Dwarf (di)haploid
pito
mutants obtained from a tetraploid potato
cultivar (
Solanum tuberosum
subsp.
tuberosum
) via anther culture
are defective in gibberellin biosynthesis
Jari P.T. Valkonen
a,*, Thomas Moritz
b, Kazuo N. Watanabe
c, Veli-Matti Rokka
daDepartment of Plant Biology,Genetic Centre,Swedish Uni6ersity of Agricultural Sciences(SLU),PO Box7080,S-750 07Uppsala,Sweden bDepartment of Forest Genetics and Plant Physiology,SLU,S-901 83Umea˚,Sweden
cDepartment of Biotechnological Science,Kinki Uni6ersity,Uchita,Wakayama,649-6493,Japan
dAgricultural Research Centre of Finland,Plant Production Research,Crops and Soil,Myllytie10,FIN-31600Jokioinen,Finland
Received 23 April 1999; received in revised form 30 June 1999; accepted 19 July 1999
Abstract
Nine dwarf (di)haploid lines (2n=2× =24) were obtained from the tetraploid (2n=4× =48), long day-adapted potato cultivar ‘Pito’ (Solanum tuberosum subsp. tuberosum) through anther culture. They grew slowly, had very short internodes, compact and ball-shaped appearance, and dark green leaves. Dwarfism was due to a recessive gene, designatedpito. Endogenous gibberellin contents were measured in the leaves of dwarf and wild-type lines by gas chromatography linked to mass spectrometry (GC-MS). High amounts of GA19, GA20, GA29, GA1, and GA8were detected in the wild-type plants, which indicated that the early 13-hydroxylation pathway was predominantly used for GA biosynthesis inS. t. subsp. tuberosum. Also GA53, GA15and GA9 were detected but not quantified. Very low endogenous amounts of all analysed GAs were detected in the pitomutants, indicating a block at an early part of the GA biosynthesis pathway. The dwarf lines strongly and quickly responded to the exogenous application of low amounts (79 nM) of bioactive GA (GA3), which restored normal growth and confirmed that thepito
dwarfs were synthesis mutants and not GA response mutants. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Gibberellin; Potato;Solanum tuberosum; Dwarf; Gas chromatography mass spectrometry GC-MS
www.elsevier.com/locate/plantsci
1. Introduction
Since the discovery of gibberellin (GA) in the fungus Gibberella fujikuroi[1] and the observation that exogenous application of GA can recover the normal growth of the recessive dwarf mutant (le) of pea (Pisum sati6um L.) [2,3], the central role of GAs as growth regulators in plants has been es-tablished. While over 100 structures of these diter-penoid compounds are known to date, only few of those containing 19 carbon atoms (C19-GA) are biologically active in plants [4]. The main biologi-cal effects are manifested during germination, stem elongation and reproductive development [5,6].
Genetic mutants altered in GA biosynthesis or response to GA have been described in many plant species and families, including Brassicaceae (Ara -bidopsis and Brassica rapa L.), Poaceae (barley, Hordeum 6ulgareL.; maize, Zea maysL.; and rice, Oryza sati6a L.), Fabaceae (pea), and Solanaceae (tomato, Lycopersicon esculentum Mill.) to men-tion a few [6 – 8]. They have been fundamental in resolving the GA biosynthesis pathways in differ-ent species. Qualitative and quantitative data on GAs in plants have been significantly increased and improved lately since the highly sensitive de-tection method, gas chromatography linked to mass spectrometry (GC-MS), has been applied to analysis of endogenous GA contents [4,8]. Current knowledge on the GA biosynthesis pathway per-mits to divide it into three parts: (i) the synthesis
* Corresponding author. Fax: +46-18-67-33-92.
E-mail address:[email protected] (J.P.T. Valkonen)
of ent-kaurene from geranyl diphosphate by co-palyl diphosphate synthase and ent-kaurene syn-thase; (ii) the conversion of ent-kaurene to GA53 by cytochrome P450 enzymes; and (iii) metabolism to bioactive GAs by dioxygenases [8,9].
