Research focused on early events in host–pathogen interactions has provided new insights into fundamental aspects of microbial pathogenicity and plant responses. Considerable progress has been made in understanding regulation of the delivery of pathogenicity determinants from bacteria into plant cells, signal cascades involved in fungal pathogenicity, the co-ordinating role of the plant cytoskeleton in plant defence and calcium flux as a primary signalling function during the hypersensitive reaction.
Addresses
Department of Biological Sciences, Wye College, Wye, Ashford, Kent TN25 5AH, UK
*e-mail: [email protected]
†e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:312–319 http://biomednet.com/elecref/1369526600200312 © Elsevier Science Ltd ISSN 1369-5266
Abbreviations avr avirulence
[Ca2+]
i cytosolic calcium concentration HR hypersensitive reaction
LRRP leucine-rich repeat protein
MAPK mitogen-activated protein kinase
PKA protein kinase A
R resistance
ROS reactive oxygen species
SLIK signalling linker protein
vir virulence
Introduction
The early events reviewed here are those occurring in plants and also pathogens following inoculation. Scanning recent research into plant diseases suggests that there has perhaps been an unhealthy focus on resistance and in par-ticular the hypersensitive reaction (HR) which is defined as ‘the rapid death of plant cells at the infection site lead-ing to restricted colonisation of the potential pathogen’. There is no doubt that understanding the HR is an impor-tant target, but the key events in the establishment of basic pathogenicity and susceptible interactions are com-paratively poorly understood — particularly for obligate fungal pathogens such as the rusts and mildews [1]. Varietal resistance involving gene-for-gene interactions is typically expressed via the HR, but the more widespread activation of non-host resistance does not usually involve plant cell death — it involves highly co-ordinated localised alterations to plant cell walls at challenge sites [1,2,3•].
Links are emerging, however, between avirulence (avr) and virulence (vir) gene function with more and more genes being found to have dual activity depending on the presence or absence of the matching Rgene in the plant [4,5••]. The modes of action of potential virulence factors
remain to be determined, but an emerging hypothesis is
that virgene function may be associated with suppression of primitive mechanisms of resistance which do not involve hypersensitive cell death [1].
Signal delivery by bacterial pathogens
Bacteria enter plants passively, often driven by rain-splash through natural openings such as stomata. Exposure to the micro-environment of the intercellular space in plant tis-sue induces expression of the type III secretion system, which comprises components of the hrpgene cluster. The
hrpgenes are required, both for pathogenicity and for the ability to cause the HR in non-host and resistant host plants [6]. The proteins encoded by hrpgenes are homolo-gous to those required for the delivery of virulence factors by several mammalian pathogens, for example Yersinia[7]. No characteristic motifs have been identified in proteins secreted by this route but recent experiments with Yersinia
show that the secretion signal may reside in untranslated RNA upstream of the translation start [8,9••]. Operation of
such a co-ordinated translation and secretion system has not yet been described in a plant pathogen [4,10].
Expression of hrpgenes, and also coregulated avrgenes, is induced by growth in defined minimal media. But expres-sion measured in the plant has regularly achieved levels far greater than in vitro, implying some specific activation beyond that achieved by low nutrient conditions [6]. In
Ralstonia, hrp regulation directly involves HrpB, which shares homology with the AraC family of transcription acti-vators [11]. Dissection of the regulatory pathway in
Ralstoniahas also demonstrated the involvement of PrhA (plant regulator of hrp genes), which controls interaction with plants in an hrpB-independent manner [6,11,12••].
Although PrhA has significant similarity with siderophores, which are typically associated with iron uptake, iron does not appear to be the signal sensed in the plant. Significantly, induction requires the presence of plant cells (not just extracts) in suspension culture, implying a role for bacterium/host cell contact.
Certain proteins with weak HR eliciting activity, such as harpins from P. syringae and E. amylovora, and PopA from
R. solanacearum, are encoded by genes within or associated with hrp clusters, and are readily secreted into culture media. By contrast, there are many reports of failure to detect similar secretion of other proteins, in particular those encoded by avrgenes, for example avrBs3or avrB
from X. campestris pv. vesicatoria (Xcv) and P. syringae
respectively.The implication is that certain key virulence determinants are not secreted into the intercellular space but are directly transferred from bacteria into plant cells using special components of the type III secretion system. Two recent developments have, however, allowed secre-tion of Avr proteins to be demonstrated in vitro. The hrp
Early events in host–pathogen interactions
cluster from E. chrysanthemi has proved to be ‘leaky’ and has been expressed in E. coli, allowing secretion of AvrB to be observed [13•]. More significantly, use of minimal
media with low pH and overexpression of regulatory hrp
genes, has allowed secretion of AvrB and also avrBs3 from
Xcv [10]. Having achieved secretion of known Avr proteins it will now be possible to search for other proteins secret-ed by the same pathway, which may have virulence functions and are, therefore, key players in the establish-ment of parasitism.
