Therapeutic Strategies
Michael E. Thase and Gary S. Sachs
The depressed phase of bipolar affective disorder is a significant cause of suffering, disability, and mortality and represents a major challenge to treating clinicians. This article first briefly reviews the phenomenology and clini-cal correlates of bipolar depression and then focuses on the major pharmacological treatment options. We strongly recommend use of mood stabilizers as the first-line treat-ment for the type I form of bipolar depression, largely because longer-term preventative therapy with these agents almost certainly will be indicated. Depressive episodes that do not respond to lithium, divalproex, or another mood stabilizer, or episodes that “breakthrough” despite preventive treatment, often warrant treatment with an antidepressant or electroconvulsive therapy. The ne-cessity of mood stabilizers in the type II form of bipolar depression is less certain, aside from the rapid cycling presentation. Both experts and practicing clinicians rec-ommend bupropion and the selective serotonin reuptake inhibitors as coequal initial choices, with venlafaxine and monoamine oxidase inhibitors, such as tranylcypromine, preferred for more resistant cases. The risk of antidepres-sant-induced hypomania or mania with concomitant mood stabilizer therapy is low, on the order of 5% to 10% during acute phase therapy. Additional therapeutic options and optimal durations of therapy also are discussed. Biol Psychiatry 2000;48:558 –572 ©2000 Society of Biological Psychiatry
Key Words:Bipolar affective disorder, depression, anti-depressants, mood stabilizers
Introduction
A
bout 90% of people who experience an episode of mania will, at some point, also suffer a major depres-sive episode (Goodwin and Jamison 1990). For many with this classic form of mental illness, the morbidity and mortality risk of the depressive episodes outstrip the morenotorious consequences of mania. Moreover, the hallmark of the type II form of bipolar disorder is that the depressive episodes are more numerous, lengthy, and/or severe than the hypomanias (Coryell et al 1987; Dunner 1993). Indis-criminant use of antidepressants can accelerate cycling by inducing manias and mixed states (Altshuler et al 1995), yet failure to treat residual depressive features may rein-force demoralization and weaken the therapeutic alliance. For these reasons, bipolar affective disorder cannot be treated effectively without careful attention to manage-ment of depressive episodes. After briefly considering the phenomenology of the disorder, this article will review the major pharmacologic treatment options for bipolar depres-sive episodes.
Overview
How does bipolar depression differ from other depressive states? At the simplest level, the risk of treatment-emer-gent mania and the frequent need to combine mood stabilizers and antidepressants distinguish these conditions (Goldberg and Kocsis 1999); however, nearly 60 years before the routine use of antidepressants, Kraepelin (1921) suggested that the phenomenology of manic depression differed from melancholia in several respects, including more pronounced volitional inhibition (i.e., “paralysis” of will) and less prominent dysphoric apprehension. Over the ensuing years, some psychopathologists have suggested a greater incidence of reverse vegetative signs (e.g., weight gain and hypersomnolence) in bipolar depression (Akiskal 1996; Goodwin and Jamison 1990; Himmelhoch et al 1972; Koukopoulos and Koukopoulos 1999), although such differences are not pronounced in studies of more severe populations (Casper et al 1985; Mitchell et al 1992). What is clear, however, is that reverse vegetative features are not unusual in bipolar depression (Goodwin and Jamison 1990).
Perris (1966) drew upon other, historical validators in distinguishing between bipolar and unipolar depressive states, including age of onset, number of lifetime episodes, and gender distribution. When compared to unipolar depression, bipolar disorder is characterized by an earlier age of onset, more frequent episodes, and a more equal From the Department of Psychiatry, University of Pittsburgh School of Medicine,
Western Psychiatric Institute and Clinic, Pittsburgh, Pennsylvania (MET) and the Department of Psychiatry, Massachusetts General Hospital, Harvard Med-ical School, Boston (GSS).
Address reprint requests to Michael E. Thase, M.D., University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinic, Department of Psychiatry, 3811 O’Hara Street, Pittsburgh PA 15213.