In the cultivated potatoes (Solanum tuberosum L., Solanaceae), GAs are of special interest be-cause they function as inhibitors of tuberization and, on the other hand, induce sprouting of dor-mant tubers and promote elongation of stolons [10,11]. However, rather little data is available on the endogenous amounts of GAs in the cultivated potato as compared with the mutants of other major crop species mentioned above. GA20 and GA1 have been quantified in the sprouts and leaves of potatoes by GC-MS [12 – 14]. Studies on GAs in stolons suggest that GA1 is the main regulator of tuberization [11]. In addition, low amounts of GA4 and GA9 have been detected in the buds of internodal cuttings and in stolons grown in vitro [11].
Genetic mutants affected in GA metabolism would be helpful in resolving the role of GAs in various important physiological and developmen-tal processes in potato. However, few such mu-tants have been described to date. A tetraploid clone (2n=4× =48) of S. tuberosum subsp. andigena Hawkes, the subspecies adapted to short-day conditions and cultivated in the An-dean region in South America, contains a reces-sive gene (ga1) that confers a dwarf phenotype in plants homozygous for this locus [15]. The dwarf plants have smaller and darker green leaves, a compact ball-shaped mass of foliage, flower very sparsely, and are photoperiod-neutral in contrast to the wild type genotypes [14,15]. The normal growth can be restored by foliar application of bioacive GA [15], which, consistent with the re-duced amounts of endogenous GAs, indicates that the dwarfs are GA synthesis mutants rather than GA response mutants [5]. Many dwarf di(-haploid) lines resembling the ga1 mutant have been obtained from the potato cultivars Barbara and Titana, of which cultivar Barbara reportedly contains subspecies andigena in the pedigree [16]. However, these dwarf lines have been considered ‘non-vigorous’ for a breeding program [16] and no report on their further characterization is available.
We have recently found several dwarf
geno-types among the (di)haploid (2n=2× =24) lines produced from the tetraploid potato cultivar Pito, a genotype representing the long day-adapted subspecies tuberosum of the cultivated potato pre-dominantly grown outside South America [17 – 19]. The (di)haploids are called haploids in the following, consistent with the use of the term haploid for sporophytes with gametic chromo-some number [20]. The aim of this study was to measure the endogenous amounts of GA in the leaves of the long day-adapted potato by GC-MS and to characterize the dwarf mutants for GA biosynthesis and response to exogenous applica-tion of bioactive GA.
2. Materials and methods
2.1. Plant material and culture
Haploid lines of cultivar Pito were produced through anther culture and regenerated as previ-ously described [18]. Regenerated plants were multiplied by internodal cuttings in vitro.
In vitro plantlets of the haploids and cultivar Pito were transferred to soil and grown in a greenhouse or in a growth chamber. In the green-house, cultivation was under natural daylight supplemented with illumination from sodium halide lamps (photoperiod 18 h; 200 mmol/s/m2, measured at crop height). The daily minimum and maximum temperatures were 19°C and 24 – 25°C, respectively. In the growth chamber, (8.8 m2; Weiss Umweltstechnik, Germany), photope-riod was 18 h (250 mmol/s/m2), temperature was 19/17°C (day/night), and relative humidity was 40%. Plants were watered daily and fertilized weekly with 0.5% N:P:K fertilizer (5:7:6) (Kukkien Y-lannos, Kemira OY, Finland).
2.2. Determination of ploidy le6els
2.3. Determination of endogenous gibberellin (GA) content
Leaf samples (1.0 g fresh weight, f.w.) were extracted over night in 20 ml of 80% methanol. Deuterated GAs (17,17-[2H
2]GA) obtained from Professor L. Mander, Canberra, Australia, were added as internal standards prior to extraction. After extraction, the sample was reduced to aqueous phase in vacuo and diluted with 20 ml of H2O. The aqueous phase was adjusted to pH 2.8 with 1 M HCl and partitioned three times with equal volumes of ethyl acetate. The ethyl acetate extracts were combined, the H2O freezed out, and thereafter reduced to dryness in vacuo. The residue was dissolved in 2 ml of H2O, pH adjusted to 8.0 with 1 M KOH, and applied to a pre-equilibrated QAE Sephadex (Pharmacia-LKB, Uppsala, Sweden) anion-exchange column (30 mm×10 mm i.d.). The column was washed with 15 ml of H2O (pH 8.0) prior to elution with 25 ml 0.2 M formic acid which was run directly onto a pre-equilibrated 500-mg C18 ISOLUTE cartridge (Sorbent AB, V. Fro¨lunda, Sweden) which was then eluted with 4 ml of methanol. The methanol eluate was dried and thereafter subjected to reversed-phase HPLC.
