Influence of the content of heavy metals and molecular weight of humic
acids fractions on the activity and stability of urease
C. Marzadori*, O. Francioso, C. Ciavatta, C. Gessa
Institute of Agricultural Chemistry, U.C.I. - S.T.A.A. University of Bologna, Bologna, Italy
Accepted 20 June 2000
Abstract
The aim of this work was to evaluate the effect of two different humic acid (HA) fractions, high molecular weight (HMW 100–300 kDa) and low molecular weight (LMW 10–20 kDa), extracted from peat on the activity and stability of Jack Bean urease. HMW HA significantly inhibited urease activity at pH 6.0 but did not influence the activity at pH 7.0 and 8.0. HMW HA stabilised urease activity over a period of 11 days to treatments with protease and with Cu21and Hg21(two powerful inhibitors of soluble urease activity). The LMW HA inhibited urease activity at pH 6.0, 7.0 and 8.0, and did not stabilise urease activity in the presence of protease. The residual activity of urease, at pH 7.0 and 8.0 in the presence of LMW HA and Cu21, was 90 and 69%; in the presence of LMW HA and Hg21it was 81 and 52%. The residual activity of urease, at pH 7.0 and 8.0, in the presence of HMW HA and Cu21was 99 and 88%; in the presence of HMW HA and Hg21it was 94 and
74%. These results showed that the two HA fractions influenced both the activity and stability of the urease differently. It is proposed that the inhibition of the urease by HMW and LMW HA is mainly due to the two heavy metals which, although immobilised on the HA, are still able to interact with the urease.q2000 Elsevier Science Ltd. All rights reserved.
Keywords: Urease; Humic acid fractions; Metals; Enzyme activity and stability
1. Introduction
Extracellular enzymatic activity is stabilised in soil by the association with organic and inorganic colloids (Burns et al., 1972a,b; McLaren et al., 1975; Nannipieri et al., 1990). Numerous authors consider the association of the extracel-lular enzymes with the humified organic substance a funda-mental requirement for their stabilisation in soil (Nannipieri et al. 1996).
Nannipieri et al. (1974) used pyrophosphate (0.1 M, pH 7.1) to extract active humic–urease complexes from soil, and reported that the extract contained 30–40% of the total soil urease activity. Ceccanti et al. (1978), using ultra-filtration and gel permeation techniques, separated the pyro-phosphate extract into five humic fractions of different molecular weights; all the fractions contained active
humic–urease complexes. The separation procedure,
which eliminated the humic molecules below 10,000 Da, gave fractions in which the total activity of the extracted urease increased by about 8%, and the specific activity of the enzyme increased more than three fold. Nannipieri et al. (1978) established that the urease activity associated with
the humic matter in higher molecular weight fractions (i.e.
.10,000 Da) was highly stable in relation to elevated temperature and proteolytic treatments. Nannipieri et al. (1980, 1985), again using a pyrophosphate extract, were able to fractionate stable humic–phosphatase and humic– protease complexes, further confirming the presence of natural humic–enzymatic complexes in the soil.
Serban and Nissembaum (1986) incubating HA, extracted from peat and peroxidase or catalase, synthesized humic– enzyme associations more stable towards heat and enzy-matic degradation than the free enzyme. Sarkar (1986) using cellulase, obtained a humic–enzymatic complex, stable in the presence of 24 mM Ca21, which was significantly more resistant to thermal and proteolytic treatments than the free enzyme. Serban and Nissembaum (1986) and Sarkar (1986) suggested that the protected enzyme was physically trapped within the humic matrix, and that the ionic bonds were of secondary importance. Ruggero and Radogna (1988) formed a humic–enzyme complex using tyrosinase and HA, but in the presence of Ca21the enzymatic activity was not resistent to the thermal and proteolytic treatments.
Burns (1986) affirmed that the principle process involved in stabilising enzymes was the co-polymerisation of the enzymes in the humic molecules during the humification processes. The adsorption or entrapment of the enzymes
0038-0717/00/$ - see front matterq2000 Elsevier Science Ltd. All rights reserved.