Received February 25, 2000; revised June 28, 2000; accepted July 3, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
proportion of men and women (Goodwin and Jamison 1990; Perris 1966). Within the bipolar disorder grouping, however, women experience more depressive episodes than men and rapid cycling is more common among women (Bauer et al 1994; Leibenluft 1996). Studies of various neurobiological correlates of depression have not yielded clear-cut differences between unipolar and bipolar depressions (Bowden 1999; Joffe et al 1999).
Other characteristics that may distinguish bipolar de-pression from other forms of dede-pression include a greater incidence of psychotic features, especially in early-onset cases (Akiskal et al 1983; Weissman et al 1984) and a greater risk of completed suicide (Goodwin and Jamison 1990). These features, which may be related to as yet unidentified neurobiologic differences or could simply represent severity “markers,” obviously have important clinical implications. A lifetime history of substance abuse is also more likely for those with bipolar disorder when compared to nonbipolar cases (Goodwin and Jamison 1990; Salloum and Thase, in press). Although often underappreciated, caffeinism and nicotine dependence should not be overlooked when assessing the bipolar patient’s substance abuse history. Not surprisingly, aver-age life expectancy appears to be significantly shorter for those with bipolar disorder when compared to those with other depressive disorders (Goodwin and Jamison 1990).
Antidepressants
One might assume that the effectiveness of antidepressant medications in bipolar depression is well established because the modern recognition of the disorder (e.g., Perris 1966) is so closely temporally linked to the antide-pressant era. To the contrary, not a single antideantide-pressant medication, nor even a particular class of antidepressant, has been demonstrated to be effective in at least two adequately powered, placebo-controlled clinical trials.
There are several reasons that the efficacy of antide-pressants in bipolar depression has received so little attention. First, there has been a sense of complacency that the results of studies of nonbipolar depression are gener-alizable and that more systematic studies are not needed. Second, regulatory agencies, including the U.S. Food and Drug Administration, have not required that separate studies of bipolar depression be conducted. Third, bipolar depressed patients are now frequently excluded from studies of antidepressants and, when included, patients may not be able to take concomitant mood stabilizers. Moreover, the proportion of bipolar patients included in any one study is typically too small to permit subanalysis. Finally, although bipolar disorder is not a rare condition, its various presentations (type 1 versus type 2, mixed, rapid cycling, psychotic) and complexities (e.g., high rates
of substance abuse and anxiety disorder comorbidities) often defy recruitment or large groups of relatively homo-geneous patients. As a result, it is only in the past decade that a concerted effort has been made to form large collaborative study groups that can conduct studies of bipolar depression in a timely manner. Multicenter studies have their own problems, such as ensuring reliability and controlling other sources of variability, but these difficul-ties are not insurmountable.
Acute-phase antidepressant pharmacotherapy of bipolar depression employs the same doses and duration to define an adequate trial that are used in treatment of other depressive disorders; however, the optimal duration of continuation phase therapy for bipolar depression has not been established and it isnotcertain that all antidepressant responders require a minimum of 6 –9 months of treat-ment. Moreover, the value of longer courses of mainte-nance antidepressant pharmacotherapy has not been proven in bipolar disorder (Sachs and Thase 2000a).
The classes of antidepressants to be reviewed below include the selective serotonin reuptake inhibitors (SSRIs), bupropion, other novel antidepressants, tricyclics (TCAs), and the monoamine oxidase inhibitors (MAOIs).
SSRIs
The SSRIs are ranked as first- or second-choice interven-tions for episodes of bipolar depression that have not responded to conservative management or following “breakthrough” prophylactic therapy with a mood stabi-lizer (American Psychiatric Association 1994; Frances et al 1996). There is no one favorite SSRI for this indication, and each agent has relative advantages and disadvantages. For example, fluoxetine is perceived by some clinicians as having an edge (over the other SSRIs) for treatment of patients with prominent anergia (Sachs and Thase 2000b) and, by virtue of its early entry in the U.S. market, it has a more extensive track record. Yet other experts are concerned that the long elimination half-life of fluox-etine’s principal metabolite, norfluoxetine, may compli-cate therapy if there is a treatment-emergent manic epi-sode (Frances et al 1996).