The HPLC system consisted of a Waters model 600 pump (Waters Associates, Milford, MA, USA) connected via a Waters 717-autosam-pler to a 4-mm Nova – Pak C18 column, 150 mm×3.9 mm i.d. (Waters Associates). The mo-bile phase was a 20-min linear gradient of 20 – 100% methanol in 1% aqueous acetic acid at a flow rate of 1 ml/min. Five fractions correspond-ing to the GAs of interest were dried, methylated with ethereal diazomethane, and after evapora-tion trimethylsilylated in 20 ml of dry pyridine/
BSTFA/trimethylchlorosilane (50:50:1, v/v) at 70°C for 30 min. The derivatization mixture was then reduced to dryness and dissolved in dichloromethane. Samples were injected in the splitless mode into an HP 5890 gas chro-matograph (Hewlett Packard, Palo Alto, CA, USA) fitted with a fused silica glass capillary column (30 m long, 0.25 mm i.d.) with a chemi-cally bonded 0.25 mm DB-5MS stationary phase (J&W Scientific, Folsom, CA, USA). The injec-tor temperature was 270°C. The column temper-ature program varied depending on which GA that was analysed. The column effluent was
in-troduced into the ion source of a JMS – SX/
SX102A mass spectrometer (JEOL, Tokyo, Japan). The interface temperature was 280°C and the ion source temperature was 250°C. The accel-eration voltage was 10 kV. Ions were generated with 70 eV at an emission current of 500 mA. For quantification, samples were analysed in ei-ther high resolution selected ion monitoring (HR-SIM) mode or in selected reaction monitor-ing mode (SRM) [23]. For all the GA analyses, calibration curves were recorded from 0.5 to 20 pg of GA with 20 pg of [2H
2]GA as the internal standard. All data were processed by a JEOL MS – MP7010 data system.
2.4. Treatment of plants with GA
Dwarf haploid lines were grown for 3 months in the greenhouse or growth chamber (two exper-iments in each) before GA treatment. The whole plant was sprayed, until moist, with 79 nM (27.5 ppm) GA3 (Sigma, St. Louis, MO, USA) dis-solved in distilled water. The treatment was re-peated four times at 2-day intervals.
3. Results
3.1. Ploidy of the anther culture-deri6ed dwarf regenerants
Eight dwarf genotypes were found among the 67 androgenetic genotypes obtained through an-ther culture of the tetraploid cultivar Pito (2n=
3.2. GA content
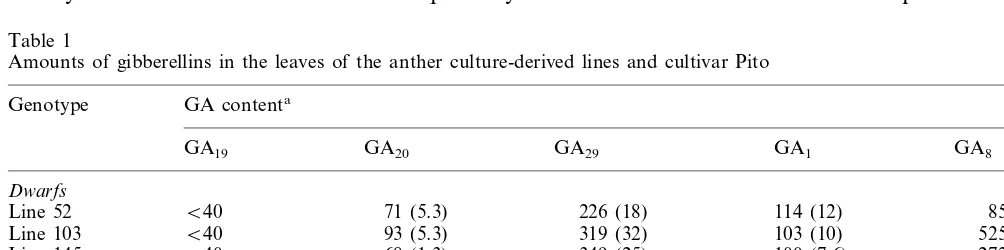
The amounts of GAs were determined by GC-MS in the leaves of three pito dwarfs picked by random (lines 52, 103 and 145), one normally growing haploid (line 142), and Pito (Table 1). The analysed GAs were GA19 (substrate for GA 20-oxidase), GA20 (metabolite of GA19 and end-product of GA 20-oxidase), GA29 (inactive 2b -hy-droxylation product of GA20), GA1 (bioactive 3b-hydroxylation product of GA20) and GA8 (in-active 2b-hydroxylation product of GA1) [6,7]. They define the ‘early 13-hydroxylation pathway’ [6,8] utilized for synthesis of bioactive GAs from GA12 that is the common precursor of all GAs [4,6]. GA29, GA19 and GA8 have not been quantified in potatoes previously.
