Importance of riparian habitats for small mammal and
herpetofaunal communities in agricultural
landscapes of southern Québec
Charles Maisonneuve
a,∗, Stéphanie Rioux
baSociété de la Faune et des Parcs du Québec, Direction de la faune et des habitats, 675 René-Lévesque Est, Québec, Que., Canada G1R 5V7 bSociété de la Faune et des Parcs du Québec, Direction régionale du Bas Saint-Laurent, 506 Lafontaine, PO Box 445,
Rivière-du-Loup, Québec, Que., Canada G5R 3C4
Received 14 December 1999; received in revised form 18 July 2000; accepted 4 August 2000
Abstract
The presence of adequate riparian strips in agricultural landscapes is generally recognized to contribute to the reduction of the impacts of agricultural practices on the water quality of streams, to regularize water temperature and to help in the creation of important wildlife habitats. This study aimed at determining the importance of riparian strips in agricultural landscapes of southern Québec for small mammal and herpetofaunal communities, and verifying farmers’ general belief that these habitats become shelters for species considered as agricultural pests. Abundance, composition and diversity of communities were compared between three types of riparian strips: herbaceous, shrubby and wooded. A total of 1460 small mammals belonging to 14 species and 329 amphibians and reptiles belonging to 11 species were captured with line trapping and drift fences. The generalist species Sorex cinereus, Zapus hudsonius, Blarina brevicauda, and Bufo americanus were abundant in all three types of riparian strips. Peromyscus maniculatus, Sorex fumeus, Clethrionomys gapperi, and Rana pipiens were associated more closely to wooded strips, whereas Rana sylvatica was captured mostly in shrubby strips. The abundance of small mammals and herpetofauna increased with complexity of vegetation structure. Small mammal diversity was higher in herbaceous and wooded riparian strips, whereas the herpetofaunal community was more diverse in shrubby strips. Proportion and abundance of pest species diminished with complexity of vegetation structure, whereas insectivores increased in abundance. Maintaining woody vegetation in riparian strips should increase abundance and diversity of wildlife within agricultural landscapes where increasing development pressure is presently contributing to the conversion of such habitats to herbaceous strips. Such a management approach should also help reducing the risk of riparian strips becoming shelters for pest species. © 2001 Elsevier Science B.V. All rights reserved.
Keywords: Agricultural landscapes; Herpetofauna; Riparian strips; Small mammals; Québec
∗Corresponding author. Tel.:+1-418-623-1650;
fax:+1-418-623-0420.
E-mail address: c [email protected] (C. Maisonneuve).
1. Introduction
Over the last few decades in most agricultural regions of the province of Québec, there was a tran-sition from local, family subsistence farming to more industrial farming practices oriented towards regional and national markets. The traditional dairy-oriented
agriculture that was previously characterized by a mo-saic of pastures, prairies and woodlots was replaced by a more specialized agriculture aimed at large-scale production, with corn as (Zea mays L.) the dominant crop in the south-west. The new agricultural practices developed for this large-scale production has led to the expansion of cultivated areas, thus exerting an in-creasing pressure on uncultivated portions of the land. Forests in agricultural landscapes have been reduced and fragmented in numerous small woodlots, and ri-parian strips which often represent the only remaining corridors for wildlife between these woodlots are also being threatened.
Riparian strips are not only recognized as impor-tant wildlife habitats for a great diversity of species (Thomas et al., 1979; Small and Johnson, 1986; De-camps et al., 1987; Naiman et al., 1993), but they also help reduce the impacts of agricultural practices on the water quality of streams by controlling bank erosion, and by filtering fertilizers, pesticides and sediments from adjacent crops (Lowrance et al., 1985, 1986; Brenner et al., 1991; Gilliam, 1994; Vought et al., 1994) and they maintain quality of aquatic habitats by regularizing water temperature (Karr and Schlosser, 1978). Thus, efforts to integrate the conservation of riparian strips in the management of agricultural lands should lead to both sound agroecosystems and environments.
However, many factors contribute to limit the value of riparian strips in agricultural landscapes of Québec. Under the provincial Protection Policy for Lakeshores, Riverbanks, Littoral zones and Flood-plains, a buffer strip of 3 m is required for the pro-tection of riparian areas in agricultural landscapes, whereas 10–20 m are required in urban or forested landscapes. Moreover, the vegetation in these narrow riparian strips is often maintained at the herbaceous stage through mowing or burning. This practice stems from the farmers’ belief that such management re-duces the risk of riparian strips becoming shelters for pest species (birds, rodents, weeds, insects). In a recent study made for the Union des producteurs agricoles (Lamarre et al., 1993), pesticide use for weed control was even recommended within riparian strips. This perception of riparian habitats repre-sents a major obstacle for efforts to integrate wildlife habitat needs in the management of agricultural landscapes.
The objectives of this study were thus to determine the importance of riparian strips for small mammal and herpetofaunal (amphibians and reptiles) communities in agricultural landscapes of southern Québec, and to verify if there is a basis to farmers’ belief concerning the risk of riparian strips being used as shelters by rodent pest species.