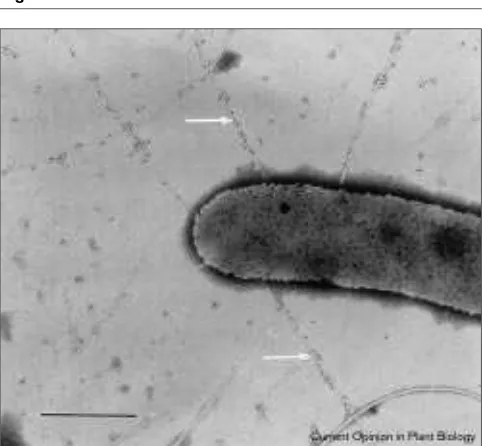
Secretion and delivery of Avr proteins, whether in vitroor in the plant, seem to require the presence of a functional Hrp pilus (Figure 1). Pili encoded by hrpgenes have now been identified in all major groups of plant pathogens. Purified HrpA protein from P. syringae pv. tomato was found to reassemble into filamentous structures [14]. Localization of HrpA by immunocytochemistry using transmission electron microscopy has revealed the presence of the pilus within plant cell walls in contact with bacteria (SY He and I Brown, personal communication). The pilus appears to be capable of crossing the cell wall barrier. The question remains, how-ever, whether proteins are delivered through the pilus or if penetration of the pilus into the wall merely anchors bacte-ria and allows localised secretion into the plant cell wall and from there, via diffusion across the polysaccharide matrix, into contact with the plant cell membrane.
Identification of virulence factors
Expression of avr genes in plants with the matching
Rgene leads to the HR in several interactions [15,16]. The Avr proteins delivered by the hrp-dependent secretion sys-tem are, therefore, the elicitors of hypersensitive cell death. Intriguingly, expression of avrgenes has also been reported to cause effects in plants which lack the comple-mentary R genes [17••,18]. Transient expression of avr
genes has been the most common approach to examine effects in plant cells but a more tractable system is provid-ed by stable expression from a highly regulatprovid-ed promoter. McNellis et al. [17••] describe such an approach, using avrRpt2 expression via a glucocorticoid-induced promoter in Arabidopsis. The expression of avrRpt2has clear effects in the absence of the matching Rgene (in this case RPS2). Induction of patchy necrosis and inhibition of root growth were both observed [17••]. Use of the induced promoter
provides an elegant model to examine effects of Avr pro-teins on plant cells.
A dual function for avrgenes in virulence as well as HR-induction is often not apparent because Avr mutants are not compromised in pathogenicity [4]. Virulence functions may be masked by redundancy and it may be that it is only by deletion of large regions of the bacterial genome, equiv-alent to the pathogenicity islands (PAIs) in animal pathogens [19], that loss of virulence will be achieved. The possibility that avrgenes may be located on mobile regions in P. syringaehas been proposed [20]. A region containing several avr genes which has multiple virulence functions
and may be considered a PAI, has been located on a 154 kb plasmid in P. syringaepv. phaseolicola. Loss of the plasmid results in loss of virulence to previously susceptible culti-vars; cured strains elicit the HR rather than causing disease (R Jackson personal communication).
Genes required for pathogenicity but not for the ability to cause the HR have also been identified in E. amylovora, P. syringae pv. tomato (Pst) and Xcv. In the former two species, homology and functional similarity have been shown for the hrp-linked pathogenicity genes: the disease specific locus dspEF [21•] (or previously dspAB [22]) in E. amylovoraand avrEin Pst. Intriguingly, the avrElocus was so named because it determined the ability of Pstto elicit the HR in the non-host soybean. In Xcvmutations in the hpaA gene (hrp associated) abolish pathogenicity but retain, in part, the ability to deliver the avrBs3-mediated HR in pepper [23•]. Like AvrBs3, the HpaA protein was
found to contain nuclear localization signals which are important for the interaction with the plant.
Amongst fungal pathogens, Cladosporium fulvum, the cause of tomato leaf mould, is unique in its intercellular biotrophic growth habit. Proteins secreted by C. fulvum
into the leaf apoplast are avirulence gene products such as Avr5 and Avr9, and virulence factors ECP1 and ECP2. Mutation of the Ecpgenes leads to loss of virulence and spore production [5••]. By means of an elegant potato
virus X-derived expression system, genotypes of tomato were identified that activated the HR following expo-sure to ECP2, which, therefore, has dual function as a
Figure 1
determinant of both pathogenicity and avirulence. Laugé et al. [5••] raise the possibility that separate
domains on ECP2 might determine the avirulence and virulence activities. Nevertheless, because ECP2 is required for full pathogenicity, the matching recognition gene designated Cf-ECP2 should be efficient and durable in protecting tomato against leaf mould disease.
Fungal infection structures
Bacterial surface features, such as hrp pili, have been found to be significant factors in pathogenicity, but the interaction between pathogenic fungi and their hosts is structurally a far more complex process. To be success-ful, the fungi have to complete a well defined series of developmental steps including (at least) spore germina-tion, appressorium formation and plant cell wall penetration. Attempts to unravel key determinants of fungal pathogenicity have targeted events involved in production of a specific infection structure, for example the appressorium [24] or haustorium [25], or applied the ‘black box’ approach of mutant hunts based on inser-tional mutagenesis using tagged sequences [26•]. Most
detailed information comes from experiments with the rice–blast fungus Magnaporthe grisea.