The strengths of the SSRIs include relatively simple dosage titration and excellent tolerability. As a class, the SSRIs have an outstanding record for safety in overdose. Studies of nonbipolar depression also suggest that the SSRIs have a low incidence of causing switches, perhaps only half the magnitude of TCAs (Peet 1994).
fluvoxamine and clozapine, mediated through inhibition of the CYP P450 IA2 isoenzyme, which requires dosage reduction of the latter (Nemeroff et al 1996). Clinical experience also suggests a pharmacodynamic enhance-ment of some of lithium’s side effects, such as tremor, diarrhea, and nausea. During longer-term therapy with the SSRIs, weight gain and sexual dysfunction occasionally can be problematic. One drawback, cost, will pass quickly as generic formulations of these compounds begin to become available over the next few years.
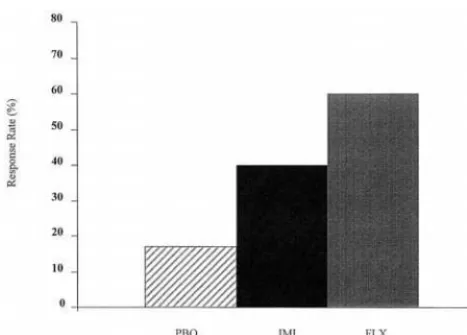
There are only two double-blind, placebo-controlled studies of an SSRI in the literature. Cohn et al (1989) contrasted fluoxetine (20 – 80 mg/day) and imipramine (75–300 mg/day) with placebo in a study of 89 patients with type I bipolar disorder, of which about 25% received concurrent lithium therapy (Figure 1). Results favored fluoxetine in terms of response (intent to treat response rates: fluoxetine, 60%; imipramine, 40%; placebo, 17%). During the first 3 weeks of therapy, switch rates were 7% for imipramine, 0% for fluoxetine, and 3% for placebo. Thereafter, however, a number of fluoxetine responders did switch to hypomania or mania, increasing the switch rate to 17% across 12 weeks of therapy.
The results of a second large multicenter, placebo-controlled trial comparing paroxetine and imipramine will be published shortly (Nemeroff et al, in press). All patients received concurrent lithium treatment. There was a modest 15% difference favoring both active agents over placebo, although this difference was not statistically significant. Day-to-day tolerability favored the SSRI over the TCA, and relatively few patients switched on either active antidepressants. A post-hoc analysis suggested that re-sponse in the group receiving lithium and placebo was associated with blood levels of 0.8 mEq/L or higher. Further, there was a significant difference favoring the
group receiving active paroxetine (when compared to those receiving placebo) among the patients with lower lithium levels.
A third double-blind study compared the addition of either paroxetine (36 mg/day) or a second mood stabilizer (divalproex,n5 19, lithium, n 5 8) for bipolar I or II patients who became depressed during long-term prophy-lactic therapy (Young et al 2000). Both strategies were effective (the average patient experienced a 601% reduc-tion in depression scores), although significantly more patients dropped out of the lithium plus divalproex group (6/16 vs. 0/11). No patient in the paroxetine plus mood stabilizer group suffered a shift into hypomania or mania. There is also a dearth of information on treatment of bipolar II depression with SSRIs. It is unfortunate that industry-sponsored trials of new antidepressants seldom utilize standardized psychiatric interviews to assess pa-tients, because it is likely that 10% or even more of the thousands of apparently nonbipolar participants would have met criteria for bipolar II disorder (Akiskal et al 1995). Clinical experience would suggest that SSRIs are useful treatments of this common condition and concur-rent mood stabilization is not always necessary. For example, Simpson and DePaulo (1991) reported an 81% response rate to fluoxetine (up to 80 mg/day) across an 80-week follow-up in a series of 16 patients with bipolar II depression. Three patients (19%) experienced switches during this extended treatment period.