2. Material and methods
2.1. Study area
2.2. Field methods
Two methods were used to trap small mammals. The first one consisted of lines of traps installed par-allel to the streams. A total of 18 sites were selected to cover a total of 3600 m in each of the habitat types. Each line had a length of 600 m. Museum special snap traps, Sherman live traps, and pitfall traps (2 l) were placed alternately every 10 m. Thus, each line comprised 20 of each of these traps. Snap traps were baited with peanut butter, and live traps with ap-ple pieces dipped in peanut butter. Pitfall traps were not baited, but filled with enough water to rapidly drown mammals. Half of these lines were operated for five consecutive nights during September 1995, the other half during September 1996. Total trapping effort was thus 1800 night-traps in each of the habitat types.
Drift fences (Corn, 1994; Kirkland and Sheppard, 1994) were used as a second trapping method to sample amphibians and reptiles, and to make ad-ditional small mammal captures. Drift fences were installed on the same 18 sites (six by habitat type) where line trapping had been carried out in the previ-ous year. Each of the arrays consisted of jute fences 45 cm high and 30 m long installed parallel to the streams. Four 25 l pitfall traps were installed flush to the ground at every 10 m and with enough water to immediately drown mammal species. Six funnel traps were also placed alongside the fences, on both sides, one set between each pitfall traps. Each of the arrays was operated for a total of 22 nights. In 1996 and 1997, sampling was carried out during 14 nights in May, four nights in June–July, and four nights in September. A trapping effort of 1320 night-traps was thus carried out in each of the three habitat types.
Except for easily identified species (squirrels, chip-munks, weasels) captured in live traps, all mammal specimens were sacrificed and kept frozen until later identification with the use of cranial and dental charac-ters. Amphibians were identified in the field, marked by cutting a toe in order to consider recaptures in eval-uation of abundance, and released.
Land use adjacent to each trapping station along line transects was noted. These could be grouped into the following four categories: cereals, pastures, prairies and fallow lands.
2.3. Statistical analyses
Shannon’s index (Zar, 1984) was used to measure diversity within each of the three riparian habitats
H= nlogn−
P
filogfi
n (1)
where n is the total number of individuals captured for all detected species combined, and fi the number of captures for species i.
Hutcheson’s test (1970) was used to compare diver-sity indices between habitat types
t= H1−H2
whereSH2 is the variance of the diversity index ob-tained as follows:
SH2 =
P
filogfi−(Pfilogfi)2/n
n2 (3)
Since habitat types with similar diversity indices may be inhabited by different communities, an overlap index (Horn, 1966) was calculated
O= where fi is the number of captures of species i, gi the number of captures of species j, n1 the total number of captures in habitat 1, and n2 the total number of captures in habitat 2. This index varies from 0, when two communities have no species in common, to a maximum of 1 when all species and relative abundance are the same in both habitats compared.
The reciprocal of Simpson’s index was used to determine niche breadth (Levins, 1968; Colwell and Futuyma, 1971; Whittaker and Levin, 1975; Brown and Parker, 1982) for each species
W =P1
pij2 (5)
where pij is the occurrence rate of species i in habitat j. This rate is obtained as follows:
pij= POij
Oij
where Oij is the number of captures of species i in habitat j. Since three habitat types were compared, a niche breadth value of 1 indicates that a species is only present in one habitat type, whereas a maximum value of 3 indicates that a species is distributed evenly in all three habitat types. This value of niche breadth can be used as a tolerance index to habitat modifica-tions; species with great niche breadths are considered tolerant and to modifications of their habitat and vice versa (Best et al., 1979; Stauffer and Best, 1980).
Proportions of insectivores and rodents within each habitat type were compared with G-tests (Scherrer, 1984: 484). When this test indicated heterogeneity between the three habitat types, multiple comparison tests (Scherrer, 1984: 488) were carried out to de-termine to which habitat this was due. The G-tests were also used to compare proportions of pest species among the small mammal communities. Two species were considered as potential pests: Microtus pennsyl-vanicus, and Mus musculus.
Comparison of observed numbers of individual species with numbers expected according to adjacent land use availability was carried out with G-tests. This test was carried out for species for which at least 20 captures were obtained in the trap lines. Microtus pennsylvanicus and M. musculus were grouped as pests species for this analysis.
