Antioxidative parameters in the seedlings of pigeonpea (
Cajanus
cajan
(L.) Millspaugh) in response to Zn and Ni stresses
K.V. Madhava Rao *, T.V.S. Sresty
Department of Botany,Andhra Uni6ersity,Visakhapatnam 530 003,A.P., India Received 26 November 1999; received in revised form 7 April 2000; accepted 7 April 2000
Abstract
The zinc (Zn) and nickel (Ni) as oxidative stress factors and associated responses of 6-day-old seedlings of two pigeonpea (Cajanus cajan (L.) Millspaugh) cultivars namely LRG30 and ICPL87 were studied. Zinc and Ni exposure increased lipid peroxidation in relation to their concentration. Reduction in dry matter accumulation of roots and shoots was noticed in Zn and Ni treatments. The activities of antioxidative enzymes such as superoxide dismutase, peroxidase and glutathione reductase registered higher values and the activity of catalase and the antioxidative substances such as ascorbic acid and total glutathione contents registered lower values in all the Zn and Ni treatments when compared to their controls. The levels of catalase, peroxidase and glutathione reductase and ascorbic acid and total glutathione contents were high in cv. LRG30 than in cv. ICPL87 in response to Zn and Ni treatments. However, the activity of superoxide dismutase, the major scavenger of O2
−
radical registered higher values in cv. ICPL87. The cv. LRG30 is less sensitive to Zn and Ni treatments compared to the cv. ICPL87. Correlation coefficients between the different antioxidant parameters and metal dose level, or dry matter accumulation, were established, assessing for an induced-oxidative stress. Additional evidence was provided by comparing the sensitivity of the two cultivars. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Antioxidative systems;Cajanus cajan(L.) Millspaugh; Cultivar differences; Lipid peroxidation; Metal accumulation; Nickel; Oxidative stress; Pigeonpea; Zinc
www.elsevier.com/locate/plantsci
1. Introduction
Heavy metals such as Cd, Pb, Hg, Cu, Zn and Ni at supra-optimal concentrations affect plant growth, development and yield [1 – 3]. The pres-ence of elevated concentrations of heavy metals in the growth medium of the germinating seeds sup-presses mobilisation and translocation of reserve materials from the reserve tissue to the growing regions and their subsequent utilisation there [4,5]. Heavy metal stress results in the production of O2
−, H
2O2and OH, which affect various cellular
processes mostly the functioning of membrane sys-tems [6 – 8]. A regulated balance between oxygen
radical production and destruction is required if metabolic efficiency and function are to be main-tained in both optimal and stress conditions [9,10]. Cells are normally protected against free oxy-radi-cals by the operation of intricate antioxidant sys-tems, comprising both enzymic systems such as superoxide dismutase (SOD), catalase and perox-idase and nonenzymic systems, acting as free radi-cal scavengers such as ascorbate, glutathione and phenolic compounds [10 – 12]. Ascorbate and glu-tathione are involved, in addition, as cofactors in the detoxifying enzymic processes.
Superoxide dismutase is a major scavenger of O2
−
and its enzymatic action results in the
forma-tion of H2O2and O2[11]. The oxidative damage to
different cellular components by H2O2 could be
minimised either by catalase and peroxidase activi-ties or by a reaction sequence known as ascor-bate – glutathione cycle involving the redox pairs
* Corresponding author.
E-mail address:madhavaroakv@worldmailer.com (K.V. Madhava Rao).
Table 1
Per cent seed germination of two pigeonpea cultivars in response to different concentraions of Zn and Ni supplieda
Metal concentration (mM) LRG30 ICPL87
93.092.54
Control 96.093.10
Zinc
2.5 85.092.63 78.091.77
76.091.91
5.0 67.093.38
59.092.61 66.593.42
7.5
Nickel
71.092.83 79.593.15
0.5
70.092.73
1.0 63.091.92
1.5 62.092.46 55.092.16
aData represent average of three experiments 9S.E.
of ascorbate – dehydroascorbate and glutathione – glutathione disulphide [10,11]. The antioxidant property of glutathione depends on the oxidation of SH group of the tripeptide (GSH) to disulphide (GSSG) form [13]. A high ratio of reduced to oxidised form of glutathione was maintained by the activity of glutathione reductase (GR) at the expense of NADPH. These reactions also reduce
or avoid the formation of reactive OH radical.
Much information is available on the effect of redox heavy metals on various antioxidant pro-cesses in plants [7,14,15]. However, the effect of excess concentrations of Zn and Ni on antioxida-tive processes are meagre [8,16,17] and needs care-ful consideration since they are usecare-ful to plants at lower concentrations and affect drastically at
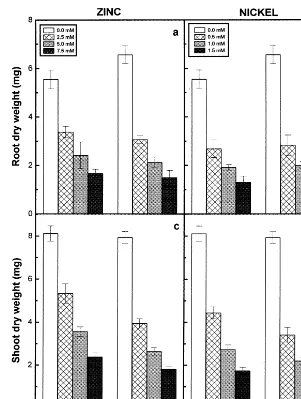
Fig. 2. Dry matter accumulation in roots (a) and (b) and shoots (c) and (d) of the 6-day-old seedlings of two pigeonpea cultivars in response to different concentrations of Zn (a and c) and Ni (b and d) supplied. Data represent based on the average values of 15 seedlings per replicate. Vertical bars indicate S.E.
vated concentrations. Therefore, the present study deals with Zn and Ni as oxidative stress factors and the associated response of the pigeonpea seedlings to such stresses. Further the study is also intended to evaluate the differential responses of two representative pigeonpea cultivars to Zn and Ni stresses.
