Successful use of L-PPT in the recovery of transgenic pea plants was found in the present study and reported by others [4,24].
Table 3
Percent of embryo axes producing independent transgenic pea plants
aData for Greenfeast (GF) includes previous experiments.
This can be verified by Southern hybridization, where clones have identical DNA fragment pat-terns. In these experiments, the 323 transgenic plants were from a total of 39 independent trans-formation events with between 1 and 45 clones of each independent type.
Transgenic plants were recovered from each genotype and from each selection system, but not in all possible combinations. In addition, plants were recovered from original axes that ranged in age from 23 to 31 DAF, but not from the oldest (34 DAF) and youngest (23 DAF) explants. In conjunction with the interim measurements of GUS activity, this still suggests a larger window of opportunity than the 3-day optimum span indi-cated in the original work [4].
ex-Table 4
Summary ofb-glucuronidase (GUS) activity in transgenic pea plants
Total no. Range of GUS activity Mean MUG value
Agrobacterium
(pmol MU mg protein−1min−1)
clones
vector (LBG) (pmol MU mg protein−1min−1)
6
66 19–14 590 5180 (2384)a
28–4888
3 2995 (1503)
71
30
75 4–1756 137 (61)
aNumber in parentheses represents the standard error of the mean.
However, the present study appears to be the first report of the use of chlorsulfuron for selection in pea transformation.
Root production on regenerated shoots of peas has been described as difficult [2,29]. To overcome this problem, other researchers have grafted shoots generated in vitro to the roots from seedlings grown in vivo. Unfortunately, escapes and chimaeras were common [2]. In the present study, rooting under selection pressure may have reduced the number of plants obtained; however, there were no escapes, as confirmed by Southern hybridization analysis (see below). In addition, no chimaeras were identified in the present study. Samples from various parts of individual plants over time (data not shown) showed consistent GUS activity. Grant et al. [28] also reported that reselection during root elongation effectively weeded out escapes.
3.2. Assessment of transgenic plants
Leaf discs from initial transgenic (T0) plants
were used to determine GUS activity in (Table 4). Even with the same Agrobacterium vector, there was considerable variation in the activity, proba-bly related to the location of gene insertion. In general, the plants recovered from transformation with LBG66 had a higher mean MUG value than those with LBG71 (Table 4). In turn, plants recov-ered from transformation with LBG75 had the lowest mean MUG values, despite the high values observed after the callus phase (Fig. 2). There is no reason to presume that gus gene expression would be the same in organized shoot tissue in the absence of selection as it was in callus under selection pressure.
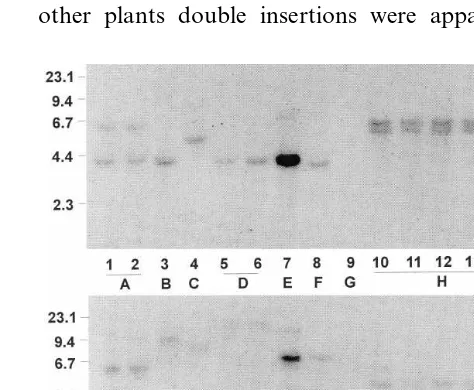
All putative transgenic plants were examined with Southern blots. Southern blots can be used to determine gene copy number, verify gene integra-tion into the plant genome and confirm
identifica-tion of independent transformaidentifica-tion events. In the t-DNA of the plasmid from LBG75, there is one EcoRI site located between the gus and ahas3r genes. When integrated into plant genomic DNA, cleavage of this site results in border fragments of random sizes depending upon the distance to the nearest genomic EcoRI site. Fig. 4 shows the results from one set of samples including DNA from transgenic plants of five different genotypes, all of which had been transformed using Agrobac
-terium vector LBG75. In this case, the same blot
was hybridized with probes from both thegusand theahas3rgenes. With both probes, the autoradio-graphs showed specific DNA fragment patterns, indicating sequence homology with the probes. Some plants had single copies of a gene, but in other plants double insertions were apparent in
Fig. 4. Southern blots of DNA from transgenic plants pro-duced by co-cultivation of thin slices of embryo axes with
Agrobacteriumvector LBG75. The DNA was cut withEcoRI and detected by radioactive probes for thegusA(top) orahas
Table 5
x2analysis of T
1segregation ratios from selected independent transformants of peas
Plant Genotype Construct No. plants x2Value Probability
Pos Neg Total
LBG66 15 4
PLP19 1512 19 0.16 0.69
PLP34 1512 LBG66 16 3 19 0.85 0.35
LBG66 20 0
PLP46a M38 20 6.67 0.01*
LBG66 11 9
VI 20
PLP22 4.27 0.04*
LBG71 17 3
DB404a 18G 20 1.07 0.30
LBG75 16 4
1512 20
MA26 0.27 0.61
18G
KM212 LBG75 12 7 19 1.42 0.23
LBG75 16 4
25E 20
DB291 0.27 0.61
M38
DB175 LBG75 17 2 19 1.72 0.19
LBG75 5 6 11 5.12 0.02*
PLP61 NZ66
LBG75 16 4 20
VI 0.27
DB148 0.61
161
Total 46 207 0.93 0.34
aSouthern blot analysis indicates two copies of thegusgene. The remainder of plants each contain one copy.