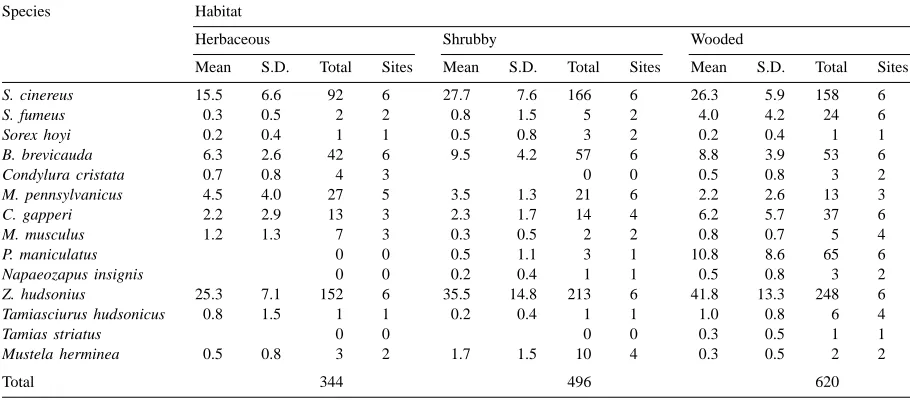
Table 1
Mean, standard deviation (S.D.) and total numbers of individuals of each species of small mammals captured within each of three riparian habitat types and number of sites on which they were detected in agricultural landscapes of southern Qu´ebec, 1995–1997
Species Habitat
Herbaceous Shrubby Wooded
Mean S.D. Total Sites Mean S.D. Total Sites Mean S.D. Total Sites
S. cinereus 15.5 6.6 92 6 27.7 7.6 166 6 26.3 5.9 158 6
S. fumeus 0.3 0.5 2 2 0.8 1.5 5 2 4.0 4.2 24 6
Sorex hoyi 0.2 0.4 1 1 0.5 0.8 3 2 0.2 0.4 1 1
B. brevicauda 6.3 2.6 42 6 9.5 4.2 57 6 8.8 3.9 53 6
Condylura cristata 0.7 0.8 4 3 0 0 0.5 0.8 3 2
M. pennsylvanicus 4.5 4.0 27 5 3.5 1.3 21 6 2.2 2.6 13 3
C. gapperi 2.2 2.9 13 3 2.3 1.7 14 4 6.2 5.7 37 6
M. musculus 1.2 1.3 7 3 0.3 0.5 2 2 0.8 0.7 5 4
P. maniculatus 0 0 0.5 1.1 3 1 10.8 8.6 65 6
Napaeozapus insignis 0 0 0.2 0.4 1 1 0.5 0.8 3 2
Z. hudsonius 25.3 7.1 152 6 35.5 14.8 213 6 41.8 13.3 248 6
Tamiasciurus hudsonicus 0.8 1.5 1 1 0.2 0.4 1 1 1.0 0.8 6 4
Tamias striatus 0 0 0 0 0.3 0.5 1 1
Mustela herminea 0.5 0.8 3 2 1.7 1.5 10 4 0.3 0.5 2 2
Total 344 496 620
3. Results
3.1. Small mammals
A total of 1460 individual small mammals belong-ing to 14 species were captured (Table 1). Total num-ber of captures increased gradually with increasing complexity of vegetation structure: 23.6% of indi-viduals were captured in herbaceous riparian strips, 34.0% in shrubby strips, and 42.5% in wooded strips (Table 2). All 14 species were detected in wooded strips, 11 species in herbaceous strips and 12 in shrubby strips. Diversity was significantly lower in shrubby strips than in wooded or herbaceous strips, which had similar diversity indices. Relatively high values were obtained for overlap indices between habitat types: 0.976 between herbaceous and shrubby strips, 0.918 between herbaceous and wooded strips, and 0.931 between shrubby and wooded strips, indi-cating that small mammal communities differed much between herbaceous and wooded strips.
Table 2
Number of individuals, number of species, and diversity indices of small mammals caught in three riparian habitat types in agricultural landscapes of southern Qu´ebec, 1995–1997
Habitat
Herbaceous Shrubby Wooded
Total number of individuals 344 496 620
Mean number of individuals/site 57.3 82.7 103.3
Minimum number of individuals/site 36 73 75
Maximum number of individuals/site 76 105 115
Total number of species 11 12 14
Mean number of species/site 6.5 6.8 9.3
Minimum number of species/site 4 6 7
Maximum number of species/site 9 9 12
Shannon’s diversity indexa 0.665A 0.613B 0.744A
aIndices followed by identical letters did not differ significantly (Hutcheson’s test).
value of 2.78 and, contrarily to the three preceding species whose abundance increased with complexity of vegetation structure, occurred mainly (44.3%) in herbaceous strips. A niche breadth value of 2.36 was obtained for Clethrionomys gapperi. The species was detected on all six sites covered in wooded strips, where 58% of all individuals captured. Over 95% of all Peromyscus maniculatus were captured in wooded strips, which led to a very low niche breadth value (1.09) for this species. A relatively low niche breadth value (1.59) was also obtained for S. fumeus with 75% of all captures made in shrubby strips. Other species represented less than 1% of captures.
Proportions of rodents and insectivores differed sig-nificantly between the three habitat types (G=9.58, d.f. = 2, P < 0.01). A multiple comparisons test indicated that rodents were significantly more abun-dant in wooded strips (61%) than in shrubby strips (52%), but failed to detect any difference in the propor-tion of rodents between herbaceous strips (59%) and
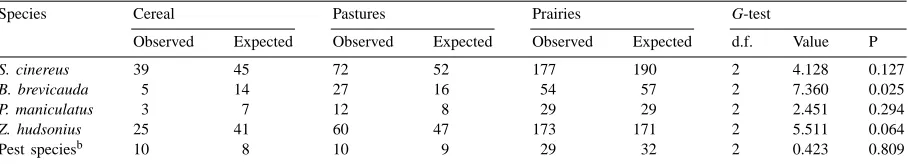
Table 3
Observed and expected numbers of individual species according to land use adjacent to trapping stations in riparian strips located in agricultural landscapes of southern Qu´ebec, 1995–1997a
Species Cereal Pastures Prairies G-test
Observed Expected Observed Expected Observed Expected d.f. Value P
S. cinereus 39 45 72 52 177 190 2 4.128 0.127
B. brevicauda 5 14 27 16 54 57 2 7.360 0.025
P. maniculatus 3 7 12 8 29 29 2 2.451 0.294
Z. hudsonius 25 41 60 47 173 171 2 5.511 0.064
Pest speciesb 10 8 10 9 29 32 2 0.423 0.809
aFisher’s exact test.
bMus musculus and Microtus pennsylvanicus combined.
the two other habitat types. Even though proportions of rodents were similar in herbaceous and wooded strips, species composition differed. M. pennsylvani-cus and M. musculus were more abundant in herba-ceous strips, P. maniculatus and C. gapperi being es-sentially present in wooded strips.