2. Materials and methods
2.1. Plant material and hea6y metal treatment
Seeds of two Cajanus cajan (L.) Millspaugh
cultivars namely LRG30 (a long duration type) and ICPL87 (a short duration type) obtained from
ICRISAT, Patancheru, India were used for the present study. Seeds of uniform size were selected and soaked in distilled water for 2 h and were surface sterilised with 0.001 M mercuric chloride for 2 min. The seeds were then washed thoroughly with glass-distilled water. The washed seeds were then spread over Petri dishes lined with two-lay-ered filter paper containing varying concentrations of Zn and Ni. For the treatments, sulphate salts of
Zn (ZnSO4.7H2O) and Ni (NiSO4.6H2O) were
used in three different concentrations of each metal representing 2.5, 5.0 and 7.5 mM for Zn and 0.5, 1.0 and 1.5 mM for Ni. The seeds grown in distilled water were referred to as controls. The seeds of the two cultivars were allowed to
of 12 h with a light intensity of 195 mmol m−2
s−1. Then the 6-day-old seedlings were collected
and separated into individual parts for growth measurements and experimental analyses. All the experiments were replicated at least three times.
2.2. Metal analysis
The Zn and Ni content in the roots and shoots of 6-day-old seedlings of pigeonpea cultivars were determined by atomic absorption spectrophotome-ter (Perkin Elmer 2380) afspectrophotome-ter wet digestion of 1 g of dried and powdered material in 5 ml of ternary
mixture of HNO3: H2SO4: HClO4 in the ratio of
10: 1: 4 (v/v/v).
2.3. Dry matter accumulation
Roots and shoots of 6-day-old pigeonpea seedlings both treated and controls were separated gently and oven-dried immediately at 80°C for 48 h, by which time constant dry weights were obtained.
2.4. Lipid peroxidation
Lipid peroxidation in roots and shoots of 6-day-old pigeonpea seedlings was determined by esti-mating the malondialdehyde content following the method of Heath and Packer [18] with slight mod-ification. One g of material was macerated in 5 ml of 0.1% trichloroacetic acid. The homogenate was
centrifuged at 10 000×g for 5 min. For every 1
ml of the aliquot of the supernatant 4 ml of 20% TCA containing 0.5% thiobarbituric acid was added. The mixture was heated at 95°C for 30 min and then cooled quickly on ice bath. The resulting
mixture was centrifuged at 10 000×g for 15 min
and the absorbance of the supernatant was mea-sured at 532 nm. Measurements were corrected for unspecific turbidity by subtracting the absorbance at 600 nm. The concentration of malondialdehyde was calculated by using extinction coefficient of
155 mM−1 cm−1.
2.5. Enzyme determinations
For the estimation of superoxide dismutase (EC 1.15.1.1) activity, 1 g each of roots and shoots were homogenised separately in 5 ml of 50 mM
phosphate buffer (pH 7.0) containing 1%
polyvinylpyrrolidone. The homogenate was
filtered and centrifuged at 15 000×g for 10 min.
The supernatant obtained was used as enzyme extract. All steps in the preparation of enzyme extract were carried out at 0 – 4°C. An aliquot of 2 ml of the enzyme extract was used for the determi-nation of protein content by using the method of Lowry et al. [19]. The activity of superoxide dis-mutase was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium, adopting the method of Beauchamp and Fridovich [20]. The 3 ml reaction mixture contained 40 mM phosphate buffer (pH 7.8), 13
mM methionine, 75 mM nitroblue tetrazolium, 2
mM riboflavin, 0.1 mM EDTA and 0.1 ml of
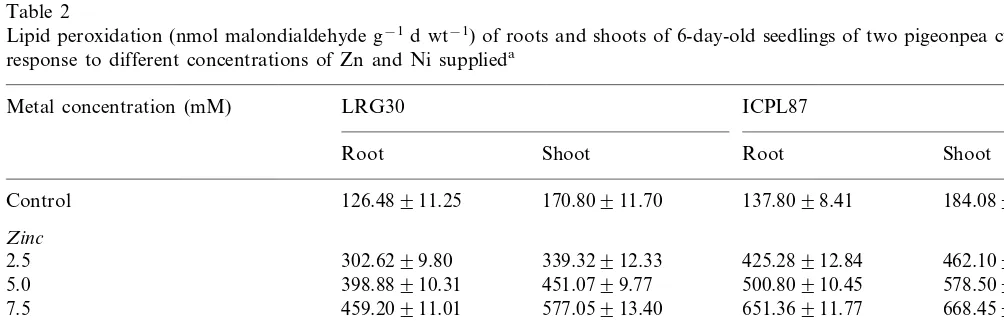
Table 2
Lipid peroxidation (nmol malondialdehyde g−1d wt−1) of roots and shoots of 6-day-old seedlings of two pigeonpea cultivars in
response to different concentrations of Zn and Ni supplieda
Metal concentration (mM) LRG30 ICPL87
Root Shoot Root Shoot
184.08910.75
Control 126.48911.25 170.80911.70 137.8098.41
Zinc
302.6299.80 339.32912.33
2.5 425.28912.84 462.10910.97
398.88910.31 451.0799.77
5.0 500.80910.45 578.50912.54
459.20911.01 577.05913.40
7.5 651.36911.77 668.45911.86
Nickel
0.5 390.64910.99 481.26910.84 475.4098.75 587.74913.24
624.15911.56
1.0 485.7697.61 604.48910.72 690.39912.58
614.72910.42 730.27912.51 720.54911.60 806.5899.67 1.5
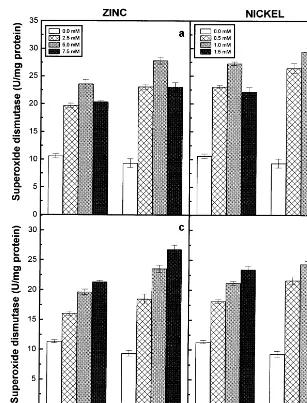
Fig. 3. Superoxide dismutase activity of the roots (a) and (b) and shoots (c) and (d) of the 6-day-old seedlings of two pigeonpea cultivars in response to different concentrations of Zn (a and c) and Ni (b and d) supplied. Data represent the average of three experiments. Vertical bars indicate S.E.
enzyme extract. Riboflavin was added at the end and the test tubes were shaken and placed 30 cm below light source consisting of two 15-W fluores-cent lamps. The reduction was started by switch-ing on the light. The reaction was allowed to take place for 30 min and was stopped by switching off the light. Then the tubes were covered with a black cloth. The absorbance of the solution was mea-sured at 560 nm and from which the absorbance of the nonirradiated reaction mixture, which served as control, was deducted. Log A560 was plotted as a function of the volume of enzyme extract used in the reaction mixture. From the resultant graph the volume of the enzyme extract corresponding to 50% inhibition of the reaction was read and considered as one enzyme unit.