The amounts of all GAs (Table 1) were greatly reduced in the dwarfs as compared to the normally growing haploid line 142 and Pito, the differences ranging from ca. 6-fold (GA1) to ca. 40-fold (GA19). The low amounts of the bioactive GA1 were quite similar (100 – 114 pg/g f.w.) in the three pito dwarfs and, on the other hand, the amounts of GA1were similarly high (653 and 685 pg/g f.w., respectively) in the haploid line 142 and the te-traploid ‘Pito‘ that grew normally. Also, low amounts of GA53 (13-hydroxylated GA12; the first precursor of the early 13-hydroxylation pathway) were detected. Due to the very low amounts and some unknown interfering substances quantifica-tion of GA53was considered inaccurate and is not presented here. For the same reason, the amounts of GA15 and GA9 produced via the non-hydroxy-lation pathway [6,7] could not be accurately deter-mined, but their existence showed that some GA was synthesized also via the alternative pathway.
3.3. Phenotypic responses to treatment with GA
Experiments in the greenhouse and growth chamber produced similar results. The pito mu-tants grew very slowly and were 9 – 10 cm high after 3 months. They had very short internodes, compact and ball-shaped appearance, and dark green leaves (Fig. 1). In a few pito mutants, small flower buds developed but were soon aborted and no flowers developed, whereas the wild-type hap-loids initiated flowering after ca. 8 weeks of growth. The pitomutants and wild-type genotypes both formed tubers during the 3 months of observation.
Spraying with GA3 induced rapid stem elonga-tion, of which the first signs were clearly visible only 1 day after the first GA treatment. On aver-age, dwarf plants elongated 17.393.3 cm (31 plants measured in two experiments) in 10 days after the first GA treatment (Fig. 1). Leaves of the GA-treated plants were slightly more pale green than the leaves of the non-treated dwarfs, and similar to the wild-type haploids. The dwarf geno-types initiated flowering 18 – 21 days after treat-ment with GA. No differences were observed in the response to GA treatment among the dwarf haploids.
4. Discussion
Detection of GA53 and the high amounts of GA19 in the haploid line 142 and cultivar Pito indicated that the long day-adapted cultivated potatoes of subspecies tuberosum utilize primarily the early 13-hydroxylation pathway for synthesis of bioactive GAs. All GAs in plants are
synthe-Table 1
Amounts of gibberellins in the leaves of the anther culture-derived lines and cultivar Pito
Genotype GA contenta
GA1 GA29
GA20
GA19 GA8
Dwarfs
B40 71 (5.3) 226 (18) 114 (12)
Line 52 85 (1.4)
B40 93 (5.3)
Line 103 319 (32) 103 (10) 525 (34)
68 (1.3)
Line 145 B40 349 (25) 100 (7.6) 272 (25)
Normally-growing controls
1635 (79)
Line 142 9105 (507) 10 006 (757) 653 (47) 18 776 (836)
1434 (85)
Pito 2576 (104) 9521 (567) 685 (67) 12 024 (132)
Fig. 1. Two plants of the haploid pitomutant (line no. 10) grown in the greenhouse for 3 months. The plant to the right was sprayed with 79 nM GA35 and 3 days before photogra-phy, whereas the plant to the left was sprayed with distilled water. Height of the GA-treated plant is ca. 30 cm.