The proportion of pest species in the three habitat types varied significantly (G=21.73, d.f.=2,P < 0.001). A multiple comparisons test indicated that this was due to a significantly higher proportion of pest species in herbaceous strips (10.0%) than in shrubby (4.7%) or wooded (2.8%) strips. Even if the total num-ber of captures increased from herbaceous to wooded strips, the abundance of pest species decreased with complexity of vegetation structure.
Table 4
Mean, standard deviation (S.D.), and total numbers of individuals of each species of herpetofauna captured in each of the three riparian habitat types and number of sites in which they were detected in agricultural landscapes of southern Qu´ebec, 1995–1997
Species Habitat
Herbaceous Shrubby Wooded
Mean S.D. Total Sites Mean S.D. Total Sites Mean S.D. Total Sites
Ambystoma maculatum 0 0 0.3 0.8 2 1 0 0
Eurycea bislineata 0.3 0.7 2 1 0.2 0.4 1 1 0.3 0.5 2 2
B. americanus 2.8 2.9 17 4 6.7 7.6 40 6 16.7 9.5 100 6
Hyla cricifer 0.2 0.4 1 1 0.7 1.1 4 2 0 0
Rana catesbeiana 0 0 0.2 0.4 1 1 0.2 0.4 1 1
Rana septentrionalis 0.5 0.8 3 2 1.3 1.5 8 3 1.0 1.2 6 3
Rana clamitans 0.5 0.8 3 2 0.2 0.4 1 1 0.3 0.5 1 1
R. sylvatica 0.2 0.4 1 1 3.8 4.9 23 4 1.0 1.0 6 4
R. pipiens 1.2 2.6 7 1 0.8 1.1 5 3 14.7 11.4 88 6
Thamnophis sirtalis 0 0 0.5 0.8 3 2 0.2 0.4 1 1
Storeria occipitomaculata 0.2 0.4 1 1 0.2 0.4 1 1 0 0
Total 35 89 205
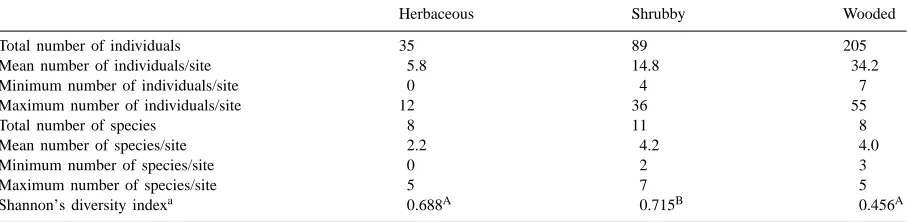
3.2. Herpetofauna
A total of 329 individual amphibians and reptiles be-longing to 11 species were captured (Table 4), i.e., nine amphibians with more than 98% of all captures, and two reptiles. All occurred in shrubby strips, whereas eight species were detected in the other habitat types (Table 5). Diversity was significantly lower in herba-ceous and wooded strips than in shrubby strips. Rel-atively high values were obtained for overlap indices between herpetofaunal communities of the different habitat types: 0.847 for herbaceous and shrubby strips,
Table 5
Number of individuals, number of species, and diversity indices of herpetofauna caught in three riparian habitat types in agricultural landscapes of southern Qu´ebec, 1995–1997
Herbaceous Shrubby Wooded
Total number of individuals 35 89 205
Mean number of individuals/site 5.8 14.8 34.2
Minimum number of individuals/site 0 4 7
Maximum number of individuals/site 12 36 55
Total number of species 8 11 8
Mean number of species/site 2.2 4.2 4.0
Minimum number of species/site 0 2 3
Maximum number of species/site 5 7 5
Shannon’s diversity indexa 0.688A 0.715B 0.456A
aIndices followed by identical letters did not differ significantly (Hutcheson’s test).
0.862 for herbaceous and wooded strips, and 0.760 for shrubby and wooded strips.
species represented less than 2% of captures and niche breadth values were not calculated.
4. Discussion
4.1. Small mammals
Abundance and richness of small mammal species clearly increased with increasing complexity of the vegetation structure in riparian strips. Similar results were obtained in shelterbelts by Yahner (1983) and Dambach (1948), who contended that linear habitats with all the vegetation strata shelter a greater abun-dance and diversity of small mammals. Vertical strat-ification of the vegetation reduces predation risk by providing a better cover (Stamp and Ohmart, 1978).
Although similar small mammal diversities were observed in herbaceous and wooded riparian strips, the overlap index of small mammal communities in these habitat types was lowest, indicative of differences in composition. These differences were ascribed to the high presence of some specialist species in wooded strips (P. maniculatus, Sorex fumeus) or low abun-dance in herbaceous strips (C. gapperi). Contradictory results obtained from other studies on P. maniculatus were due to the existence of subspecies with extreme habitat requirements. P. maniculatus bairdii is associ-ated with cultivassoci-ated fields and prairies in the United States (Hooper, 1942; Hansen and Warnock, 1978; Fleharty and Navo, 1983), where it is considered as a pest (Linduska, 1949), whereas P. maniculatus gracilis and P. maniculatus abietorum mostly occupy forested habitats in the Maritimes and up to the St. Lawrence River (Hooper, 1942; Iverson et al., 1967; Banfield, 1974). S. fumeus is considered more habitat selective than S. cinereus and is more abundant in humid de-ciduous or mixed forests with soils covered with deep humus (Hamilton, 1940; Wrigley, 1969), in agreement with the present results. C. gapperi is generally con-sidered a specialist species characteristic of wooded habitats (Iverson et al., 1967; Miller and Getz, 1977; Yahner, 1983); it has a relatively high water turn-over rate and poorly efficient kidneys, impeding its survival in low humidity (Getz, 1968). It has been shown that Peromyscus leucopus, another forest dwelling species (Getz, 1961b; Kaufman and Fleharty, 1974; Snyder and Best, 1988), can use riparian herbaceous strips as
corridors between woodlots (Cummings and Vessey, 1994).