Peroxidase (EC 1.11.1.7) activity was deter-mined following the method described by Kar and Mishra [21]. Two hundred mg of each sample was homogenised with 10 ml of 0.1 M phosphate
buffer (pH 6.8) and centrifuged at 17 000×g in a
refrigerated centrifuge. The supernatant was taken as the enzyme extract. The assay mixture for the
peroxidase comprised 125 mmol of phosphate
buffer (pH 6.8), 50mmol of pyrogallol, 50mmol of
H2O2 and 1 ml of enzyme extract. This was
con-tent of the enzyme extract was calculated follow-ing the method of Lowry et al. [19].
Catalase (EC 1.11.1.6) activity was estimated by the permanganate method of Povolotskaya and Sedenka [22] as followed by Gopalachari [23]. Two hundred mg of roots and shoots were weighed separately and ground in pre-cooled glass mortar with few ml of cold phosphate buffer (pH 7.0). The extract was filtered through glass wool and made up to 25 ml with the same buffer solution. Two ml of enzyme extract was taken in a conical
flask and 1 ml of 0.045 M H2O2 was added.
Exactly after 5 min 1 ml of 12% H2SO4was added
to stop the enzyme activity and titrated with 0.05
N KMnO4. The end point was denoted by the
pink colour of the solution, which lasts for 30 s. A
blank was run simultaneously as above substitut-ing 2 ml of the enzyme extract with 2 ml
phos-phate buffer. The activity of catalase was
calculated by using the following equation:
mg H2O2 destroyed in 5 min by 1 g plant
tissue=25
2 ×0.85×
V W
where, V is the difference between blank and titre
values; W is fresh weight in grams; 2 is extract taken in ml.
The factor 0.85 represents 1 ml of 0.05 N
KMnO4 equal to 0.85 mg of H2O2.
The assay of glutathione reductase (EC 1.6.4.2) in the crude tissue homogenate was carried out according to Smith et al. [24]. One gram each of
Table 3
Correlation coefficients between different parameters of antioxidative mechanisms and increasing concentrations of externally supplied metal ions or dry matter accumulation of roots and shoots of two pigeonpea cultivars
Zinc concentration Dry weight Nickel concentration Dry weight supplied
supplied
ICPL87
LRG30 LRG30 ICPL87 LRG30 ICPL87 LRG30 ICPL87
Roots
Superoxide dismutase 0.767 0.744 −0.913* −0.913* 0.704 0.627 −0.895* −0.864 Catalase −0.889* −0.894* 0.986** 0.993** −0.922* −0.890* 0.999** 0.993**
0.802 −0.949* −0.898* 0.429
0.813 0.514
Peroxidase −0.630 −0.731
−0.979**
Ascorbic acid −0.964** 0.987** 0.997** −0.967** −0.911* 0.989** 0.999**
−0.998** 0.959** 0.922* −0.991**
Total glutathione −1.000** −0.989** 0.964** 0.967**
0.964** −0.996** −0.994** 0.989**
0.939* 0.982**
Glutathione reductase −0.950* 0.977**
Shoots
0.979**
Superoxide dismutase 0.974** −0.998** −0.993** 0.965** 0.938* −0.899* −0.997**
−0.888* 0.986** 0.942* −0.930*
−0.919* −0.890*
Catalase 0.893* 0.949*
0.902*
Peroxidase 0.957* −0.978** −0.998** 0.907* 0.988** −0.996** −0.926*
−0.984** 0.982** 0.962** −0.968** −0.958* 0.991** 0.995**
Ascorbic acid −0.998**
−0.995** 0.979** 0.940* −0.923*
−0.995** −0.982**
Total glutathione 0.882* 0.908*
0.894* −0.983**
Glutathione reductase 0.913* −0.992** 0.909* 0.947* −0.927* −0.928*
*PB0.05; **PB0.01.
roots and shoots of the pigeonpea seedlings were homogenised separately with a mortar and pestle using 5 ml of 0.1 M potassium phosphate buffer (pH 7.5) containing 0.5 mM EDTA. The brie was filtered with cheese cloth and the filtrate was
cen-trifuged for 10 min at 20 000×g to sediment
particulate matter. The supernatant is referred to as crude extract. The protein content of the en-zyme extract was estimated following the method of Lowry et al. [19]. The reaction mixture con-tained 1.0 ml of 0.2 M potassium phosphate buffer (pH 7.5) containing 1 mM EDTA, 0.5 ml 3 mM
5,5%-dithiobis (2-nitrobenzoic acid) in 0.01 M
phos-phate buffer (pH 7.5), 0.25 ml H2O, 0.1 ml 2 mM
NADPH, 0.05 ml enzyme extract and 0.1 ml 20 mM oxidised glutathione (GSSG). The compo-nents were added in the order listed to a cuvette and the reaction was initiated by the addition of GSSG. The increase in absorbance at 412 nm was continuously monitored for 5 min. The rate of enzyme activity was calculated using standard curve prepared by known amounts of glutathione reductase.