nant in the long day-adapted ‘Irish potato’ (sub-species tuberosum). The previous authors [13,14] who analysed foliar samples of the short day-adapted subspecies andigena for turnover of vari-ous exogenvari-ously applied GAs also found that the early 13-hydroxylation pathway was predominant. The relative molar amounts of different GAs analysed in our study indicated significant accu-mulation of the inactive 2b-hydroxylation prod-ucts (GA29and GA8) in leaves, which has not been analysed in the previous studies. It may be a consequence from the control of the amounts of the bioactive GA1. This hypothesis stems from the surprisingly similar amounts of GA1 in the three dwarf haploids on one hand, and in the wild-type haploid line 142 and Pito on the other hand. Furthermore, in the line 142 and ‘Pito’, the amounts of GA1 were much lower than the amounts of the precursor GA20 and the inactive turnover product GA8. Also, in stolons of subspe-cies tuberosum, the amounts of GA1 are strictly controlled, regulating tuber initiation [11]. Feed-back control of the GA 20-oxidase mRNA levels by GA1(tested by exogenous application with the bioactive GA3) has been suggested in a recent study using the ga1 mutant of subspecies andigena [25]. Unfortunately the data are difficult to relate to the results of this study because only the differ-ences in steady-state mRNA levels and not in the endogenous amounts of GA were analysed in the ga1 mutant [25].
Two alternative dioxygenase-catalysed pathways have been suggested, which results in sequential oxidation of GA12 to GA15 or GA14 instead of GA53 [6]. For example, oxidation of GA12to GA15 has been described in tobacco (Nicotiana tabacum L., Solanaceae) [26]. The early 3b-hydroxylation pathway (GA14-pathway) contains a bioactive in-termediate GA4 that can be further oxidized to GA1. The non-early 3b,13-hydroxylation pathway (GA15-pathway) contains an intermediate GA9 that is oxidized to GA20 or GA4 [6]. Traces of GA15 and GA9were detected in the potato leaves analysed in this study, which is consistent with the detection of GA9 and GA4 in stolons of the same potato subspecies in a previous study [11]. These data indicate that the non-early 3b ,13-hydroxyla-tion pathway exists in the long day-adapted sub-species tuberosum. However, the significance of this pathway is unclear because only low amounts of GAs are synthesized (this study, [11]) and no sized from the tetracyclic hydrocarbon
ent-kau-rene by a series of oxidative reactions catalysed by monooxygenases or 2-oxoglutarate dependent dioxygenases [6,7]. The first reactions are catalysed by monooxygenases, resulting in the formation of GA12 and GA53. Further oxidization steps result-ing in, e.g. GA19, GA20, GA29 or GA1, and GA8, respectively, are carried out by dioxygenases. GA1 is the main bioactive GA in most species, causing the major effects such as stem elongation [5], whereas GA29 and GA8are inactive 2b -hydroxyla-tion products from GA20 and GA1, respectively [7].
predomi-up- or down-regulation during development and under different growth conditions has been ob-served [11].
The very low endogenous amounts of GA in the pito mutants indicated that GA biosynthesis was blocked. The extremely low amounts of GA19 and detection of some GA53 suggested that the gene pito attributable to the defective GA synthesis is probably involved in the early (monooxygenase-catalysed) part of the pathway [4,6,8]. The strong and quick response to the exogenous application of low amounts (79 nM) of GA3 confirmed that the dwarf haploids were, indeed, synthesis mutants and not GA response mutants; the latter show reduced or no response to the bioactive GAs [5]. GA1is considered to be the major bioactive GA in plants [5], while GA3, a more persistent 1,2-dehy-dro analogue of GA1originally described from the fungus Gibberella fujikori, is synthesized and highly bioactive also in plants [24,27].