The largest series of SSRI treatment of bipolar II depression was published by Amsterdam et al (1998a). This study compared acute and continuation phase therapy results of bipolar II (n5 89) and nonbipolar (n5 750) patients during treatment with fluoxetine monotherapy (20 mg/day). There were no major differences in response rates or tolerability between the two diagnostic groups, although complaints of both somnolence and agitation were significantly greater in the bipolar II group. Also, about 4% of the bipolar II patients switched during each of the acute and continuation phases of the study, as com-pared to a switch rate of,1% in the nonbipolar group.
In practice, the decision to use concurrent mood stabi-lization during treatment of bipolar II depression must be made on a case-by-case basis, with age of onset, cycle length, past history of rapid cycling, patient gender, and prior frequency and severity of hypomanias important considerations.
Bupropion
This amino ketone derivative is ranked by experts as the coequal of the SSRIs for treatment of bipolar depression (American Psychiatric Association 1994; Frances et al 1996). Such a high ranking is particularly noteworthy
when juxtaposed with the market position of bupropion relative to the SSRIs in the United States. This reflects both the need for better antidepressants for bipolar depres-sion and a history of research and clinical experience with bupropion dating nearly 20 years (Shopsin 1983; Wright et al 1985).
Bupropion is presumed to work via noradrenergic and dopaminergic mechanisms and thus provides a clinically and conceptually useful alternative to SSRIs (Ascher et al 1995). First, being devoid of direct serotoninergic effects results in a unique tolerability profile, with a virtual absence of weight gain and sexual side effects. Second, the effects of bupropion on these two catecholamine neuro-transmitter systems may be especially useful for treatment of patients with anergia and pronounced vegetative rever-sal (e.g., Goodnick et al 1998). Third, there has been some evidence for a number of years that bupropion may have a particularly low risk of inducing manic switches (Haykel and Akiskal 1990; Sachs et al 1994; Shopsin 1983), although not all reports have confirmed this beneficial property (Fogelson et al 1992).
Bupropion dosing is limited to 400 (sustained-release formulation) or 450 (immediate-release formulation) mg/ day because of a dose-dependent risk of seizures (Johnston et al 1991). Therapeutic blood level monitoring could permit higher dose therapy for selected patients (Golden et al 1988), although this is not a standard practice. Overall, the side effect tolerability of bupropion is comparable to SSRIs, despite the aforementioned differences in weight gain and sexual dysfunction. Common side effects of bupropion include dry mouth and constipation (presum-ably noradrenergically mediated, because the compound is not anticholinergic), as well as headaches and insomnia.
A single small controlled trial contrasted bupropion with the noradrenergically active TCA desipramine (Sachs et al 1994). In this study, 15 patients were assigned to initial double-blind therapy and four patients received a second, cross-over trial after nonresponse to the first medication. All 15 patients received concurrent therapy with lithium, divalproex, or carbamazepine. The two antidepressants were comparably effective (five of 10 patients treated with desipramine responded vs. five of nine treated with bupropion); however, five patients treated with the TCA switched, whereas only one patient switched on bupropion.
A properly controlled, double-blind comparison of bu-propion and an SSRI in bipolar depression is long overdue. Studies of patients with nonbipolar depression suggest parity of efficacy (Feighner et al 1991; Kavoussi et al 1997), although the results might be different in bipolar patients predisposed to switching or rapid cycling. This issue is too important to leave to inference! One multi-center study conducted by the Stanley Foundation research
network is underway, and we have recently begun a second large study as part of the NIMH-funded multi-center initiative, the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). The results of these studies should be available within 4 to 5 years.
Other Newer Antidepressants
This heterogeneous grouping includes venlafaxine, nefaz-odone, mirtazapine, and reboxetine. Not surprisingly, none of these agents has been evaluated systematically in bipolar I depression. Nevertheless, they provide useful alternatives for patients who do not respond to or cannot tolerate SSRIs and bupropion.