2.6. Determination of ascorbic acid content
Ascorbic acid content was estimated according to the method of Roe [25], which is based on the
reduction of 2,6-dichlorophenol indophenol (2,6-DCIP) by ascorbic acid. One gram each of roots and shoots of the two pigeonpea cultivars were macerated separately with 10 ml of 5% metaphos-phoric acid using a mortar and pestle. The ho-mogenate was filtered through filter paper and the filtrate was made up to 20 ml with 5% metaphos-phoric acid. Duplicate samples of 10 ml aliquots were titrated with 0.025% 2,6-DCIP reagent until a pink end point that persists for 15 s was ob-tained. The quantity of ascorbic acid was calcu-lated by comparing with the values obtained by the known quantities of standard ascorbic acid.
2.7. Determination of total glutathione content
Total glutathione (GSH+GSSG) content was
assayed by enzymatic recycling procedure of Ti-etze [26] as followed by Griffith [27]. Two hundred mg of roots and shoots of the pigeonpea seedlings were homogenised separately in 1.5 ml of 2% metaphosphoric acid containing 2 mM EDTA and
1 g/1 ml polyvinylpolypyrrolidone by using a
pre-cooled mortar and pestle and then centrifuged at
17 000×g for 10 min. The pH of the extract was
brought to 5.5 with 10% sodium citrate.
50 units of glutathione reductase per ml were prepared by using 125 mM sodium phosphate buffer (pH 7.5) containing 6.3 mM Na-EDTA. Total glutathione content was estimated by mixing
700ml of solution i and 100ml of solution ii and the
sample extract of 200 ml to give a final volume of
1.0 ml, directly in a cuvette with 1 cm light path
and equilibrated to 30°C. To this solution 10ml of
solution iii was added and then the absorbance was monitored at 412 nm. The total glutathione con-tent present in the aliquot was evaluated by com-parison of the rate observed to a standard curve generated with known amounts of glutathione.
The t-test was used to compare the growth
performance of two pigeonpea cultivars in terms of their root or shoot dry weights. In addition
signifi-cance of differences between the components of antioxidative parameters and external concentra-tions of metal ions supplied or dry weight of the organs was evaluated by using correlation coeffi-cients.
3. Results
3.1. Effect of Zn and Ni on per cent seed germination
The per cent germination (considered as 0.4 cm radicle emergence after 24 h of germination) of the two pigeonpea cultivars exposed to externally sup-plied Zn and Ni was shown in Table 1. The
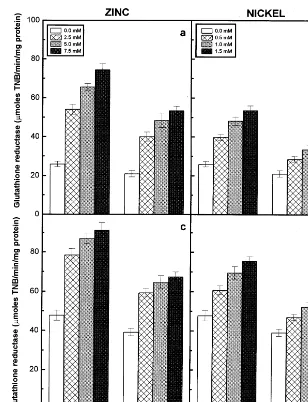
Fig. 6. Glutathione reductase activity of the roots (a) and (b) and shoots (c) and (d) of the 6-day-old seedlings of two pigeonpea cultivars in response to different concentrations of Zn (a and c) and Ni (b and d) supplied. Data represent the average of three experiments. Vertical bars indicate S.E.
cent germination decreased with increasing con-centrations of heavy metal treatments.
3.2. Effect of Zn and Ni on symptoms of phytotoxicity
The symptoms of toxicity appeared as reduction in seedling growth. The growth of the main root was affected much and as a result it exhibited the formation of fibrous roots. The roots and stem portion of the shoots were partially turned to brown colour. At high doses of metal ions the roots appear dark brown in colour.
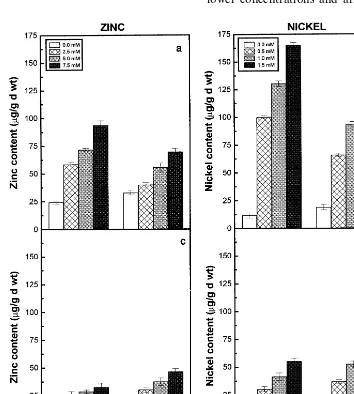
3.3. Metal accumulation
3.4. Effect of Zn and Ni on dry matter accumulation
The effect of Zn and Ni on dry weights of roots and shoots of the pigeonpea seedlings are shown in Fig. 2. Both the metals caused a conspicuous decrease of dry matter accumulation in roots and shoots and this becomes more steep with increas-ing concentrations of Zn and Ni supplied. Between the two cultivars of pigeonpea, the reduction in dry matter accumulation was more significant in ICPL87 than in LRG30 in response to Zn and Ni
treatments. It was further evident from the t
-val-ues calculated on per cent decrease in relation to
controls of roots or shoots. The t-values
(LRG30"ICPL87) obtained for the root growth
of 2.5 and 5.0 mM Zn-treated seedlings were 4.30 and 2.75 and the values obtained for the root growth of 0.5 and 1.0 mM Ni-treated seedlings were 2.34 and 3.00 at 0.10 level of significance. Similar responses of the two pigeonpea cultivars to Zn and Ni treatments were also obtained for the
shoot growth and thet-values (LRG30"ICPL87)
obtained were 5.21 and 3.39 for the respective Zn treatments and 5.85 and 2.60 for the respective Ni treatments at 0.10 level of significance. However,
the t-values obtained for the root or shoot dry
weights at higher concentrations of Zn (7.5 mM)
and Ni (1.5 mM) were not significant at P=
0.10 level. The Ni treatments registered lower val-ues of dry matter accumulation than Zn-treated ones.
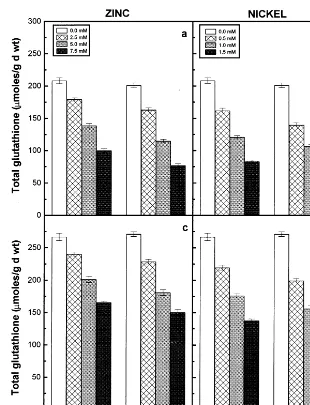
Fig. 8. Total glutathione content of the roots (a) and (b) and shoots (c) and (d) of the 6-day-old seedlings of two pigeonpea cultivars in response to different concentrations of Zn (a and c) and Ni (b and d) supplied. Data represent the average of three experiments. Vertical bars indicate S.E.