Generally considered a typical species of prairies and other open grass-dominated habitats (Iverson et al., 1967; Grant, 1971; Morse, 1973; Yahner, 1983; Manson et al., 1999), M. pennsylvanicus was more abundant in herbaceous riparian strips, but many in-dividuals were also caught in shrubby and wooded strips, leading to the fourth highest niche breadth value obtained. Some studies found M. pennsylvan-icus in forested habitats (Dowler et al., 1985), and others attributed its presence to irregular incursions in times of high population densities and suggested that it avoids forest habitats (Grant, 1971; Tamarin et al., 1984). In shelterbelts, the species is present in openings and avoids the more wooded sections (Yahner, 1982). The shrubby and wooded riparian strips studied here were generally regularly broken by small openings, which probably was the reason for the presence of M. pennsylvanicus.
Linduska (1949) suggested that M. musculus, con-sidered a pest species, rarely inhabits linear herba-ceous habitats during the summer, but occupies these habitats after adjacent fields have been harvested. A good part of the present sampling was carried out after harvest, and only 14 individuals were captured, half of these in herbaceous strips, indicating a probable selection for this habitat type. M. musculus sometimes prefers linear marginal habitats to cultivated prairies, hayfields and corn fields (Clark et al., 1996; Kirsch, 1997). The low abundance of this species in this study could reflect its distribution in the agricultural landscape, in agreement with Kaufman and Kaufman (1990) who suggested that M. musculus is rarely, if ever, abundant in prairies and in cultivated fields. Linear habitats such as riparian strips could simply be corridors between farm buildings (Merriam, 1988; Clark et al., 1996).
vegetation on habitat selection by Z. hudsonius and B. brevicauda (Quimby, 1951; Getz, 1961a; Choate and Fleharty, 1973), and the proximity of streams could explain their preponderance in this study. In spite of its generalist nature, B. brevicauda was less abun-dant than expected in riparian strips adjacent to cereal fields. Although this species may avoid corn fields, this avoidance does not reduce its use of linear habi-tats adjacent to corn fields (Kirsch, 1997). Low avail-ability of prey species within buffer strips adjacent to cereal fields could probably have influenced our results.
4.2. Herpetofauna
As for small mammals, the abundance of the her-petofauna increased with increasing complexity in vegetation structure, and the diversity of herpetofaunal communities of herbaceous and wooded strips were similar. However, contrarily to what was obtained for small mammals, richness and diversity were greater in shrubby riparian strips. The lowest overlap index obtained between riparian strip-types was between shrubby and wooded riparian strips. This difference was mostly due to the presence of species in shrubby strips which were absent from wooded strips (Am-bystoma maculatum, Hyla crucifer, Storeria occipit-omaculata) and to a greater abundance of R. pipiens in wooded strips.
Only three out of 11 species were caught on more than 20 occasions. This low sample size reduced the possibility of any conclusion in terms of habitat se-lection. B. americanus clearly was most generalist, having the highest niche breadth value and being detected in all sites but two located in herbaceous riparian strips, in agreement with previous studies (Leclair, 1985; Dubé, 1994; deMaynadier and Hunter, 1998).
R. pipiens is generally considered a species of open habitats, fields and prairies (Dole, 1965; Cook, 1984), vegetation structure being the main factor for habi-tat selection with a preference for good gramineous cover (Beauregard and Leclair, 1988), no shrubs and reduced litter (Dubé, 1994). The majority (88%) of the individuals were caught in wooded riparian strips, giv-ing this species a relatively low niche breadth value. The wooded riparian strips in this study often had a rich herbaceous cover without any shrub layer, which
would provide adequate habitat for R. pipiens. It is, however, more difficult to interpret the absence of this species in herbaceous riparian strips.
More than 75% of R. sylvatica were captured in shrubby riparian strips, leading to a relatively low niche breadth value. This species is generally asso-ciated with wooded habitats (Heatwole, 1961; Bellis, 1962) and is considered intolerant to habitat modi-fications (deMaynadier and Hunter, 1998). Air and substrate moisture are important factors in the pro-cess of habitat selection (Marshall and Buell, 1955; Roberts and Lewin, 1979), the dense vegetation in the shrubby riparian strips studied probably explained the preponderance of R. sylvatica there.