The phenotype and the reduced amounts of bioactive GA1 in the haploid pito dwarfs closely resembled the previously described recessive ga1 mutant detected in an accession of the short day-adapted, tetraploid subspecies andigena [15]. The pito dwarfs contain ca. 6.5-fold lower amounts of GA1 than the wild type genotypes, whereas ca. 25-fold reduction has been observed in the ga1 dwarfs [14]. The dwarfs of subspecies andigena tuberize under long and short days, i.e. are day-length neutral, in contrast to the wild-type geno-types that tuberize only under short days [14,15]. This is consistent with the role of GA as an inhibitor of tuber initiation [10,11]. Because sub-species tuberosum (e.g. cultivar Pito) is day-length neutral and tuberizes under long days, no effect due to reduced GA biosynthesis was expected and studied in this respect in the pito dwarfs. The block of GA biosynthesis in the ga1 mutant is reported to occur between GA12 and GA53 [13], and the dwarf phenotype can be reversed and flowering capacity recovered by foliar application of bioactive GA [15]. Thus, the pito and ga1 mutants share many similarities, even though they originate in different subspecies of S. tuberosum based on the pedigree data available [15,17].
It is assumed that because the media used for anther culture, haploid induction and regeneration of shoots contained no GA in our study [18], many dwarf genotypes defective in GA biosynthe-sis probably regenerated and grew slowly and were
not transferred to rooting medium. However, de-tection of the recessive pito mutant by haploid induction from a tetraploid cultivar demonstrated that this method, which avoids interference by genes inherited from other genotypes in crosses, is powerful in revealing recessive gene mutations car-ried in potato cultivars. The ga1 and pito mutants originate in two different subspecies of S. tubero-sum and are the only GA synthesis mutants re-ported in the large and economically important genusSolanumto date. Therefore, genetic localiza-tion, isolation and characterization of thega1 and pito genes will be an interesting subject for future studies. These efforts should benefit from working on pito at the diploid level using the haploids described in this study.
Acknowledgements
We thank Kirsti Salmi for assistance in tissue culture and flow cytometry, Inga-Britt Carlsson for assistance in the GA analyses, Professor E. Varis for information about the pedigree of culti-var Pito, and Dr Anders Kculti-varnheden and Dr Sabina Vidal for critically reading the manuscript. Financial support to J.V. from The Academy of Finland (grants c6994 and c36256) and the Swedish Agricultural and Forestry Science Coun-cil (SJFR, grant 32.0667/97), to V.M.R from The Finnish Ministry of Agriculture and Forestry, to T.M. from the Swedish Natural Science Council (NFR), and to J.V. and T.M. from the Swedish Foundation of Strategic Research (SSF) is grate-fully acknowledged.
References
[1] T. Yabata, Biochemistry of the ‘bakanae’ fungus of rice, Agric. Hortic. 10 (1935) 17 – 22.
[2] G. Mendel, Experiments in Plant Hybridization, Royal Horticultural Society of London (translation 1938), Har-vard University Press, Cambridge, MA, USA, 1866. [3] P.W. Brian, H.G. Hemming, The effect of gibberellic
acid on shoot growth and development, Physiol. Plant. 8 (1955) 669 – 681.
[4] T. Lange, Molecular biology of gibberellin synthesis, Planta 204 (1998) 409 – 419.
[5] J.J. Ross, Recent advances in the study of gibberellin mutants, Plant Growth Regul. 15 (1994) 193 – 206. [6] J.J. Ross, I.C. Murfet, J.B. Reid, Gibberellin mutants,
[7] P. Hedden, The oxidases of gibberellin biosynthesis: their function and mechanism, Physiol. Plant. 101 (1997) 709 – 719.
[8] P. Hedden, W.M. Proebsting, Genetic analysis of gib-berellin biosynthesis, Plant Physiol. 119 (1999) 365 – 370. [9] C.A. Helliwell, C.C. Sheldon, M.R. Olive, A.R. Walker, J.A.D. Zeevaart, W.J. Peacock, E.S. Dennis, Cloning of the Arabidopsis ent-kaurene oxidase gene GA3, Proc. Natl. Acad. Sci. USA 95 (1998) 9019 – 9024.
[10] E.E. Ewing, P.C. Struik, Tuber formation in potato: induction, initiation, and growth, Hortic. Rev. 14 (1992) 89 – 198.
[11] X. Xu, A.M. van Lammeren, E. Vermeer, D. Vreugden-hil, The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro, Plant Physiol. 117 (1998) 575 – 584.