3.5. Effect of Zn and Ni on lipid peroxidation
The formation of malondialdehyde content was considered as a measure of lipid peroxidation. The lipid peroxidation of roots and shoots of the two pigeonpea cultivars increased with increasing con-centrations of externally supplied Zn and Ni ions (Table 2). The lipid peroxidation was more active in cv. ICPL87 in response to Zn and Ni treat-ments. The lipid peroxidation was greater in the Ni-treated seedlings than in the Zn-treated ones.
3.6. Effect of Zn and Ni on antioxidati6e enzymes
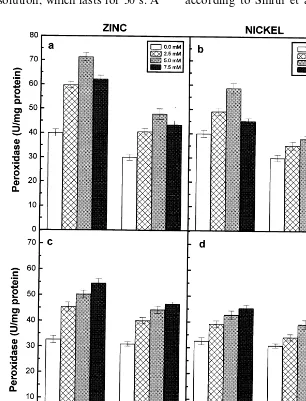
Superoxide dismutase and peroxidase activities
of the roots and shoots of the two pigeonpea cultivars exhibited a rise in relation to increasing concentrations of externally supplied Zn and Ni and registered higher values when compared to their respective controls (Figs. 3 and 4). However, the roots of the 7.5 mM Zn and 1.5 mM Ni-treated seedlings of the two pigeonpea cultivars exhibited lower values of superoxide dismutase and peroxidase activities when compared to other treatments of Zn and Ni (Fig. 3a and b and Fig. 4a and b). The correlation coefficients obtained for the two enzyme activities in the roots of two cultivars and external concentrations of Zn or Ni
supplied were not significant at P=0.05 level. The
signifi-cant negative correlation with the dry matter accu-mulation, whereas the Ni-treated seedlings did not show any significant correlation with the dry
mat-ter accumulation. On the other hand, the
superoxide dismutase and peroxidase activities of the shoots of two cultivars correlated positively with external concentrations of metal ions sup-plied and negatively with the dry matter accumula-tion (Table 3). Among the two cultivars of pigeonpea, greater levels of superoxide dismutase activity was exhibited by roots and shoots of ICPL87, however higher levels of peroxidase was noted in LRG30 in response to Zn and Ni
treat-ments. Among the two metal ions,
Ni-induced higher levels of superoxide dismutase and lower levels of peroxidase compared to Zn treat-ments.
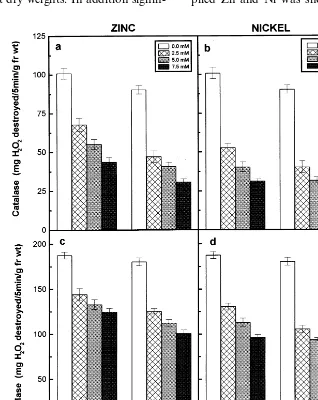
The catalase activity of the roots and shoots of the Zn- and Ni-treated seedlings of the two pi-geonpea cultivars dropped substantially with in-creasing concentrations of externally supplied metal ions and registered lower values when compared to their respective controls (Fig. 5). The catalase activity of the roots and shoots of the two pigeonpea cultivars showed a signifi-cant negative correlation with external con-centrations of Zn or Ni supplied and a positive
correlation with dry matter accumulation
(Table 3). Among the two cultivars of pigeonpea,
LRG30 registered higher values of catalase
activity than ICPL87 in response to Zn and Ni
treatments. The seedlings that were grown
in different concentrations of Ni showed lower levels of catalase activity compared to Zn-treated ones.
Glutathione reductase activity of the roots and shoots of the two cultivars increased with
increas-ing concentrations of externally supplied
Zn and Ni and registered higher values when compared to their controls (Fig. 6). The glu-tathione reductase activity of the roots and shoots showed a strong positive correlation
with external concentrations of Zn or Ni
and a negative correlation with dry matter accu-mulation (Table 3). Among the two cultivars of pigeonpea, LRG30 registered higher values of glu-tathione reductase activity than ICPL87 in re-sponse to both the metal treatments. Among the treatments, Ni-induced lower levels of glutathione
reductase activity compared to Zn-treated
ones.
3.7. Effect of Zn and Ni on antioxidati6e
compounds
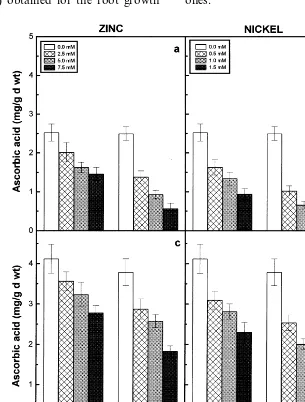
The ascorbic acid and total glutathione contents of the roots and shoots of two cultivars decreased with increasing concentrations of externally sup-plied Zn or Ni and registered lower values when compared to their respective controls (Figs. 7 and 8). Further the ascorbic acid and total glutathione contents of roots and shoots of the two pigeonpea cultivars showed a significant negative correlation with the increasing concentrations of metal ions supplied and a positive correlation with dry matter accumulation (Table 3). Among the two cultivars of pigeonpea, LRG30 registered higher values of both the antioxidant compounds than ICPL87 in response to Zn and Ni treatments. Among the two
metal treatments, the Ni-treated pigeonpea
seedlings registered lower values of ascorbic acid and total glutathione contents.