* ProbabilitiesB0.05 indicate significant difference from the expected 3:1 ratio.
Table 6
Comparison ofb-glucuronidase (GUS) activity in primary transformants (T0) and their offspring (T1)
Construct Plant genotype Original transformant Mean MUG value (pmol mg protein−1min−1)
T1 plantsa
T0plant
PLP19 8240 6665
LBG66 1512
18G DB314 4888
LBG71 3329
1756
LBG75 1512 MA17 874
M38
LBG75 DB174 142 37
KM211 267
18G 232
LBG75
LBG75 NZ66 PLP61 11 10
26 23
DB163 25E
LBG75
aMean value from 20 plants.
both blots. Each of the distinct band patterns, indicated by different letters, represents an inde-pendent transformation event. Plants with identi-cal patterns were considered to have arisen from the same transformation event and could be traced back to the same original slice. In the case of plants 1 and 2 (event A), two bands were visible when the blot was hybridized with the probe for gus, but only one band was visible with theahas3r probe. In the latter case, two fragments produced by the EcoRI cleavage may have co-incidentally been of similar lengths. Even with a difference of 200 bp, two bands could not be distinguished at this level. Use of another restriction enzyme could clarify the number of insertions in this instance.
T1 seeds representing all three Agrobacterium
vectors and six of the seven pea genotypes were advanced to the next generation, to examine the heritability of the inserted genes. Table 5 shows the results of the x2 analysis. Only three of eleven
groups of progeny deviated significantly (PB0.05) from the expected results of 3:1 positive:negative for a single gene. Of these, the original PLP46 had two copies of the gene (as seen on a Southern blot) which may have segregated independently, as all offspring were positive. This distribution is consis-tent with that expected for two insertion loci segre-gating independently (15:1). Also, the small sample size of 11 T1 plants from PLP61 reduced
indi-cated a ratio of 3.5:1 with no significant deviation from the expected 3:1 ratio. This too would sug-gest that chimaeras were not a problem with this material. In addition, the similar MUG values of the T0 plants and their T1 progeny suggest high
heritability (Table 6).
Transgenic plants were recovered from all of the genotypes tested in this study. Three different selection systems were effective in the recovery of transgenic plants. The insertedgusgene was inher-ited in the T1 progeny of all plants tested. The
method is reproducible, despite the long time pe-riod required and low frequency of success. On the basis of these results, it seems likely that more agronomically-relevant genes could be directly in-serted into a pea cultivar of choice.
Acknowledgements
This work was partially funded by the Saskatchewan Pulse Growers, the Manitoba Pulse Growers Association, Inc. and the Alberta Pulse Growers Commission. We gratefully acknowledge the technical assistance of Ms Maureen Anderson, Mr David Baliski and Ms Kangfeng Mei.
References
[1] J. Puonti-Kaerlas, T. Eriksson, P. Engstro¨m, Production of transgenic pea (Pisum sati6umL.) plants byAgrobac
-terium tumefaciens-mediated gene transfer, Theor. Appl. Genet. 80 (1990) 246 – 252.
[2] S.J. Bean, P.S. Gooding, P.M. Mullineaux, D.R. Davies, A simple system for pea transformation, Plant Cell Rep. 16 (1997) 513 – 519.
[3] A. deKathen, H.-J. Jacobsen, Agrobacterium tumefa
-ciens-mediated transformation ofPisum sati6umL. using
binary and cointegrate vectors, Plant Cell Rep. 9 (1990) 276 – 279.
[4] H.E. Schroeder, A.H. Schotz, T. Wardley-Richardson, D. Spencer, T.J.V. Higgins, Transformation and regener-ation of two cultivars of pea (Pisum sati6um L.), Plant
Physiol. 101 (1993) 751 – 757.
[5] P.L. Polowick, S.D. Baliski, J.D. Mahon, Comparison of three Agrobacterium vectors and selection agents in the transformation of Pisum sati6um (pea), 4th Canadian
Plant Tissue Culture and Genetic Engineering Confer-ence, Saskatoon, SK, June 1 – 4, 1996, p. 59.
[6] R.S.S. Datla, J.K. Hammerlindl, L.E. Pelcher, W.L. Crosby, G. Selvaraj, A bifunctional fusion between b -glucuronidase and neomycin phosphotransferase: a broad-spectrum marker enzyme for plants, Gene 101 (1991) 239 – 246.
[7] R.S.S. Datla, F. Bekkaoui, J.K. Hammerlindl, G. Pilat, D.I. Dunstan, W.L. Crosby, Improved high-level consti-tutive foreign gene expression in plants using an AMV RNA4 untranslated leader sequence, Plant Sci. 94 (1993) 139 – 149.
[8] G. Vancanneyt, R. Schmidt, A. O’Connor-Sanchez, L. Willmitzer, M. Rocha-Sosa, Construction of an intron-containing marker gene: splicing of the intron in trans-genic plants and its use in monitoring early events in
Agrobacterium-mediated plant transformation, Mol. Gen. Genet. 220 (1990) 245 – 250.