PII: S 0 0 3 8 - 0 7 1 7 ( 0 0 ) 0 0 1 6 3 - 2
www.elsevier.com/locate/soilbio
in the humic matrix was considered to be less important. Rowell (1974) used the polymerisation ofp-benzochinone with trypsin, pronase, subtilisin, papain, carboxypeptidase-A, urease and acid phosphatase to obtain soluble and inso-luble complexes resistant to both thermal and enzymatic denaturation. The enzymatic activity of the complexes varied from 0 to 62% of the total activity of the added enzymes. Sarkar and Burns (1983, 1984) obtained stable synthetic humic–b-glucosidase complexes with increased enzymatic activity by using resorcinol and pyrogallol. However, neither Rowell (1974) nor Sarkar and Burns (1983, 1984) were able to obtain stable complexes when the enzyme was adsorbed by the preformed humic-like polymer. Gianfreda et al. (1995a) formed complexes between tannic acid and urease in the presence of FeCl3,
MnCl2 and OH–Al polymers and precipitates of Fe2O3
and MnO2. They showed that soluble metals favoured the
formation of soluble and insoluble tannate–urease
complexes which were more active than those formed in the absence of metals.
Little information is available regarding the direct effect of different molecular size fractions of humics on enzymatic activity. Soil humic acids (HA) can be considered to consist of a large number of colloidal polydispersed substances that differ with regard to molecular weight and chemical char-acteristics (Stevenson, 1994; Francioso et al., 1996). The aim of this work is to measure the effect of humic substances, extracted from peat and fractionated according to their molecular weight, on the activity and stability of a pure urease derived from Jack Bean.
2. Materials and methods
2.1. Extraction and fractionation of humic acids
Humic acids were extracted from Irish Sphagnum peat. Two kilogram of peat, air dried, was extracted with 40 l of 0.1 M NaOH plus 0.1 M Na4P2O7 at 208C under N2 and
stirred for 6 h. The suspension was centrifuged at 5000g for 30 min and filtered through a 0.22mm filter with the use of Minitam S System (Millipore). The solution was acidified with 15% HCl until pH ,2, then centrifuged at 5000gfor 20 min to precipitate the HA. One hundred g HA was redissolved in about 30 l of NaCl 0.005 M, pH 7.0. Separation of HA into different molecular weight fractions was carried out by means of a tangential ultrafiltration system (Molecular/Po MP3 Spiral Wound UF, Spectrum, Houston, Texas), provided with a refrigerant system (48C). Two HA fractions, high and low nominal molecular weights were used in the experiments: 100–300 kDa (HMW) and 10–20 kDa (LMW). All the samples were dialysed (0.5 kDa) against distilled H2O and freeze-dried. Other
details and elemental analysis of HA were reported by Fran-cioso et al. (1996). The content of Cu21and Zn21in HMW HA was 72 and 210mg g21dry weight, respectively, while
for LMW HA it was 960 and 1850mg g21 dry weight,
respectively.
2.2. Urease preparation and assay of urease activity
A solution of 0.20 mg ml21 commercial Jack Bean
lyophilised urease (SIGMA Chemical Co., St. Louis, MO.) was prepared in 10 ml distilled H2O and mixed with
40 ml Gomori buffer 2 mM pH 7.0 (Gomori is formed by a mix of Tris and maleic acid). The urease solution, 50 ml, was passed through an ultrafiltration cell (AMICON), using a membrane with a cut-off of 100 kDa (the MW of urease is 480 kDa), until a concentration of 10 ml was obtained. Forty ml of buffer were re-added to the ultrafiltration cell and the procedure was repeated five times. This process replaces the buffers contained in the commercial lyophilised preparation with Gomori buffer. The range of Gomori buffer (pH 5.0– 8.5) was large enough to cover the range of the experimental pH values. The final concentration of the enzyme stock solution was 0.20 mg ml21. The assay solution tested during the experiments contained aliquots of enzyme stock solution of 0.4–0.5 ml. The content of protein of the lyophilised commercial extract was 30mg 0.4 ml21enzyme stock solu-tion as determined using a protein assay kit (Bio-Rad Protein Assay).
Triplicate measurements of the urease activity were made using a stat method (Crison Compact Titrator 2000 pH-meter, Alella, Spain). The activity was measured by record-ing the volume of a 10 mM HCl solution necessary to main-tain a constant pH in the assay solution (10 ml) conmain-taining urease or urease–HA and 400 mM urea as a substarte. The activity measurements were begun after linearity of the addition curve was reached (i.e. after 1 min.) in order to allow uniform urea concentration. One unit (U) of enzyme activity is defined as the amount of enzyme needed to hydrolyse 1mmol urea min21at 258C. The calculation of the urease activity units was carried out taking into consid-eration the pH of the samples, the volume of HCl added and the acidity constants of the products of urea hydrolysis, using the procedure suggested by Blakeley et al. (1969).