Several environmental cues have been reported to favour appressorium differentiation. In the bean rust fungus, topographical stimuli, including ridges as low as 0.5µm in height have been implicated. The mechanisms underly-ing the rapid perception of surface topography and co-ordinated response remain to be discovered [24]. In
M. grisea, appressoria form on any hard hydrophobic sur-face and their production is stimulated by the addition of cutin monomers or cAMP. Signalling through a cAMP dependent pathway is critical for appressorium morpho-genesis. Mutations in genes that encode either a G protein
αsubunit or adenylate cyclase block appressorium differ-entiation and also have other effects on fungal growth. The adenylate cyclase operates upstream of alternative protein kinase A (PKA) holoenzymes which are separate-ly required for growth, appressorium production and plant cell wall penetration. Genetical analysis has demonstrated that the cAMP-dependent signal interacts with a mitogen-activated protein (MAP) kinase called Pmk1 to allow development of a mature appressorium [27]. Mutation in a second MAP kinase encoded by MPS1 does not impair differentiation of the appressorial structure but compro-mises function, as indicated by failure to penetrate through the underlying plant cell wall [28••].
Analysis of intracellular haustoria, which are arguably the most important structures required for nutrient uptake by the economically devastating pathogens the rusts and mildews, remains constrained by inherent problems in deal-ing with the obligate parasites. Purification of haustoria has, however, allowed the isolation of rust genes induced during the early stage of haustorium formation [25,29].
Plant defence — local cell wall alterations
An interesting feature of MPS1 mutants of M. griseais that their failure to penetrate through plant cell walls is associated with the activation of papilla deposition and localized plant cell wall alteration. This mechanism of defence does not involve the HR and is commonly observed in the expression of resistance which does not involve gene-for-gene interactions. Cell walls are strengthened at sites of attempted penetration by the incorporation and oxidative cross-linking of proteins and various phenolic subunits including hydroxycinnamic acid amides. In barley, coumaroylagmatine derivatives were identified at reaction sites expressing mlo-based resistance to Erysiphe graminis and in onion, feruloyl-methoxytyramine (FMT) and related amides accumulated in epidermis challenged by Botrytis allii
[3•,30]. Alterations to the onion cell wall are preceded by
earlier polarisation of the cytoskeleton to sites of attempt-ed penetration (Figure 2). The formation and movement of vesicles containing FMT now provides a useful model for the examination of exocytosis in plant cells and the overall co-ordination of such a highly targeted response.
In bean leaves, the rapid and highly co-ordinated increases occurring in activities of callose synthase and peroxidase, and deposition of some of their substrates in mesophyll cell walls and papillae adjacent to hrpmutant bacteria have now been defined by immunocytochemistry [2]. Localized reactions were associated with a minor burst of H2O2, but
accumulation of the reactive oxygen species (ROS) was much less than observed during the HR [31•,32], supporting Figure 2
the idea that the activation of cell death programmes requires high threshold levels of ROS. Both peroxidase and neutrophil-like NADPH oxidase activities have been implicated as generators of ROS such as H2O2[33•,34].
The HR in gene-for-gene interactions: calcium
and confusing kinases
Recent overviews [1,35,36] attempt to place the HR in the context of other defence responses and emphasise the remarkable variability in the timing and nature of bio-chemical events occurring in different plant–pathogen interactions (despite them all being described as examples of the HR!). Although the timing and sequence of events following gene-for-gene recognition may be very different, they ultimately lead via alternative routes to hypersensi-tive cell death. Given the variability apparent at the structural, cellular level it is not surprising that a bewilder-ing range of early biochemical responses and pathways has been described. Three key components have been pro-moted: elevated cytosolic calcium, Ca2+ binding proteins
(calmodulin) and protein phosphorylation (kinases).
Calcium and calmodulin
Evidence for an elevated cytosolic calcium ([Ca2+] i)
trig-gering the activation of defence mechanisms has been derived primarily from treatment of cell suspensions with microbes [37,38] or elicitors [39,40•,41,42]. Although
con-venient, studies in culture do not necessarily reflect the continued presence and contribution of the pathogen to the interaction. It is clearly preferable to monitor calcium fluxes within whole tissue. An in plantastudy, involving ratio imaging of microinjected epidermal strips from cow-pea after challenge with the basidospores of Uromyces, provides strong evidence for a role of Ca2+in
hypersensi-tive cell death. HR specific changes were observed before the fungus penetrated the plant cell wall and were proba-bly mediated by peptide elicitors diffusing from invading hyphae [43••].
Measurement of changes in [Ca2+]
i during the P. syringae/Arabidopsis: AvrRpm1/RPM1 interaction using aequorin-generated bioluminescence, has demonstrated the in plantageneration of a biphasic intracellular calci-um signature (M Grant, I Brown, J Mansfield, unpublished data). Our data suggest that elevation of [Ca2+]
i occurs almost instantaneously after delivery of
AvrRpm1 and is a primary function of the resistance gene product RPM1 which contains leucine rich repeats (LRRs). Calcium appears to serve as a post-recognition molecular switch, transducing the initial RPM1-mediat-ed response stimuli to multiple downstream effectors. A role for RPM1 in activation of calcium channels is sup-ported by the demonstration that RPM1 is a peripheral membrane associated protein [44••], which interacts with
a putative membrane protein in the two-hybrid system (cited in [44••]). Furthermore, the proposed requirement
for localisation of AvrRpm1 or AvrB to the plant plasma membrane for activity [44••], suggests that calcium
elevation may be mediated via the actions of a mem-brane-localised protein complex.