4. Discussion
Zinc and Ni uptake as revealed by their con-tents in the roots and shoots of two pigeonpea cultivars increased with increasing concentrations of respective metal ions supplied (Fig. 1). The accumulation of Zn and Ni in the roots of cv. LRG30 registered higher values compared to the roots of cv. ICPL87. It was demonstrated that tolerant plants accumulate more amount of heavy metals in their roots [28]. Zinc and Ni treatments also affected the dry matter accumulation in roots and shoots of the two pigeonpea cultivars (Fig. 2). Toxic levels of heavy metal ions have been re-ported to induce negative effects on some key metabolic processes coupled to growth in higher plants [29,30]. Between the two cultivars, the ef-fects were more conspicuous at lower concentra-tions and they were not significant at highest concentrations of Zn and Ni.
oxida-tive damage and improve resistance to oxidaoxida-tive stress. Between the two pigeonpea cultivars, the lipid peroxidation was more active in ICPL87 in response to Zn and Ni treatments. Further, lipid peroxidation was more in Ni-treated seedlings than in Zn-treated ones.
Superoxide dismutase is considered to play an important role in the cellular defence strategy against oxidative stress caused by toxic metal con-centrations [30]. The superoxide dismutase activity was stimulated in roots and shoots of the two pigeonpea cultivars in relation to increasing con-centrations of Zn or Ni supplied (Fig. 3). How-ever, the correlation coefficients obtained for superoxide dismutase activity of the roots and external concentrations of Zn or Ni were not
significant at P=0.05 level (Table 3). Among the
two cultivars of pigeonpea, greater levels of super-oxide dismutase activity was exhibited by ICPL87, indicating that this cultivar may be prone to
con-siderable amount of H2O2-induced oxidative
stress, if further detoxifying mechanisms fail to adjust adequately in the cells when compared to cv. LRG30 in response to Zn and Ni treatments. Among the two metal ions, Ni stimulated greater levels of superoxide dismutase activity than Zn. Peroxidases are known to play significant role in oxidative stress conditions [33]. Peroxidase activity was stimulated in the seedlings of two pigeonpea cultivars treated with different concentrations of Zn and Ni (Fig. 4). The increase of peroxidase activity was shown to be strongly correlated with
metal ion concentration [29,34] and related
biomass production [32]. Increased peroxidase was observed after the application of Ni to the leaves of Silene italica [35] and Phaseolus 6ulgaris [36]
and Zn to the leaves of Silene cucubalus [37] and
Phaseolus 6ulgaris [29]. Interestingly the perox-idase activity of the roots of two cultivars did not show any significant correlation with the external concentrations of Zn or Ni supplied (Table 3). Among the two cultivars of pigeonpea, lowest activity of peroxidase was noticed in ICPL87 in response to Zn and Ni treatments. However among the Zn and Ni treatments, Zn-induced higher levels of peroxidase activity than Ni.
The catalase activity in the two pigeonpea culti-vars showed a substantial decrease with increasing concentrations of externally supplied Zn or Ni (Fig. 5). In a long-term stress exposure, it was observed that Zn inhibits the catalase activity in
beans [38]. It was suggested that the heavy metal-induced decrease in catalase activity can be at-tributed to inhibition of the synthesis of catalase and other oxidase proteins during rice seed germi-nation [39]. On the other hand, it was demon-strated that catalase activity was stimulated in order to take part in an efficient defence mecha-nism against Cu-induced oxidative stress in the primary leaves of beans [7]. No stimulation of catalase activity was observed in both the pigeon-pea cultivars studied in response to oxidative dam-age under Zn and Ni stresses (Fig. 5). Among the two cultivars of pigeonpea, LRG30 registered greater values of catalase activity than ICPL87 in response to both the metal treatments. The seedlings that were grown in different concentra-tions of Ni showed lower levels of catalase activity than Zn.
Ascorbic acid plays a prominent role together with glutathione in scavenging free oxy-radicals [13]. Zinc and Ni treatments decreases the ascorbic acid and total glutathione contents of the roots and shoots of the two pigeonpea cultivars studied (Figs. 7 and 8). The decrease of these two antioxi-dants depends on the Zn or Ni concentrations supplied and it was evident by the establishment of a significant negative correlation between ascorbic acid or total glutathione content and increasing concentrations of externally supplied metal ions (Table 3). The reactive free oxygen radicals were assumed to be involved in the oxidation of ascor-bic acid to dehydroascorascor-bic acid, leading to reduc-tion in the ascorbic acid content of plants [40]. Metal-induced decline in the pool of glutathione has also been observed for many plants and fungi [41 – 43].
The antioxidant property of the glutathione de-pends on the oxidation of SH group of the tripep-tide (GSH) to disulphide (GSSG) form [13]. The participation and activation of glutathione reduc-tase in the conversion of oxidised to reduced glu-tathione was noted by several workers [10,13]. Glutathione reductase activity was stimulated by Zn and Ni treatments in the roots and shoots of the two pigeonpea cultivars (Fig. 6). The increase in the activity of glutathione reductase can atleast
partly keep the GSSG/GSH ratio at possible low
levels, since GSH plays a prominent role in the
scavenging of H2O2 [13,44]. The ions of several
metals such as Zn2+, Cd2+, Pb2+, Ni2+ and
dis-turbs the interconversion of oxidised and reduced glutathione, which results in lowering of the levels of available antioxidants in the cells [45]. The decline in the glutathione content of plants may also result from the inhibition of the enzymes involved in glutathione synthesis by toxic metal ions [46]. In addition the depletion of glutathione pool may also considered to be its role in the synthesis of metal binding peptides, the phy-tochelatins [47,48]. The decrease in ascorbic acid and total glutathione contents were relatively less in cv. LRG30 than in cv. ICPL87 in response to Zn and Ni treatments. A high ratio of reduced to oxidised form of glutathione has been maintained in cv. LRG30 than in cv. ICPL87, as indicated by the higher levels of the recycling enzyme, the glutathione reductase in cv. LRG30 compared to cv. ICPL87 in response to both Zn and Ni treat-ments.