2.3. Stability of free urease and urease–HA
The stability of free urease, HMW and urease-LMW at pH 6.0, 7.0 and 8.0 was monitored at 0, 1, 2, 4, 7, 9 and 11 days. Stability was also monitored at pH 7.0 in sterile conditions using filtered (MILLEX-GP 0.22mm
Millipore) solutions and autoclaved (30 min 1218C
0.2 MPa) reagents and glassware.
The incubation of the samples and the urease activity assay were carried out in 50 ml plastic test-tubes, at 258C, containing 0.4 ml of enzyme stock solution, 7.6 ml Gomori 2 mM and 1 ml HA at a concentration of 15mmol C ml21. No HA was added to the free urease samples that conse-quently contained 8.6 ml of Gomori buffer. The enzymatic assay started with the addition of 1 ml 4 M urea. The concentration of urease in the assay solution was the same, unless specified, for all the experiments. From preli-minary experiments it was found that urease activity, present in the solution containing HA (urease–HA), needed 15 min for equilibration. Therefore, the enzymatic assays in the presence of HA were started after a contact time between HA and urease of 15 min.
2.4. pH Activity profile and kinetic
The pH activity profile for free urease, urease-HMW and urease-LMW was obtained by changing the pH of the buffer in the 6.0–8.0 range and carrying out the measurements following the pH-stat method at the corresponding value of pH.
Enzyme kinetics for free urease, urease-HMW and urease-LMW were determined at pH 6.0 and pH 7.0 using 30mg of urease, and at pH 8.0 using 38mg of urease. The
activity was measured as a function of substrate concentra-tion andKmandVmaxvalues were determined by fitting the
experimental data to the Lineweaver–Burk equation.
2.5. Enzyme stability in the presence of Cu21and Hg21
Stability in the presence of Cu and Hg chloride was measured after adding the metals (at a concentration of 7.0×1028Cu21and 2.0×1027M Hg21) to the assay solu-tion 15 min after the addisolu-tion of HA. The concentrasolu-tions chosen have been reported to cause approximately 50% inhibition of urease activity at pH 7.0 (Hughes et al., 1968). The addition of Cu and Hg chloride did not change the pH of the assay solution. The trials with the EDTA, 3 mM, were conducted using Gomori buffer 2 mM.
3. Results and discussion
3.1. HA influence on urease activity and kinetic behaviour
The activity of free urease, HMW and urease-LMW as a function of pH is shown in Fig. 1. At pH 6.0 the activity of urease-LMW was equivalent to 69% (11 U
^0.2) of that of the free enzyme while at pH 8.0 the activity decreased to 42% (6 U ^0.1). At pH 6.0 and 6.5 urease-HMW showed a reduction in activity, with respect to free enzyme, but the reduction was less than that shown by urease LMW. At pH values at or above 7.0, HMW did not influence the enzyme activity.
The Km and the Vmax calculated for free urease,
urease-HMW and urease-LMW are shown in Table 1. The kinetic parameters of the urease changed with pH; withKm values
increasing from pH 6.0 to pH 8.0. In contrast, the Vmax
values of the three preparations, were maxima at pH 7.0 and at a minimum at pH 6.0. The Km values of
urease-HMW were sigmificantly lower with respect to free urease, at pH 6.0, 7.0 and 8.0. LMW significantly increased Km
values at both pH 7.0 and pH 8.0, but had no influence at pH 6.0. Compared to free urease, theVmaxvalues decreased
in the presence of LMW at all pH values, while HMW decreased the Vmax only at pH 6.0. Several studies on
enzyme immobilisation have reported that both specific activity and substrate affinity are considerably reduced after interaction of an enzyme with a carrier (Burns, 1986). However, no variation was actually found in the kinetic parameters of a Jack Bean urease adsorbed on a
Fig. 1. Effect of pH on the activity of free urease and urease–HA.