5. Conclusions
This study clearly shows the importance of maintaining a diversity of riparian strips in order to maintain high wildlife diversity within agricultural landscapes. Shrubby riparian strips had a higher di-versity of herpetofauna, whereas a higher didi-versity of small mammals was found in herbaceous and wooded riparian strips. Even though diversity of small mam-mals was similar in these last two habitats, small mammal communities differed in composition and relative abundance. Moreover, species considered as habitat specialists and intolerant to habitat modifica-tions were present in all of the habitats studied. Thus, all three types of riparian strips were important for different species or groups of species. This underlines the interest of having a fair ratio of each of these habitats to maintain the highest possible diversity in agricultural landscapes. In these highly fragmented landscapes, riparian strips often represent wildlife corridors between remaining habitat islands (Weg-ner and Merriam, 1979; Fahrig and Merriam, 1985; Henderson et al., 1985; La Polla and Barrett, 1993; Burbrink et al., 1998).
by the presence of shrubs and trees in riparian strips, in agreement with similar studies in which the most abundant small mammal species generally inhabited forested habitats or pastures and were not considered as pest species (Dambach, 1948; Yahner, 1983). The abundance of species likely to be pests was relatively low in herbaceous riparian strips, and about twice as high in shrubby and wooded strips. Dambach (1948) concluded that herbaceous field borders have more rodent pest species than shrubby borders, and there are indications that increases in M. musculus numbers are induced by the removal of woody vege-tation (Geier, 1978; Best et al., 1979; Geier and Best, 1980).
Not only are pest species less abundant in ripar-ian strips with woody vegetation, the abundance of insectivore species is also greater. Total mammalian and amphibian insectivore numbers in wooded ri-parian strips were 2.4 times greater than in herba-ceous strips. Moreover, some rodent species also contribute in the control of insect species (Parmenter and MacMahon, 1988): a small mammal commu-nity of shrews and rodents may consume up to 6800 preys/ha/day (Churchfield and Brown, 1987). The presence of linear habitats like wooded riparian strips may also favour bats (Verboom and Huitema, 1997). The diversification of the vegetation structure may therefore contribute to integrated control of pest in-sects spending part of their annual cycle in riparian habitats.
Acknowledgements
We are particularly grateful to the numerous landowners who granted us access to their fields. We would also like to thank A. Desrosiers, M. Leclerc, R. Mc Nicoll and S. St-Onge who participated in the identification of small mammals and provided assistance in the field with L. Choinière, A. Cossette, G. Couture, S. Gagnon, M. Gosselin, L. Lessard, M. Paquin, and É. Sénécal. We would also like to thank L. Bélanger for his collaboration to the project and for giving us access to the data on vegetation characteris-tics and riparian strip widths. Funding for the project was provided by the Minsitère de l’Environnement et de la Faune du Québec.
References
Adler, G.H., 1985. Habitat selection and species interactions: an experimental analysis with small mammal populations. Oikos 45, 380–390.
Banfield, A.W.F., 1974. Les mammifères du Canada. Presses de l’Université Laval and University of Toronto Press, Canada. Beauregard, N., Leclair, R., 1988. Multivariate analysis of the
summer habitat structure of R. pipiens Schreber in Lac Saint-Pierre (Quebec, Canada). In: Szaro, R.C., Severson, K.E., Patton, D.R. (Coord.), Management of Amphibians, Reptiles and Small Mammals in North America. USDA Forestry Service, General Technical Report RM-166, pp. 129–141.
Bellis, E.D., 1962. The influence of humidity on wood frog activity. Am. Midl. Nat. 68, 139–148.
Best, L.B., Stauffer, D.F., Geier, A.R., 1979. Evaluating the effects of habitat alteration on birds and small mammals occupying riparian communities. In: Strategies for protection and management of floodplain wetlands and other riparian ecosystems. USDA Forestry Service, General Technical Report WO-12, pp. 117–124.
Brenner, F.J., Mondok, J.J., McDonald, R.J., 1991. Impact of riparian areas and land use on four non-point source pollution parameters in Pennsylvania. J. Penn. Acad. Sci. 65, 65–78. Brown, L.N., 1967. Ecological distribution of six species of shrews
and comparison of sampling methods in the Central Rocky Mountains. J. Mamm. 48, 617–623.
Brown, W.S., Parker, W.S., 1982. Niche dimensions and resource partitioning in a Great Basin desert snake community. In: Scott, N.J. (Ed.), Herpetological Communities. US Department of International Fisheries and Wildlife Service, Wildlife Research Report 13, pp. 59–81.
Burbrink, F.T., Phillips, C.A., Heske, E.J., 1998. A riparian zone in central Illinois as a potential dispersal corridor for reptiles and amphibians. Biol. Conserv. 86, 107–115.
Choate, J.R., Fleharty, E.D., 1973. Habitat preference and spatial relations of shrews in a mixed grassland in Kansas. Southwest. Nat. 18, 110–112.
Churchfield, S., Brown, V.K., 1987. The impact of small mammals in successional grasslands. Biol. J. Linnean Soc. 31, 273–290. Clark, B.K., Clark, B.S., Munsterman, W.E., Homerding, T.R., 1996. Differential use of roadside fencerows and contiguous pastures by small mammals in southeastern Oklahoma. Southwest. Nat. 41, 54–59.
Colwell, R.K., Futuyma, D.J., 1971. On the measurement of niche breadth and overlap. Ecology 52, 567–576.
Cook, F.R., 1984. Introduction aux amphibiens et reptiles du Canada. Musée national de Sciences Naturelles, Musées Nationaux du Canada, Ottawa.
Corn, P.S., 1994. Straight-line drift fences and pitfall traps. In: Heyer, W.R., Donnelly, M.A., McDiarmid, R.W., Hayek, L.-A.C., Foster, M.S. (Eds.), Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians. Smithsonian Institute Press, Washington, DC.