[12] M.G. Jones, R. Horgan, M.A. Hall, Endogenous gib-berellins in the potato (Solanum tuberosum), Phytochem-istry 27 (1988) 7 – 10.
[13] J.H. van den Berg, P.J. Davies, E.E. Ewing, A. Halinska, Metabolism of gibberellin A12and A12-aldehyde and the identification of endogenous gibberellins in potato (Solanum tuberosumssp.andigena) shoots, J. Plant Phys-iol. 146 (1995) 459 – 466.
[14] J.H. van den Berg, I. Simko, P.J. Davies, E.E. Ewing, A. Halinska, Morphology and [14C] gibberellin A12 metabolism in wild-type and dwarf Solanum tuberosum
ssp. andigenagrown under long and short photoperiods, J. Plant Physiol. 146 (1995) 467 – 473.
[15] J.B. Bamberg, R.E. Hannemann Jr., Characterization of a new gibberellin related dwarfing locus in potato (Solanum tuberosumL.), Am. Potato J. 68 (1991) 45 – 52. [16] R.C.B. Hutten, Basic Aspects of Potato Breeding via the Diploid Level, PhD Thesis, Agricultural University, Wa-geningen, The Netherlands, 1994, pp. 23 – 31.
[17] H. Stegemann, D. Schnick, Index 1985 of European Potato Varieties, Mitteil. Bundesanstalt Land-Forstwirtsch Braunschweig, Paul Parey, Berlin, Germany.
[18] V.-M. Rokka, L. Pietila¨, E. Pehu, Enhanced production of dihaploid lines via anther culture of tetraploid potato (Solanum tuberosum L. ssp. tuberosum) clones, Am. Potato J. 73 (1996) 1 – 12.
[19] J.P.T. Valkonen, V.-M. Rokka, K.N. Watanabe, Exami-nation of the leaf-drop symptom of virus-infected potato using anther culture-derived haploids, Phytopathology 88 (1998) 1073 – 1077.
[20] R. Ortiz, S.J. Peloquin, Use of 24-chromosome potatoes (diploids and dihaploids) for genetical analysis, in: J.E. Bradshaw, G.R. MacKay (Eds.), Potato Genetics, CAB International, Wallingford, UK, 1994, pp. 133 – 154. [21] D.W. Galbraith, K.R. Harkins, J.M. Maddox, N.M.
Ayres, D.P. Sharma, E. Firoozabady, Rapid flow cyto-metric analysis of the cell cycle in intact plant tissue, Science 220 (1983) 1049 – 1051.
[22] K. Arumuganathan, E.D. Earle, Estimation of nuclear DNA contents of plants by flow cytometry, Plant Mol. Biol. Rep. 9 (1991) 229 – 241.
[23] T. Moritz, J. Olsen, Comparison between high-resolution selected ion monitoring, selected reaction monitoring and four-sector tandem mass spectrometry in quantitative analysis of gibberellins in milligram amounts of plant tissue, Anal. Chem. 67 (1995) 1711 – 1716.
[24] C.R. Spray, M. Kobayashi, Y. Suzuki, B.O. Phinney, P. Gaskin, J. MacMillan, Thedwarf-1 (d1) mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway, Proc. Natl. Acad. Sci. USA 93 (1996) 10 515 – 10 518.
[25] E. Carrera, S.D. Jackson, S. Prat, Feedback control and diurnal regulation of gibberellin 20-oxidase transcript levels in potato, Plant Physiol. 119 (1999) 765 – 773. [26] E.T. Jordan, P.M. Hatfield, D. Hondred, M. Talon,
J.A.D. Zeevaart, R.D. Viestra, Phytochrome A overex-pression in transgenic tobacco, Plant Physiol. 107 (1995) 797 – 805.
[27] S. Fujioka, H. Yamane, C.R. Spray, B.O. Phinney, P. Gaskin, J. MacMillan, N. Takahashi, Gibberellin A3 is biosynthesized from gibberellin A20via gibberellin A5in shoots of Zea mays L, Plant Physiol. 85 (1990) 9031 – 9035.