The changes in the activities of superoxide dis-mutase, peroxidase and glutathione reductase in Zn- and Ni-treated seedlings of the two pigeonpea cultivars can be regarded as a consequence of metal-induced oxidative stress. Though, the cv. ICPL87 registered higher levels of superoxide dis-mutase activity, the pattern of dry matter and lipid
peroxidation changes indicated that the cv.
ICPL87 seems to be more susceptible to Zn and Ni treatments than cv. LRG30. The higher levels of superoxide dismutase activity may probably led
to greater levels of H2O2accumulation in the roots
and shoots of cv. ICPL87 than in cv. LRG30 in response to Zn and Ni treatments. Further, the
detoxification mechanisms of H2O2in cv. ICPL87
were less active than in cv. LRG30 as revealed by the lower levels of catalase and peroxidase and ascorbic acid and total glutathione contents in cv. ICPL87. The pigeonpea cultivar LRG30 in addi-tion to better growth performance registered lower levels of superoxide dismutase and higher levels of catalase and peroxidase activities and ascorbic acid and total glutathione contents resulting in relatively less oxidative damage, confirming that the cv. LRG30 is less sensitive to Zn and Ni treatments compared to cv. ICPL87. Further, it may be presumed that most of the oxidative stress in these cultivars results from decreased catalase activity and lower contents of ascorbic acid and total glutathione. When the less sensitive and the sensitive cultivars were compared, the levels of heavy metals are higher in the roots and lower in
the shoots of less sensitive cultivar suggesting that roots might act as a kind of heavy metal filters for the protection of plants.
Acknowledgements
T.V.S.S. is grateful to University Grants Com-mission, New Delhi for financial assistance.
References
[1] H.W. Woolhouse, Toxicity and tolerance in the response of plants to metals, in: O.L. Lange, P.S. Nobel, C.B. Osmond, M. Ziegfer (Eds.), Encyclopaedia of Plant Physiology, Responses to the Chemical and Biological Environment, vol. 12c, Springer, Berlin, 1983, pp. 245 – 300.
[2] A.M.B. Pahlsson, Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants: a literature review, Water Air Soil Pollut. 47 (1989) 287 – 319.
[3] T.V.S. Sresty, K.V. Madhava Rao, Ultrastructural alter-ations in response to zinc and nickel stress in the root cells of pigeonpea, Environ. Exp. Bot. 41 (1999) 3 – 13. [4] F.H. Hsu, H.C. Chang, Inhibitory effects of the heavy
metals on seed germination and seedling growth ofMis
-canthusspecies, Bot. Bull. Acad. 33 (1992) 335 – 342. [5] A. Mishra, M.A. Choudhuri, Differential effect of Pb2+
and Hg2+ on inhibition of germination of seeds of two
rice cultivars, Indian J. Plant Physiol. (New series) 2 (1997) 41 – 44.
[6] C.H. Foyer, H. Lopez-Oelgado, J.F. Dat, I.M. Scott, Hydrogen peroxide- and glutathione-associated mecha-nisms of acclimatory stress tolerance and signaling, Phys-iol. Plant. 100 (1997) 241 – 254.
[7] J.E.J. Weckx, H. Clijsters, Oxidative damage and defense mechanisms in primary leaves ofPhaseolus6ulgarisas a
result of root assimilation of toxic amounts of copper, Physiol. Plant. 96 (1996) 506 – 512.
[8] J.E.J. Weckx, H.M.M. Clijsters, Zinc phytotoxicity in-duces oxidative stress in primary leaves of Phaseolus
6ulgaris, Plant Physiol. Biochem. 35 (1997) 405 – 410.
[9] B. Halliwell, The toxic effects of oxygen on plant tissues, in: L.W. Oberley (Ed.), Superoxide Dismutase, vol. 1, CRC Press, Boca Raton, FL, 1982, pp. 89 – 123. [10] C.H. Foyer, P. Descourvie´res, K.J. Kunert, Protection
against oxygen radicals: an important defence mecha-nism studied in transgenic plants, Plant Cell Environ. 17 (1994) 507 – 523.
[11] I. Fridovich, The biology of oxygen radicals, Science 201 (1978) 875 – 880.
[12] C. Bowler, M. Van Montagu, D. Inze´, Superoxide dis-mutase and stress tolerance, Annu. Rev. Plant Physiol. Plant Mol. Biol. 43 (1992) 83 – 116.
[14] C.H.R. De Vos, M.J. Vonk, R. Vooijs, H. Schat, Glu-tathione depletion due to copper induced phytochelatin synthesis causes oxidative stress inSilene cucubalus, Plant Physiol. 98 (1992) 853 – 858.
[15] S. Mazhoudi, A. Chaoui, M.H. Ghorbal, E. El Ferjani, Response of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum Mill.), Plant Sci. 127 (1997) 129 – 137.
[16] A. Chaoui, S. Mazhoudi, M.H. Ghorbal, E. El Ferjani, Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseo
-lus6ulgarisL.), Plant Sci. 127 (1997) 139 – 147.
[17] H. Schickler, H. Caspi, Response of antioxidative en-zymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum, Physiol. Plant. 105 (1999) 39 – 44.
[18] R.L. Heath, L. Packer, Photoperoxidation in isolated chloroplasts. 1. Kinetics and stiochiometry of fatty acid peroxidation, Arch. Biochem. Biophys. 125 (1968) 189 – 198.
[19] O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.L. Randall, Protein measurement with the folin phenol reagent, J. Biol. Chem. 193 (1951) 265 – 275.
[20] C. Beauchamp, I. Fridovich, Superoxide dismutase: im-proved assay and an assay applicable to PAGE, Anal. Biochem. 44 (1971) 276 – 287.
[21] M. Kar, D. Mishra, Catalase, peroxidase and polyphenol oxidase activities during rice leaf senescence, Plant Phys-iol. 57 (1976) 315 – 319.