Dambach, C.A., 1948. A study of the ecology and economic value of crop field borders. Ohio State Univ. Biol. Sci. 2, 1–205. Decamps, H., Joachim, J., Lauga, J., 1987. The importance for
birds of the riparian woodlands within the alluvial corridor of the River Garonne, s.w. France. Reg. Rivers Res. Mgmt. 1, 301–316.
deMaynadier, P.G., Hunter, M.L., 1998. Effects of silvicultural edges on the distribution and abundance of amphibians in Maine. Conserv. Biol. 12, 340–352.
Dole, J.W., 1965. Spatial relations in natural populations of the leopard frog, Rana pipiens Schreber, in northern Michigan. Am. Midl. Nat. 74, 464–478.
Dowler, R.C., Katz, H.M., Katz, A.H., 1985. Comparison of live trapping methods for surveying small mammal populations. Northeast. Environ. Sci. 4, 165–171.
Dubé, C., 1994. Inventaire de l’herpétofaune de la plaine d’inondation de quatre bassins de la région de Montréal: le lac Saint-Louis, le lac des Deux Montagnes, le lac Saint-Pierre et le Haut-Richelieu en 1992 et 1993. Pour le ministère de l’Environnement et de la Faune du Québec, Direction de la faune et des habitats et Service de l’aménagement et de l’exploitation de la faune de Montréal.
Dueser, R.D., Shugart, H.H., 1978. Microhabitats in a forest-floor small mammal fauna. Ecology 59, 89–98.
Fahrig, L., Merriam, G., 1985. Habitat patch connectivity and population survival. Ecology 66, 1762–1768.
Fleharty, E.D., Navo, K.W., 1983. Irrigated cornfields as habitat for small mammals in the sandsage prairie region of western Kansas. J. Mamm. 64, 367–379.
Geier, A.R., 1978. Habitat selection by small mammals of riparian communities: evaluating the effects of habitat alterations. M.Sc. Thesis. Iowa State University, Ames, IA.
Geier, A.R., Best, L.B., 1980. Habitat selection by small mammals of riparian communities: evaluating effects of habitat alterations. J. Wildl. Mgmt. 44, 16–24.
Getz, L.L., 1961a. Factors influencing the local distribution of shrews. Am. Midl. Nat. 65, 67–88.
Getz, L.L., 1961b. Notes on the local distribution of Peromyscus leucopus and Zapus hudsonius. Am. Midl. Nat. 65, 486–500. Getz, L.L., 1968. Influence of water balance and microclimate on
the local distribution of the red-backed vole and white-footed mouse. Ecology 49, 276–286.
Gilliam, J.W., 1994. Riparian wetlands and water quality. J. Environ. Qual. 23, 896–900.
Grant, P.R., 1971. The habitat preference of Microtus pennsylvanicus, and its relevance to the distribution of this species on islands. J. Mamm. 52, 351–361.
Hamilton Jr., W.J., 1940. The biology of the Smoky Shrew (Sorex f. fumeus Miller). Zoologica 25, 473–492.
Hansen, L.P., Warnock, J.E., 1978. Response of two species of Peromyscus to vegetation succession on land strip mined for coal. Am. Midl. Nat. 100, 416–423.
Heatwole, H., 1961. Habitat selection and activity of the wood frog, Rana sylvatica Le Conte. Am. Midl. Nat. 66, 301–313. Henderson, M.T., Merriam, G, Wegner, J., 1985. Patchy
environments and species survival: Chipmunks in an agricultural mosaic. Biol. Conserv. 31, 95–105.
Hooper, E.T., 1942. An effect on the Peromyscus maniculatus Rassenkreis of land utilization in Michigan. J. Mamm. 23, 193– 196.
Horn, H.S., 1966. Measurement of overlap in comparative ecological studies. Am. Nat. 100, 419–424.
Hutcheson, K., 1970. A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 29, 151–154.
Iverson, S.I., Seabloom, R.W., Hnatiuk, J.M., 1967. Small-mammal distributions across the prairie-forest transition of Minnesota and North Dakota. Am. Midl. Nat. 78, 188–197.
Karr, J.R., Schlosser, I.J., 1978. Water resources and the land-water interface. Science 201, 229–234.
Kaufman, D.W., Fleharty, E.D., 1974. Habitat selection by nine species of rodents in north-central Kansas. Southwest. Nat. 18, 443–452.
Kaufman, D.W., Kaufman, G.A., 1990. House mice (Mus musculus) in natural and disturbed habitats in Kansas. J. Mamm. 71, 428–432.
Kirkland, G.L., Sheppard, P.K., 1994. Proposed standard protocol for sampling of small mammal communities. In: Merritt, J.F., Kirkland, G.L., Rose, R.K. (Eds.), Advances in the Biology of Shrews, Vol 18. Carnegie Mus. Nat. Hist., Spec. Publ., pp. 277–283.
Kirsch, E.M., 1997. Small mammal community composition in cornfields, roadside ditches, and prairies in eastern Nebraska. Nat. Areas J. 17, 204–211.