In contrast to the intracellular AvrRpm1/RPM1 recogni-tion, the C. fulvum/tomato interaction is mediated through resistance gene (Cf) proteins such as Cf5 and Cf9, which also contain LRRs, but are predicted to be predominantly extracellular [45]. Elicitors from race 5 or race 9 isolates of C. fulvum cause a rapid increase in [Ca2+]
i within minutes of their addition to tomato cells
containing the cognate resistance genes, Cf5 or Cf9
[42,46••]. Patch clamping studies have identified a
plas-ma membrane Ca2+ channel activated upon challenge
with elicitor from race 5. This hyperpolarisation-activated Ca2+ channel appears to be modulated by a G-protein
dependent mechanism [42]. Interestingly, race 5 con-comitantly inhibits a plasma membrane Ca2+-ATPase,
thus providing a possible feedback mechanism limiting Ca2+ efflux back to the apoplast [47] (see [48••] for an
excellent review). Elevated [Ca2+]
imay also play a direct
role in activation of NADPH oxidase through binding to the unique EF hand motifs in the amino-terminal region of the Arabidopsis gp91phox homologue [49]. Significantly,
no direct association of Cf5 or Cf9 with their avirulence gene products has been demonstrated. Based upon the necrosis-inducing activity of several Avr9 peptides and their association with a high affinity binding site present in both susceptible and resistant tomato genotypes, it has been proposed that an Avr9-binding protein mediates an interaction between Avr9 and Cf9 [50•].
Calmodulin, Ca2+ activated phosphatases and kinases
probably integrate the elevated calcium signal into the resultant diverse array of signalling pathways. The exquis-ite specificity of calcium-based responses is illustrated by the rapid and selective transcriptional activation of specif-ic calmodulin isoforms in soybean cell cultures, and the effects of expression of a hyperactive mutant calmodulin in transgenic tobacco [51••,52].
Confusing kinases
Surprisingly, the kinases activated during early signalling events in gene-for-gene interactions are also involved in non-specific elicitation of defence responses as well as other diverse abiotic stimuli such as wounding and mechanical stress [46••,53•,54•,55,56]. Challenge of
trans-genic tobacco cell suspensions expressing Cf9 with purified Avr9 elicitor resulted in the rapid activation of tobacco SIP kinase [46••], originally identified as a
MAPK activated by salicylic acid [57•], and WIPK, a
wound inducible kinase also required for jasmonate-based wound signal transduction [55]. The same kinases were activated during N–gene recognition of tobacco mosaic virus, but with significantly different activation kinetics [53•]. In gene-for-gene interactions, and after
wounding, activation of the MAPKs appears to require phosphotyrosine phosphorylation [46••,53•,54•,57•].
modification, and understanding its regulation is an important future objective.
The post-transcriptional activation of SIPK and WIPK, although dependent upon elevated cytosolic Ca2+,
calmod-ulin and phosphorylation, were not necessary for ROS generation [46••]. However, a Ca2+-dependent protein kinase
has been implicated in the assembly of a functional NADPH-oxidase complex during avr5/Cf5 mediated defence responses in tomato [58]. These data suggest a very early bifurcation of signalling pathways downstream of the Ca2+
mediated calmodulin signalling. The intriguing questions now are: how do multifunctional kinases discriminate
between such diverse stimuli and at what stages are irre-versible cell death programmes activated?
Conclusions and a generalised model for early
interactions in plant cells
Despite enormous effort, no LRR protein (LRRP) resis-tance gene product has been shown to interact directly with an Avr product. Perhaps the LRRPs instead interpret a subset of specific signals generated from other cellular proteins, which we designate signalling linker proteins (SLIKs). The presence of an elicitor (e.g. Avr protein), or its activity, may compromise the normal configuration or function of a SLIK or a SLIK complex, and this would be
Figure 3
AvrRpm1
?
SLIK (NDR1)
PBS1/? Avr AvrPphB AvrPto
AvrRpm1
Bacterial type III secretion apparatus
AvrPto Pto
?
SLIK (?)
Prf
PBS1
AvrPphB AvrPto Pto
AVR9
Ca2+
Ca2+ Ca2+
Peronospora parasitica
Cytoplasm AVR9
?