Table 1
Kinetic parameters (KmandVmax) of free urease and urease–HA complexes (( )standard deviation)
pH Free urease Urease-HMW Urease-LMW
Km(mM) Vmax(U mg21) Km(mM) Vmax(U mg21) Km(mM) Vmax(U mg21)
6 7.4 (0.2) 701 (15) 5.2 (0.4) 453 (12) 7.2 (0.1) 453 (5.6)
7 12.2 (0.2) 1041 (50) 10.6 (0.7) 952 (45) 18.9 (2.1) 712 (31)
clay organic complex (Boyd and Mortland, 1985) and on a organo-mineral–urease complex (Gianfreda et al., 1995b). Our results (Fig. 1 and Table 1), are only partially in agree-ment with these observations and although not strictly invol-ving the immobilisation of urease on HA, show that the humic fractions influenced both urease activity and kinetics. With increasing pH, the effect of the HMW HA on the activity of the urease became insignificant and the kinetic parameters, i.e. Km, were improved. In contrast, the LMW
HA had a constant negative influence on activity at all the pH values.
3.2. Stability of free urease and urease–HA
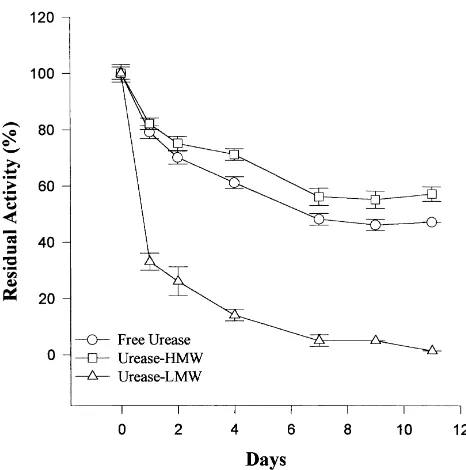
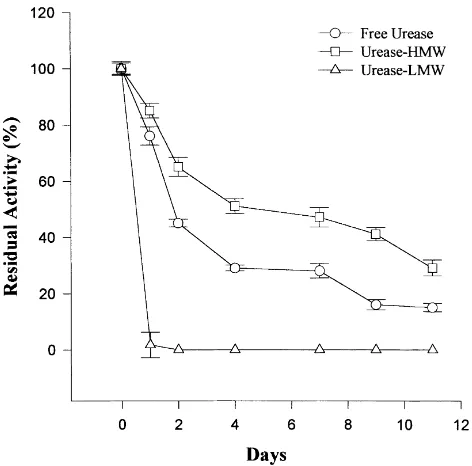
Figs. 2–4 show the stability over 11 days of free urease, urease-HMW and urease-LMW. The LMW HA did not stabilise urease activity and by day 11 the residual activity was 0% at all the pH values. The residual activity of free urease (urease control) at day 11 was 47, 52 and 25% of the
Fig. 2. Stability over 11 days of incubation of free urease and urease–HA at pH 6.
Fig. 3. Stability over 11 days of incubation of free urease and urease–HA at pH 7.
Fig. 4. Stability over 11 days of incubation of free urease and urease–HA at pH 8.
initial activity at pH 6.0, 7.0, and 8.0, respectively. Residual activity at day 11 for HMW HA was 57% (pH 6.0), 75% (pH 7.0) and 77% (pH 8.0). With increasing pH the long-term stability of free urease decreased and that of urease-HMW increased.
Among the factors which may contribute to the degrada-tion of unprotected free extracellular urease is the acdegrada-tion of proteases. Only the HMW HA provided urease some resis-tance to the pronase (Fig. 5). After 96 h of incubation the residual activity of urease-HMW was 13% of the initial whereas the residual activity of urease-LMW and free urease was 0%. The ability of HMW to stabilise the urease activity could be determined by its ability to inhibit the pronase. Ladd and Butler (1969) and Nannipieri et al.
(1978) reported that different HA were able to inhibit the pronase, and that the molecular rigidity associated with aromatic and condensed HA was responsible for the inhibition of the protease (Butler and Ladd, 1971).
Previous 1H NMR analysis carried out on identical
HMW extract indicated a proton structure in the aromatic proton region with the appearance of a very well resolved signal which may correspond to aromatic
hydrogen in different chemical environments. The 1H
NMR analysis of LMW indicated the presence of prominent resonance corresponding to the protons of the methyl groups in highly branched aliphatic struc-tures, further confirming the more aliphatic character of the LMW (Francioso et al., 1996)
Urease can also undergo degradation as a result of the oxidation of –SH groups present on the cysteines: groups which are fundamental to the activity (Hughes et al., 1968). The ability of HMW to partially protect urease over a period of time from this kind of action was measured in a sterile environment at pH 7.0 (Fig. 6). In fact, 33% of the initial activity was retained by urease-HMW after 11 days of incu-bation compared with 15 or 0% of free urease and urease-LMW, respectively.