[9] P.R. Hirsch, C.L. Wang, M.J. Woodward, Construction of a Tn5 derivative determining resistance to gentamicin and spectinomycin using a fragment cloned from R1033, Gene 48 (1986) 203 – 209.
[10] S. Rogers, H. Klee, M. Byrne, R. Horsch, R. Fraley, Improved vectors for plant transformation: expression cassette vectors and new selectable markers, Methods Enzymol. 153 (1987) 253 – 277.
[11] M. Bevan, BinaryAgrobacteriumvectors for plant trans-formation, Nucleic Acids Res. 12 (1984) 8711 – 8721. [12] W. Wohlleben, W. Arnold, I. Broer, D. Hillemann, E.
Strauch, A. Pu¨hler, Nucleotide sequence of the phos-phinothricin N-acetyltransferase gene fromStreptomyces
6iridochromogenesTu¨494 and its expression inNicotiana
tabacum, Gene 70 (1988) 25 – 37.
[13] J.E. Brandle, M.J. Morrison, J. Hattori, B.L. Miki, A comparison of two genes for sulfonylurea herbicide resis-tance in transgenic tobacco seedlings, Crop Sci. 34 (1994) 226 – 229.
[14] E.E. Hood, S.B. Gelvin, L.S. Melchers, A. Hoekema, NewAgrobacteriumhelper plasmids for gene transfer to plants, Transgenic Res. 2 (1993) 208 – 218.
[15] D.C.W. Brown, A. Atanassov, Role of genetic back-ground in somatic embryogenesis inMedicago, Plant Cell Tissue Organ Cult. 4 (1985) 111 – 122.
[16] Z.-M. Cheng, J.A. Schnurr, J.A. Kapaun, Timentin as an alternative antibiotic for suppression of Agrobacterium tumefaciensin genetic transformation, Plant Cell Rep. 17 (1998) 646 – 649.
[17] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue culture, Phys-iol. Plant 15 (1962) 473 – 497.
[18] O.L. Gamborg, R.A. Miller, K. Ojima, Nutrient require-ments of suspension cultures of soybean root cells, Exp. Cell Res. 50 (1968) 151 – 158.
[19] R.A. Jefferson, T.A. Kavanagh, M.W. Bevan, GUS fu-sions: b-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants, EMBO J. 6 (1987) 3901 – 3907.
[20] R.A. Jefferson, Assaying chimeric genes in plants: the GUS gene fusion system, Plant Mol. Biol. Rep. 5 (1987) 387 – 405.
[21] M.M. Bradford, A rapid and sensitive method for quan-titation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72 (1976) 248 – 254.
[23] J. Hattori, D. Brown, G. Mourad, H. Labbe´, T. Ouel-let, G. Sunohara, R. Rutledge, J. King, B. Miki, An acetohydroxy acid synthase mutant reveals a single site involved in multiple herbicide resistance, Mol. Gen. Genet. 246 (1995) 419 – 425.
[24] J.E. Grant, P.A. Cooper, A.E. McAra, T.J. Frew, Transformation of peas (Pisum sati6um L.) using
im-mature cotyledons, Plant Cell Rep. 15 (1995) 254 – 258. [25] V. Babic, R.S. Datla, G.J. Scoles, W.A. Keller,
Devel-opment of an efficient Agrobacterium-mediated trans-formation system for Brassica carinata, Plant Cell Rep. 17 (1998) 183 – 188.
[26] R. Schmidt, L. Willmitzer, High efficiency Agrobac
-terium-mediated transformation of Arabidopsis thaliana
leaf and cotyledon explants, Plant Cell Rep. 7 (1988) 583 – 586.
[27] B.L. Miki, P.R. Fobert, P.J. Charest, V.N. Iyer, Proce-dures for introducing foreign DNA into plants, in: B.R Glick, J.E. Thompson (Eds.), Methods in Plant Molec-ular Biology and Biotechnology, CRC Press, Boca Ra-ton, FL, 1993, pp. 67 – 88.
[28] J.E. Grant, P.A. Cooper, B.J. Gilpin, S.J. Hoglund, J.K. Reader, M.D. Pither-Joyce, G.M. Timmerman-Vaughan, Kanamycin is effective for selecting trans-formed peas, Plant Sci. 139 (1999) 159 – 164.
[29] P. Bo¨hmer, B. Meyer, H.-J. Jacobsen, Thidiazuron-in-duced high frequency of shoot induction and plant re-generation in protoplast derived pea callus, Plant Cell Rep. 15 (1995) 26 – 29.
![Fig. 1. Construction of binary plasmid pPBI3008. The binaryplasmid pPBI3008 was constructed from: a 2332 bpPstpXS1 [9] subcloned into pUC8, a 5119 bpfragment from pMON505 containing the RK2 and pBR322origins of replication [10] and a 1986 bp HindIII–I frag](https://thumb-ap.123doks.com/thumbv2/123dok/1034962.928950/2.612.69.230.434.571/construction-binaryplasmid-constructed-subcloned-bpfragment-containing-replication-hindiii.webp)