(5) AvrRPP5 Ca2+
Cladosporium fulvum
Apoplast
SLIK (EDS1)
Calmodulin, calcium dependent protein kinases RPS5/RPM1
RPM 1 Prf RPS5
RPP5 RPP14 RPS4 RPP2
? SLIK(EDS1)
RPP5
AVR9 binding protein (SLIK)
Cf9
Current Opinion in Plant Biology
(1)
(2)
(3) (4)
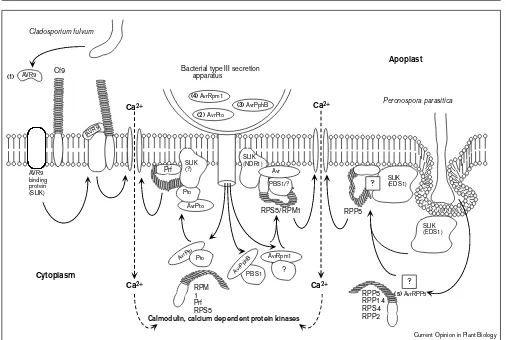
Speculative model for early signalling events occurring during selected gene-for-gene interactions, based upon current genetic and
biochemical evidence. Components which have been characterised are named (e.g. PBS1 [63•]), other proteins anticipated to be involved are designated with question marks. As no direct interaction has been demonstrated between avirulence gene products (Avr) and LRRPs (indicated by proteins with tails), we propose an integrative pathway involving signal linker proteins (SLIKs) and SLIK complexes, which may function as chaperones or signalling intermediates in coupling avirulence gene function to LRRPs. For example, in the simplest interaction, the Avr9-binding protein acts as a SLIK linking Avr9 to the LRRP, Cf9 and formation of the complex triggers Ca2+influx (dashed
arrow). Similarly, Pto may function as part of a SLIK complex to transduce AvrPto recognition to Prf. In our model, gene products
demonstrated to modify signalling pathways (EDS1 and NDR1) are positioned as SLIKs to integrate specifically a distinct subset of Avr signals towards their matching respective LRRPs (products of R
recognised specifically by an LRRP which would, there-fore, indirectly match the Avr protein. Comparative studies on R gene clusters have revealed that the sequences encoding putative solvent-exposed residues in the LRRPs are hypervariable, exhibiting elevated ratios of nonsynony-mous to synonynonsynony-mous substitutions [59]. Such a structure would allow the dynamic evolution of diverse arrays of lig-and acceptor sites.
The proposed interactions between Avr, SLIK and LRR proteins are summarised in Figure 3, in which it is postu-lated that certain genes known to be required for resistance, such asEDS1and NDR1, many encode SLIKs which operate upstream of the LRRPs. The role of EDS1 and NDR1 as SLIKs would explain their specific effects on groups of gene-for-gene interactions involving different LRRPs [60,61•]. The Pto kinase protein, which is known
to bind directly to AvrPto, may interact with a SLIK com-plex which includes both AvrPto and Prf (an LRRP), a hierarchy which is supported by results from gain of func-tion experiments with Pto [62]. Recent genetic evidence suggests that the PBS1 protein, which is required for AvrPphB/ RPS5-mediated resistance in Arabidopsis [63•],
may function in a role analogous to Pto and interact with NDR1 in the SLIK complex. Whatever the final pheno-type, the earliest response modulated by an LRRP appears to be elevated [Ca2+]
i.
With such a signalling hierarchy, certain non-specific elic-itors, such as oligo-uronides, might affect SLIKs which interact with more than one LRRP and, therefore, acti-vate a diverse range of plant responses, only some of which may lead to the HR [36]. Returning to our initial point about unravelling susceptibility, it is envisaged that Avr proteins are able to act as virulence factors by block-ing the ability of the SLIK to interact with LRRPs and, thereby, effectively suppressing plant defence responses. As outlined in Figure 3, without SLIK activity, basic par-asitim is established.
Acknowledgements
We wish to acknowledge support from the BBSRC and EC grant BIO-CT97-2244. Thanks are also due to colleagues who provided details of recent results.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Heath MC: Signalling between pathogenic rust fungi and resistant or susceptible host plants.Ann Bot1997, 80:713-720.
2. Brown I, Trethowan J, Kerry M, Mansfield J, Bolwell GP: Localization of components of the oxidative cross-linking of glycoproteins and of callose synthase in papillae formed during the interaction between non-pathogenic strains of Xanthomonas campestrisand French bean mesophyll cells. Plant J1998, 15:333-343.
3. McLusky SR, Bennett MH, Beale M, Lewis MJ, Gaskin P, Mansfield
• JW: Cell wall alterations and localized accumulation of feruloyl-3¢ -methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis alliiare associated with actin polarisation,
peroxidase activity and suppression of flavonoid biosynthesis.
Plant J1999, 17:523-534.
A combination of biochemistry and cell biology used to examine localized accumulation of novel phenolics has generated a useful model to examine secretion processes.
4. Alfano JR, Collmer A: The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, avr proteins, and death.
J Bacteriol1997, 179:5655-5662
5. Laugé R, Joosten MHA, Haanstra JPW, Goodwin PH, Lindhout P,
•• de Wit PJGM: Successful search for a resistance gene in tomato targeted against a virulence factor of a fungal pathogen.Proc Natl Acad Sci USA, 1998, 95:9014-9018.
The link between avirulence and virulence functions harnessed in order to identify a potentially durable resistance gene.
6. Van Gijsegem F: In plantaregulation of phytopathogenic bacteria virulence genes: relevance of plant-derived signals.Eur J Plant Pathol1997, 103:291-301.