Lamarre, G., Laroche, R., Barrington, S., Madramootoo, C., 1993. Sites de démonstration pour évaluer l’impact d’une bande riveraine en milieu agricole. Projet no. 22-13765-580-042. Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec, Faculté des sciences agricoles et environnementales de l’Université McGill, pour le compte de la Fédération de l’Union des producteurs agricoles de Saint-Jean Valleyfield. La Polla, V.N., Barrett, G.W., 1993. Effects of corridor width
and presence on the population dynamics of the meadow vole (Microtus pennsylvanicus). Landscape Ecol. 8, 25–37. Leclair, R., 1985. Les amphibiens du Québec: biologie des
espèces et problématique de conservation des habitats. Dép. Chimie-Biologie, Univ. du Québec à Trois-Rivières, pour le ministère du Loisir, de la Chasse et de la Pêche.
Levins, R., 1968. Evolution in Changing Environments: Some Theoretical Explorations. Princeton University Press, Princeton, NJ.
Linduska, J.P., 1949. Ecology and land-use relationships of small mammals on a Michigan farm. Ph.D. Thesis. Michigan State College of Agriculture and Applied Science, 244 pp. Lowrance, R., Leonard, R., Sheridan, J., 1985. Managing riparian
ecosystems to control nonpoint pollution. J. Soil Water Conserv. 40, 87–92.
Lowrance, R., Sharpe, J.K., Sheridan, J.M., 1986. Long-term sediment deposition in the riparian zone of a coastal plain watershed. J. Soil Water Conserv. 41, 266–271.
Manson, R.H., Ostfeld, R.S., Canham, C.D., 1999. Responses of small mammal community to heterogeneity along forest-old-field edges. Landscape Ecol. 14, 355–367.
Merriam, G., 1988. Landscape dynamics in farmland. Trends Ecol. Evol. 3, 16–20.
Miller, D.H., Getz, L.L., 1977. Factors influencing local distribution and species diversity of forest small mammals in New England. Can. J. Zool. 55, 806–814.
Morse, D.H., 1973. Habitat utilization by meadow voles on small islands. J. Mamm. 54, 792–794.
Naiman, R.J., Decamps, H., Pollock, M., 1993. The role of riparian corridors in maintaining regional biodiversity. Ecol. Appl. 3, 209–212.
Parmenter, R.R., MacMahon, J.A., 1988. Factors limiting popula-tions of arid-land darkling beetles (Coleoptera: Tenebrionidae): predation by rodents. Environ. Entomol. 17, 280–286. Quimby, D.C., 1951. The life history and ecology of the jumping
mouse, Zapus hudsonius. Ecol. Monogr. 21, 61–95.
Rickard, W.H., 1960. The distribution of small mammals in relation to the climax vegetation mosaic in eastern Washington and Northern Idaho. Ecology 41, 99–106.
Roberts, W, Lewin, V., 1979. Habitat utilization and population densities of the amphibians of northeastern Alberta. Can. Field-Nat. 93, 144–154.
Scherrer, B., 1984. Biostatistique. Gaëtan Morin éditeur, Chicoutimi, Québec, Canada.
Small, M.F., Johnson Jr., W.N. 1986. Wildlife management in riparian habitats. In: Bissonette, J.A. (Ed.), Is Good Forestry Good Wildlife Management. Maine Agriculture Experiment Station, Miscellaneous Publication No. 689, Orono, Maine, pp. 69–79.
Snyder, E.J., Best, L.B., 1988. Dynamics of habitat use by small mammals in prairie communities. Am. Midl. Nat. 119, 128– 136.
Stamp, N.E., Ohmart, D., 1978. Resource utilization by desert rodents in the Lower Sonoran Desert. Ecology 59, 700–707.
Stauffer, D.F., Best, L.B., 1980. Habitat selection by birds of riparian communities: evaluating effects of habitat alterations. J. Wildl. Mgmt. 44, 1–15.
Tamarin, R.H., Reich, L.M., Moyer, C.M., 1984. Meadow vole cycles within fences. Can. J. Zool. 62, 1796–1802.
Thomas, J.W., Maser, C, Rodiek, J.E., 1979. Riparian zones. In: Thomas, J.W. (Ed.), Wildlife Habitats in Managed Forests — The Blue Mountains of Oregon and Washington. USDA, Forest Service, Agriculture Handbook No. 553, pp. 40–47.
Verboom, B, Huitema, H., 1997. The importance of linear landscape elements for the pipistrelle Pipistrellus pipistrellus and the serotine bat Eptesicus serotinus. Landscape Ecol. 12, 117–125.
Vought, L.B.-M., Dahl, J., Pedersen, C.L., Lacoursière, J.O., 1994. Nutrient retention in riparian ecotones. Ambio 23, 363–366. Wegner, J.F., Merriam, G., 1979. Movement by birds and small
mammals between a wood and adjoining farmland habitat. J. Appl. Ecol. 16, 349–358.
Whittaker, R.H., Levin, S.A., 1975. Niche: theory and application. Benchmark Papers in Ecology, Vol. 3. Halsted Press, Stroudsburg, PA.
Wrigley, R.E., 1969. Ecological notes on the mammals of southern Quebec. Can. Field-Nat. 83, 201–211.
Wrigley, R.E., Dubois, J.E., Copland, H.W.R., 1979. Habitat, abundance, and distribution of six species of shrews in Manitoba. J. Mamm. 60, 505–520.
Yahner, R.H., 1982. Microhabitat use by small mammals in farmstead shelterbelts. J. Mamm. 63, 440–445.
Yahner, R.H., 1983. Small mammals in farmstead shelterbelts: habitat correlates of seasonal abundance and community structure. J. Wildl. Mgmt. 47, 74–84.