Venlafaxine is not grouped with the SSRIs because at higher doses (e.g., 150 –375 mg/day) it appears to have clinically significant effects on noradrenergic neurotrans-mission (Harvey et al 2000). This “dual reuptake” effect at higher doses may result in some additional therapeutic benefit relative to SSRIs (Clerc et al 1994; Dierick et al 1996; Poirier and Boyer 1999), although venlafaxine therapy at doses above 300 mg/day is associated with an 8%–10% incidence of increased blood pressure (Thase 1998). The practitioners surveyed by Sachs et al (2000) ranked venlafaxine as the next choice for bipolar depres-sions not responding to SSRIs or bupropion.
Amsterdam et al (1998b) reported on a series of 17 patients with bipolar II depression treated with the imme-diate release form of venlafaxine (75–225 mg/day). They compared the patients receiving venlafaxine monotherapy with a demographically comparable group of 31 nonbipo-lar depressed patients treated in the same protocol. The bipolar II subgroup responded somewhat more rapidly than the nonbipolar subgroup, and there were no switches to mania observed during the first 8 weeks of treatment.
The original immediate release formulation of venlafax-ine, characterized by a relatively short half-life, low plasma protein binding, and the need for divided daily dosing, was somewhat less well tolerated than the SSRIs (see, for example, Preskorn [1995] for a comparison of side effect rates after adjusting for placebo). The extended release formulation obviates the need for divided dose and may lessen these problems, although there is not yet extensive experience with this formulation at doses above 225 mg/day. Discontinuation symptoms can be problem-atic after abrupt termination of a therapeutic trial of either formulation of venlafaxine (Fava et al 1997).
Nefazodone and mirtazapine, although unrelated struc-turally, are often paired, because both agents block post-synaptic 5-HT2 receptors, both have symptomatically
receptors, mirtazapine is both more sedating than nefaz-odone and does not have the gastrointestinal side effects associated with SSRI therapy. Weight gain can be trou-blesome with mirtazapine, however, with an effect early in therapy comparable to amitriptyline (Montgomery et al 1998). Nefazodone is a relatively potent inhibitor of CYP P450 3A4, resulting in a greater potential for drug– drug interactions than mirtazapine.
Mirtazapine and nefazodone also differ from both the SSRIs and each other in effects on sleep architecture (Rush et al 1998; Thase 1999). Although both drugs improve sleep maintenance, mirtazapine (like many other antidepressants) suppresses rapid eye movement (REM) sleep whereas nefazodone therapy may actually increase REM sleep time. Therefore, nefazodone and mirtazapine should not be viewed as interchangeable treatments for bipolar depression.
Reboxetine, a selective norepinephrine reuptake inhib-itor, will soon be introduced in the United States and already is available in Canada and Europe. This agent (therapeutic dose: 8 –10 mg/day) provides a better-toler-ated alternative to noradrenergically selective TCAs, such as nortriptyline and desipramine. Some evidence, as yet far from conclusive, suggests a greater effect than the SSRI fluoxetine on a measure of social activation (Mas-sana et al 1999). If true, this could provide a welcomed addition for treatment of patients with pronounced anergic and volitional inhibition. Of course, it is also possible that noradrenergic activation associated with reboxetine ther-apy could have more pronounced adverse effects on the sleep or psychomotor activity of bipolar patients relative to SSRIs. To date, experience treating bipolar depression with reboxetine is limited and the risk of inducing manic switches cannot yet be estimated with confidence.