7. Hueck CJ: Type III protein secretion systems in bacterial pathogens of animals and plants.Microbiol Mol Biol Rev1998, 62:379-433.
8. Anderson DM, Schneewind O: An mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica.Science1997, 278:1140-1143.
9. Anderson DM, Schneewind O: Yersinia enterocoliticatype III
•• secretion: an mRNA signal that couples translation and secretion of YopQ.Mol Microbiol1999, 31:1139-1148.
Important analysis of secretion controls and the selectivity of the type III apparatus (see also [8]).
10. Rossier O, Wegelnik K, Hahn K, Bonas U: The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens. Proc Natl Acad Sci USA 1999, in press. 11. Marenda, M, Brito B, Callard D, Genin S, Barberis P, Boucher C, Arlat M:
PrhA controls a novel regulatory pathway required for the specific induction of Ralstonia solanacearum hrpgenes in the presence of plant cells.Mol Microbiol1998, 27:437-453.
12. Brito B, Marenda M, Barberis P, Boucher C, Genin S:prhJand hrpG,
•• two new components of the plant signal-dependent regulatory cascade controlled by PrhA in Ralstonia solanacearum.Mol Microbiol1999, 31:237-251.
Continued dissection of regulation pathways associated with plant cell con-tact (see also [11]).
13. Ham JH, Bauer DW, Fouts DE, Collmer A: A cloned Erwinia
• chrysanthemiHrp (type III protein secretion) system functions in
Escherichia colito deliver Pseudomonas syringaeAvr signals to plant cells and to secrete Avr proteins in culture.Proc Natl Acad Sci USA1998, 95:10206-10211.
Leakiness of the E. chrysanthemitype III system was revealed here — but why does it leak?
14. Roine E, Saarinen J, Kalkkinen N, Romantschuk M: Purified HrpA of
Pseudomonas syringaepv. tomatoDC3000 reassembles into pili.
FEBS Letts1997, 417:168-172.
15. Bonas U, van den Ackerveken G: Gene-for-gene interactions: bacterial avirulence proteins specify plant disease resistance.
Curr Opin Microbiol1999, 2:94-98.
16. Mudgett MB, Staskawicz BJ: Protein signaling via type III secretion pathways in phytopathogenic bacteria.Curr Opin Microbiol1998, 1:109-114.
17. McNellis TW, Mudgett MB, Li K, Aoyama T, Horvath D, Chua NH,
•• Staskawicz BJ: Glucocorticoid-inducible expression of a bacterial avirulence gene in transgenic Arabidopsisinduces hypersensitive cell death.Plant J1998, 14:247-257.
The stable and controlled expression of AvrRpt2 protein is a very significant step towards understanding the function of Avr proteins in plants, with or without the matching Rgene.
18. Stevens C, Bennett MA, Athanassopoulos E, Tsiamis G, Taylor JD, Mansfield JW: Sequence variations in alleles of the avirulence gene avrPphE.R2 from Pseudomonas syringaepv. phaseolicola
lead to loss of recognition of the AvrPphE protein within bean cells and a gain in cultivar-specific virulence.Mol Microbiol1998, 29:165-177.
20. Kim JF, Charkowski AO, Alfano JR, Collmer A, Beer SV: Sequences related to transposable elements and bacteriophages flank avirulence genes of Pseudomonas syringae.Mol Plant–Microbe Interact1998, 11:1247-1252.
21. Bogdanove AJ, Kim JF, Wei Z, Kolchinsky P, Charkowski AO, Conlin
• AK, Collmer A, Beer SV: Homology and functional similarity of an hrp-linked pathogenicity locus, dspER, or Erwinia amylovoraand the avirulence locus avrE of Pseudomonas syringaepathovar
tomato.Proc Natl Acad Sci USA1998, 95:1325-1330.
Demonstration of dual avirulence and pathogenicity functions in the dsp
locus. Highlights the emerging theme of common pathogenic ancestors to diverse genera of phytopathogens (see also [22]).
22. Gaudriault S, Malandrin L, Paulin JP, Barny MA: DspA, an essential pathogenicity factor of Erwinia amylovorashowing homology with AvrE of Pseudomonas syringae, is secreted via the Hrp secretion pathway in a DspB-dependent way.Mol Microbiol1997, 26:1057-1069.
23. Huguet E, Hahn K, Wengelnik K, Bonas U: hpaAmutants of
• Xanthomonas campestrispv.vesicatoriaare affected in pathogenicity but retain the ability to induce host-specific hypersensitive reaction.Mol Microbiol1998, 29:1379-1390. Potential pathogenicity gene has emerged from detailed molecular charac-terisation of the hrpcluster in Xcv.
24. Dean RA: Signal pathways and appressorium morphogenesis.
Annu Rev Phytopathol1997, 35:211-234.
25. Hahn M, Mendgen K: Characterization of in planta-induced rust genes isolated from a haustorium-specific cDNA library.Mol Plant–Microbe Interact1997, 10:427-437.