3.3. Enzyme stability in the presence of Cu21and Hg21
Hughes et al. (1968) reported that Cu21 and Hg21 are strong urease inhibitors through interaction with –SH groups present in the cysteine moieties. Table 2 shows the ability of HMW and LMW to protect the urease from inhibition of Cu21 and Hg21. At pH 6.0, the presence of HMW and LMW did not protect urease
against either Cu21 or Hg21. At pH 7.0 and 8.0,
HMW gave significant protection to urease activity. At the same pH, the LMW did not significantly protect urease activity against Cu21 and Hg21. By analysing the data in Table 2 it can be seen that HMW is the most efficient humic fraction in protecting the urease activity at pH 7.0 and 8.0. However, it is not possible to affirm that this behaviour depends on the higher affi-nity of this fraction for Cu21 and Hg21. The LMW
fraction has a greater content of phthalate and
carboxylic groups than the HMW fraction, that have a particular affinity for Cu21 and Hg21 (Francioso et al., 1996). A greater protecting ability should, therefore, be expected with the LMW fraction. We are unable to give a satisfactory explaination for this apparent anomaly, but these results could be correlated to a different
capa-city of Cu21 and Hg21 adsorbed on the HMW and
LMW to interact with the urease.
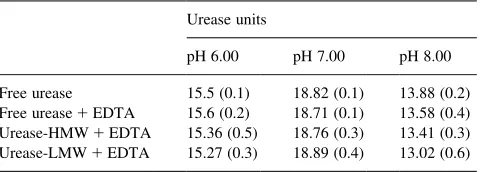
Table 3 shows the effect of EDTA on the urease activity in the presence of HMW and LMW. The results show that EDTA was able to completely eliminate the urease inhibi-tory action of LMW and HMW, and suggest that the metals adsorbed on HMW and LMW play an important role in the urease inhibition by HA.
Fig. 6. Stability over 11 days of incubation of free urease and urease–HA in a sterile environment at pH 7.
Table 2
Effect of added Cu21and Hg21on free urease and urease–HA complexes
(( )standard deviation)
Urease units Urease units Cu21 Urease units Hg21
pH 6
Free urease 16.97 (0.3) 14.03 (0.3) 12.44 (0.1) Urease-LMW 12.68 (0.3) 11.43 (0.3) 9.00 (0.2) Urease-HMW 14.88 (0.1) 12.62 (0.2) 11.01 (0.2) pH 7
Free urease 24.71 (0.5) 14.00 (1.2) 13.71 (0.4) Urease-LMW 17.21 (0.1) 15.58 (0.2) 11.90 (0.2) Urease-HMW 24.89 (0.5) 24.82 (0.5) 22.37 (0.6) pH 8
Acknowledgements
This work has been supported by funds of MURST ex quota 60% — Prof. Carlo Gessa and ex quota 40% — Prof. Claudio Ciavatta.
References
Blakeley, R.L., Webb, E.C., Zerner, B., 1969. Jack Bean urease (EC 3.5.1.5). A new purification and reliable rate assay. Biochemistry 8, 1984–1990.
Boyd, S.A., Mortland, M.M., 1985. Urease activity on a clay–organic complex. Soil Science of American Journal 49, 619–622.
Burns, R.G., Pukite, A.H., Mc Laren, A.D., 1972a. Concerning the location and persistence of soil urease. Soil Science of America Proceedings 36, 308–311.
Burns, R.G., El-Sayed, M.H., Mc Laren, A.D., 1972b. Extraction of a urease–active organo-complex from soil. Soil Biology and Biochem-istry 4, 107–108.
Burns, R.G., 1986. Interaction of enzymes with soil mineral and organic colloids. In: Huang, M., Schnitzer, M. (Eds.). Interactions of Soil Mineral with Natural Organic and Microbes, Soil Science Society of America, Madison, WI, pp. 429–451.
Butler, J.H., Ladd, J.N., 1971. Importance of molecular weight of humic and fulvic acids in determining their effects on protease activity. Soil Biology and Biochemistry 3, 249–257.