[22] K.L. Povolotskaya, D.M. Sedenka, A method for collec-tive determination of ascorbic, polyphenol and perox-idase activities, Biochimia 21 (1956) 133 – 136.
[23] N.C. Gopalachari, Changes in the activities of certain oxidising enzymes during germination and seedling de-velopment of Phaseolus mungo and Sorghum 6ulgare,
Indian J. Exp. Biol. 1 (1963) 98 – 100.
[24] I.K. Smith, T.L. Vierheller, C.A. Thorne, Assay of glu-tathione reductase in crude tissue homogenates using 5,5%-dithiobis (2-nitrobenzoic acid), Anal. Biochem. 175 (1988) 408 – 413.
[25] J.H. Roe, Chemical determination of ascorbic, dehy-droascorbic and diketogluconic acids, in: D. Glick (Ed.), Methods of Biochemical Analysis I, Interscience, New York, 1964, pp. 115 – 139.
[26] F. Tietze, Enzymatic method for quantitative determina-tion of nanogram amounts of total and oxidised glu-tathione: applications to mammalian blood and other tissues, Anal. Biochem. 27 (1969) 502 – 522.
[27] O.W. Griffith, Determination of glutathione and glu-tathione disulfide using gluglu-tathione reductase and 2-vinylpyridine, Anal. Biochem. 106 (1980) 207 – 212. [28] J.A.C. Verkleij, P. Koevoets, J. Van’t Riet, R. Bank, Y.
Nijdam, W.H.O. Ernst, Poly (g-glutamylcysteinyl)
glycines or phytochelatins and their role in cadmium tolerance ofSilene6ulgaris, Plant Cell Environ. 13 (1990)
913 – 921.
[29] F. Van Assche, C. Cardinaels, H. Clijsters, Induction of enzyme capacity in plants as a result of heavy metal toxicity: dose – response relations inPhaseolus6ulgarisL.
treated with zinc and cadmium, Environ. Pollut. 52 (1988) 103 – 113.
[30] F. Van Assche, H. Clijsters, Effects of metals on enzyme activity in plants, Plant Cell Environ. 13 (1990) 195 – 206. [31] A. Polle, H. Rennenberg, Significance of antioxidants in plant adaptation to environmental stress, in: L. Fowden, F.T. Mansfield, J. Stoddard (Eds.), Plant Adaptation to Environmental Stress, Chapman and Hall, London, 1993, pp. 263 – 273.
[32] J. Vangronsveld, H. Clijsters, Toxic effects of metals, in: M.E. Farago (Ed.), Plants and the Chemical Elements — Biochemistry, Uptake, Tolerance and Toxicity, VCH, New York, 1994, pp. 149 – 177.
[33] S. Karataglis, M. Moustakas, L. Symeonidis, Effects of heavy metals on isoperoxidases of wheat, Biol. Plant. (Praha) 33 (1991) 3 – 9.
[34] S. Mukherji, B.C. Dasgupta, Characterisation of copper toxicity in lettuce seedlings, Physiol. Plant. 27 (1972) 126 – 129.
[35] R. Gabbrielli, T. Pandolfini, O. Vergnano, Peroxidase involvement in tolerance mechanisms, G. Bot. Ital. 21 (1987) 200 – 201.
[36] F. Van Assche, C. Put, H. Clijsters, Heavy metals induce specific isozyme patterns of peroxidase inPhaseolus6ul
-garisL, Arch. Int. Physiol. Biochem. 94 (1986) 60. [37] W. Mathys, The role of malate, oxalate and mustard oil
glucosides in the evolution of zinc — resistance in herbage plants, Physiol. Plant. 40 (1977) 130 – 136. [38] J.E.J. Weckx, J. Vangronsveld, H.M.M. Clijsters, Heavy
metal induction of ethylene production and stress en-zymes: 1. Kinetics of the responses, in: J.C. Pech, A. Latche´, C. Balague´ (Eds.), Cellular and Molecular As-pects of the Plant Hormone Ethylene, Kluwer, Dor-drecht, 1993, pp. 238 – 239.
[39] P.K. Das, M. Kar, D. Mishra, Nickel nutrition of plants. 1. Effects of nickel on some oxidase activities during rice (Oryza sati6a L.) seed germination, Z. Pflanzenphysiol. 90 (1978) 225 – 233.
[40] I. Fridovich, P. Handler, Detection of free radicals gen-erated during enzymic oxidation by the initiation of sulphite oxidation, J. Biol. Chem. 236 (1961) 1836 – 1840. [41] E. Grill, E.L. Winnaker, M.H. Zenk, Phytochelatins: a class of heavy metal binding peptides from plants are functionally analogous to metallothioneins, Proc. Natl. Acad. Sci. U.S.A. 84 (1987) 439 – 443.
[42] A. Tukendorf, W.E. Rauser, Changes in glutathione and phytochelatins in roots of maize seedlings exposed to cadmium, Plant Sci. 70 (1990) 155 – 166.
[43] P. Meuwly, W.E. Rauser, Alteration of thiol pools in roots and shoots of maize seedlings exposed to cadmium: adaptation and developmental cost, Plant Physiol. 99 (1992) 8 – 15.
[44] H. Zopes, S. Klapheck, L. Bergmann, The function of homoglutathione and hydroxymethylglutathione for the scavenging of hydrogen peroxide, Plant Cell Physiol. 34 (1993) 515 – 521.
[45] N.T. Christie, M. Costa, In vitro assessment of the toxicity of metal compounds. IV. Deposition of metals in cells: interactions with membranes, glutathione, metal-lothionein and DNA, Biol. Trace Elem. Res. 6 (1984) 139 – 158.
[47] E. Grill, E.L. Winnaker, M.H. Zenk, Phytochelatins: the principal heavy metal complexing peptides of higher plants, Science 230 (1985) 674 – 676.
[48] J.C. Steffens, The heavy metal-binding peptides of plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 41 (1990) 553 – 575.