26. Balhadère PV, Foster AJ, Talbot NJ: Identification of pathogenicity
• mutants of the rice blast fungus Magnaporthe griseaby insertional mutagenesis. Mol Plant–Microbe Interact1999, 12:129-142. Indicates the potential of and also limitations to insertional mutagenesis as a means of unravelling fungal pathogenicity.
27. Hamer JE, Talbot NJ: Infection-related development in the rice blast fungus Magnaporthe grisea. Curr Opin Microbiol1998, 1:693-697. 28. Xu U-R, Staiger CJ, Hamer JE: Inactivation of the mitogen-activated
•• protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses.Proc Natl Acad Sci USA1998, 95:12713-12718. Genetical analysis of M. griseatranslated into biochemical mechanisms con-trolling development of functional appressoria.
29. Hahn M, Neef U, Struck C, Göttfert M, Mendgen K: A putative amino acid transporter is specifically expressed in haustoria of the rust fungus Uromyces fabae. Mol Plant–Microbe Interact1997, 10:438-445.
30. von Röpenack E, Parr A, Schulze-Lefert P: Structural analyses and dynamics of soluble and cell wall-bound phenolics in a broad spectrum resistance to the powdery mildew fungus in barley.
J Biol Chem1998, 273:9013-9022.
31. Bestwick CS, Brown IR, Bennett MH, Mansfield JW: Localization of
• hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringaepv.
phaseolicola.Plant Cell1997, 9:209-221.
Important demonstration of the accumulation of H2O2during the HR and localized responses (without plant cell death) to hrp mutant bacteria. Conclusions on the role of peroxidase as a ROS generator in this system may be flawed because of lack of specificity of inhibitors used, KCN and azide.
32. Bestwick CS, Brown IR, Mansfield JW: Localized changes in peroxidase activity accompany hydrogen perodixe generation during the development of a nonhost hypersensitive reaction in lettuce.Plant Physiol1998, 118:1067-1078.
33. Martinez C, Montillet JL, Bresson E, Agnel JP, Dai GH, Daniel JF,
• Geiger JP, Nicole M: Apoplastic peroxidase generates superoxide anions in cells of cotton cotyledons undergoing the hypersensitive reaction to Xanthomonas campestris pv. malvaraceumrace 18.Mol Plant–Microbe Interact1998, 11:1038-1047.
Good evidence for the role of peroxidase in generating ROS in cotton. In this interaction NADPH oxidase does not seem to be as important.
34. Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM: Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms.
Plant Physiol1998, 116:1379-1385.
35. Mansfield JW, Bennett MH, Bestwick CS, Woods-Tor AM: Phenotypic expression of gene-for-gene interaction involving fungal and bacterial pathogens: variation from recognition to response. In The Gene-for-Gene Relationship in Host–Parasite Interactions. Edited by Crute IR, Burden JJ, Holub EB. London, UK: CAB International; 1997:265-291.
36. Somssich IR, Hahlbrock K: Pathogen defence in plants — a paradigm of biological complexity.Trends Plant Sci1998, 3:86-90.
37. Atkinson MM, Midland SL, Sims JJ, Keen NT: Syringolide 1 triggers Ca2+influx, K+efflux, and extracellular alkalization in soybean
cells carrying the disease-resistance gene RPg4.Plant Physiol
1996, 112:297-302.
38. Levine A, Pennell RI, Alvarez ME, Palmer R, Lamb C: Calcium-mediated apoptosis in a plant hypersensitive disease resistance response.Curr Biol1996, 6:427-437.
39. Zimmermann S, Nurnberger T, Frachisse J-M, Wirtz W, Guern J, Hedrich R, Scheel D: Receptor-mediated activation of a plant Ca2+
-permeable ion channel involved in pathogen defense.Proc Natl Acad Sci USA1997, 94:2751-2755.
40. Piedras P, Hammond-Kosack KE, Harrison K, Jones JDG: Rapid,
• Cf9- and Avr9-dependent production of active oxygen species in tobacco suspension cultures.Mol Plant–Microbe Interact1998, 11:1155-1166.
Use of a heterologous cell suspension system to analyse early events in Cf-9 mediated signalling without potential interference from numerous Cf-9 homologues
41. Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D: Elicitor stimulated ion fluxes and O2–from the oxidative burst are
essential components in triggering defense gene activation and phytoalexin synthesis in parsley.Proc Natl Acad Sci USA1997, 94:4800-4805.
42. Gelli A, Higgins VJ, Blumwald E: Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal
elicitors.Plant Physiol1997, 113:269-279.
43. Xu HX, Heath MC: Role of calcium in signal transduction during
•• the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus.Plant Cell1998, 10:585-597. Ratio imaging of microinjected epidermal strips from cowpea after challenge with basidospores of Uromyces phaseoli provides the first compelling in plantaevidence for a role of elevated Ca2+ in the HR.
44. Boyes DC, Nam J, Dangl JL: The Arabidopsis thalianaRPM1
•• disease resistance gene product is a peripheral plasma membrane protein that is degraded coincident with the hypersensitive response.Proc Natl Acad Sci USA1998, 95:15849-15854.
The authors provide the first evidence for the localisation of a NBS-LRR resistance gene product. In addition they demonstrate the possibility of reg-ulation of RPM1 in a typical Danglesque feedback-loop model.