Ceccanti, B., Nannipieri, P., Cervelli, S., Sequi, P., 1978. Fractionation of humus–urease complex. Soil Biology and Biochemistry 10, 39–45. Francioso, O., Sanchez-Cortes, S., Tugnoli, V., Ciavatta, C., Sitti, L.,
Gessa, C., 1996. Infrared, Raman, and nuclear magnetic resonance (1H, 13C, and 31P) spectroscopy in the study of fractions of peat humic acids. Applied Spectroscopy 50, 1165–1173.
Gianfreda, L., Rao, M.A., Violante, A., 1995a. Formation and activity of
urease–tannate complexes affected by aluminum iron, and manganese. Soil Science Society of American Journal 59, 805–811.
Gianfreda, L., De Cristofaro, A., Rao, M.A., Violante, A., 1995b. Kinetic behaviour of synthetic organo- and organo-mineral–urease complexes. Soil Science Society of American Journal 59, 811–815.
Hughes, R.B., Katz, S.A., Stubbins, S.E., 1968. Inhibition of urease by metal ions. Enzymologia 36, 332–334.
Ladd, J.N., Butler, J.H., 1969. Inhibitory effect of soil humic compounds on the proteolitic enzyme pronase. Australian Journal of Soil Research 7, 241–251.
McLaren, A.D., Pukite, A.H., Barshad, I., 1975. Isolation of humus with enzymatic activity from soil. Soil Biology and Biochemistry 119, 178– 180.
Nannipieri, P., Ceccanti, B., Cervelli, S., Sequi, P., 1974. Use of 0.1 M pyrophosphate to extract urease from a podzol. Soil Biology and Biochemistry 6, 359–362.
Nannipieri, P., Ceccanti, B., Cervelli, S., Sequi, P., 1978. Stability and kinetic properties of humus–urease complexes. Soil Biology and Biochemistry 10, 143–147.
Nannipieri, P., Ceccanti, B., Cervelli, S., Matarese, E., 1980. Extraction of phosphatase, urease, protease, organic carbon, and nitrogen from soil. Soil Science of American Journal 44, 1011–1016.
Nannipieri, P., Ceccanti, B., Bianchi, D., Bonmati, M., 1985. Fractionation of hydrolase–humus complexes by gel chromatography. Biology and Fertility of Soil 1, 25–29.
Nannipieri, P., Ceccanti, B., Grego, S., 1990. Ecological significance of the biological activity in soil. In: Bollag, J.M., Stotzky, G. (Eds.). Soil Biochemistry, Marcel Dekker, New York, pp. 293–355.
Nannipieri, P., Sequi, P., Fusi, P., 1996. Humus and enzyme activity. In: Piccolo, A. (Ed.). Humic Substances in Terrestrial Ecosystems, pp. 293–324.
Rowell, M., 1974. Production and characterization of enzymes stabilized as humic acid analogues. PhD dissertation. University of Saskatchewan. Ruggero, P., Radogna, V.M., 1988. Humic acid–tyrosinase interactions as a
model of soil humic–enzyme complex. Soil Biology and Biochemistry 20, 353–359.
Sarkar, J.M., Burns, R.G., 1983. Immobilization ofa-d-glucosidase andb -d-glucosidase polyphenolic complexes. Biotechnology Letters 5, 619– 624.
Sarkar, J.M., Burns, R.G., 1984. Synthesis and properties ofa -d-glucosi-dase–phenolic copolymers as analogues of soil humic–enzyme complexes. Soil Biology and Biochemistry 16, 619–625.
Sarkar, J.M., 1986. Formation of [14C]cellulase–humic complexes and their stability in soil. Soil Biology and Biochemistry 18, 251–254. Serban, A., Nissembaum, A., 1986. Humic acid association with peroxidase
and catalase. Soil Biology and Biochemistry 18, 41–44.
Stevenson, F.J., 1994. Colloidal properties of humic substances. In: Steven-son, F.J. (Ed.). Humus Chemistry, Wiley, New York, pp. 325–349. Table 3
Effect of EDTA 3 mM on activity of free urease and urease–HA complexes (( )standard deviation)
Urease units
pH 6.00 pH 7.00 pH 8.00
Free urease 15.5 (0.1) 18.82 (0.1) 13.88 (0.2)