45. Dixon MS, Hatzixanthis K, Jones DA, Harrison K, Jones JDG: The tomato Cf-5 disease resistance gene and six homologs show pronounced allelic variation in leucine-rich repeat copy number.
Plant Cell1998, 10:1915-1925.
46. Romeis T, Piedras P, Zhang S, Klessig DF, Hirt H, Jones JD: Rapid
•• Avr9- and Cf-9-dependent activation of MAP kinases in tobacco cell cultures and leaves. Convergence of resistance gene, elicitor, wound, and salicylate responses.Plant Cell1999, 11:273-288. Evidence for gene-for-gene specific activation of two MAP kinases which are not unique to defense responses but also implicated in other stress response pathways. Additionally these these kinases exhibit calcium depen-dency and they are not involved in pathways signalling the generation of NADPH dependent ROS.
47. Lam CHB, Xing T, Higgens VJ, Blumwald E: Effect of race-specific elicitors of cladosporium fulvum on the tomato plasma membrane Ca2+-ATPase.Physiol Mol Plant Path1998, 52:309-321.
48. Blumwald E, Aharon GS, Lam BC-H: Early signal transduction
•• pathways in plant–pathogen interactions.Trends Plant Sci1998, 3:342-346.
Excellent biochemical perspective of early signalling events in plant–pathogen interactions.
49. Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C: A plant homolog of the neturophil NADPH oxidase gp91phoxsubunit
gene encodes a plasma membrane protein with Ca2+binding
50. Kooman-Gersmann M, Vogelsang R, Vossen P, Henno W, van den
• Hooven EM, Honee G, de Wit PJGM: Correlation between binding affinity and necrosis-inducing activity of mutant AVR9 peptide elicitors.Plant Physiol1998, 117:609-618.
The authors continue to accumulate strong evidence for the possibility that Avr9 binds to a receptor other than Cf9.
51. Heo WD, Lee SH, Kim MC, Kim JC, Chung WS, Chun HJ, Lee KJ,
•• Park CY, Park HC, Choi JY, Cho MJ: Involvement of specific calmodulin isoforms in salicylic acid-independent activation of plant disease resistance responses.Proc Natl Acad Sci USA
1999, 96:766-771.
Elegant experiments with transgenic tobacco implicate a specific subset of calmodulin isoforms in signalling pathways leading to plant disease resistance.
52. Harding SA, Roberts DM: Incompatible pathogen infection results in enhanced reactive oxygen and cell death responses in transgenic tobacco expressing a hyperactive mutant calmodulin.
Planta1998, 206:253-258.
53. Zhang S, Klessig DF: Resistance gene N-mediated de novo
• synthesis and activation of a tobacco mitogen-activated protein kinase by tobacco mosaic virus infection.Proc Natl Acad Sci USA
1998, 95:7433-7438. See annotation for [57•].
54. Zhang S, Du H, Klessig DF: Activation of the tobacco SIP kinase by
• both a cell wall-derived carbohydrate elicitor and purified proteinaceous elicitins from Phytophthora spp.Plant Cell1998, 10:435-450.
See annotation for [57•].
55. Seoa S, Sanoc H,Ohashia Y: Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase.Plant Cell1999, 11:289-298. 56. Ligterink W, Kroj T, zurNieden U, Hirt H, Scheel D:
Receptor-mediated activation of a MAP kinase in pathogen defense of plants.Science1997, 276:2054-2057.
57. Zang S, Klessig DF: The tobacco wounding-activated MAP kinase
• is encoded by SIPK.Proc Natl Acad Sci USA1998, 95:7433-7438. Together with [46••,53•,54•], this paper provides compelling evidence for
SIPK signalling in gene-for-gene, non-specific elicitation and wounding. Their results beg the question, how are these multiple stimuli integrated and discriminated through the MAPK?
58. Xing T, Higgins VJ, Blumwald E: Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells.Plant Cell1997, 9:249-259.
59. Michelmore RW, Meyers BC: Clusters of resistance genes in plants evolve by divergent selection and a birth-and-death process.
Genome Research1998, 8:1113-1130.
60. Aarts N, Metz M, Holub E, Staskawicz BJ, Daniels MJ, Parker JE: Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis.Proc Natl Acad Sci USA1998, 95:10306-10311. 61. Falk A, Feys B, Frost L, Jones JDG, Daniels MJ, Parker JE: EDS1, an
• essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases.Proc Natl Acad Sci USA1999, 96:3292-3297.
The cloning of EDS1, integral to signalling through a defined sub-set of R
genes, provides a base for biochemical dissection of pathways to resistance.
62. Rathjen JP, Chang JH, Staskawicz BJ, Michelmore RW:
Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of AvrPto. EMBO J 1999, 3232-3240. 63. Warren RF, Merritt MM, Holub E, Innes RW: Identification of three
• putative signal transduction genes involved in R gene-specified disease resistance in Arabidopsis.Genetics1999, 152:401-412. Genetic dissection of a specific gene-for-gene pathway leads to identifica-tion of a locus, pbs1, which may be involved in specific regulation of