Tricyclic Antidepressants
Studies of the TCAs have been reviewed in detail else-where (Goodwin and Jamison 1990; Srisurapanont et al 1995; Zornberg and Pope 1993). In this article, we are principally concerned with the efficacy and tolerability of the TCAs as a counterpoint to the newer agents or MAOIs. Of greatest concern has been a relatively high risk of manic switches during TCA therapy in relation to rather modest antidepressant effects (Himmelhoch et al 1991; Sachs et al 1994). Potential for lethality in overdose and a relatively high day-to-day side effect burden are also major concerns. Despite their many limitations, however, the TCAs are not completely obsolete for treatment of bipolar depression and they remain useful third- or fourth-line medications for selected patients (Frances et al 1996). Two of the TCAs, nortriptyline and desipramine, have somewhat less pronounced antihistaminic and
anticholin-ergic effects than the others, and therapeutic trials of these agents (as well as imipramine) can be guided by accurate, readily available plasma levels. A fourth TCA, clomipra-mine, is sufficiently serotoninergic to have efficacy in obsessive-compulsive disorder (Griest et al 1995) and may also have therapeutic potential for bipolar depression (Sachs 1996). Moreover, there is some evidence that clomipramine is a more potent antidepressant (at higher doses) than several of the newer agents (Danish University Antidepressant Group 1986, 1990, 1993).
Monoamine Oxidase Inhibitors
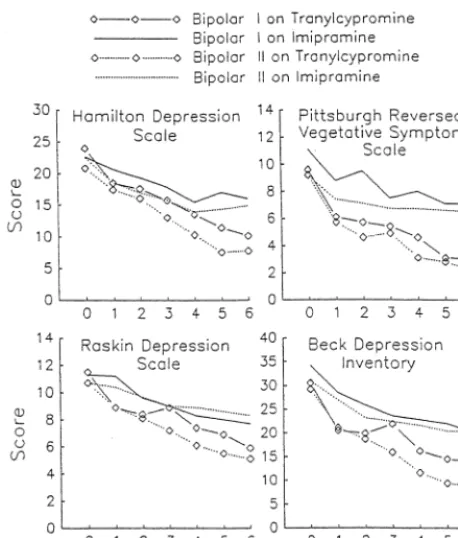
All other things equal, the MAOI tranylcypromine could be considered a treatment of first choice for bipolar depression (Frances et al 1996; Sachs 1996). The track record of this agent extends for nearly 30 years, spanning open-label (Himmelhoch et al 1972), placebo-controlled (Himmelhoch et al 1982), and imipramine-comparator (Himmelhoch et al 1991; Thase et al 1992b) clinical trials. The most compelling evidence of the efficacy of tranyl-cypromine (40 – 60 mg/day) in bipolar depression has come from studies delimited to patients with anergic and reversed neurovegetative features (Himmelhoch et al 1972, 1982; 1991; Thase et al 1992b). In the most recent study, tranylcypromine (30 – 60 mg/day) was significantly more effective than imipramine (100 –300 mg/day) and was equally effective in bipolar I and II subtypes (Figure 2; Himmelhoch et al 1991). Nonresponders in this study were crossed over to a second trial with the alternate medication (Thase et al 1992b). Twelve imipramine non-responders received double-blind therapy with tranyl-cypromine, of which nine patients (75%) responded. By contrast, only one of four tranylcypromine nonresponders responded to imipramine. Such positive results have begged the question: How useful is tranylcypromine when the depressive syndrome is not anergic? Moreover, given current practice patterns, how useful is an MAOI when an SSRI has already failed?
bipo-lar patient who has not responded to a SSRI or bupropion (Sachs 1996).
The MAOIs phenelzine and isocarboxazid also are useful antidepressants, although they have not been stud-ied systematically in bipolar depression (Thase et al 1995). Studies of nonbipolar patients with prominent reversed vegetative features do suggest that these agents can be used if tranylcypromine is either not available or poorly tolerated (Larsen 1991; Quitkin et al 1993).
In the studies of Himmelhoch et al (1991) and Thase et al (1992b) approximately 20% of the patients who re-sponded to tranylcypromine monotherapy switched to hypomania or mania within 12 weeks. Therefore, MAOIs should not be prescribed to bipolar patients without concomitant mood stabilizers. Clinical experience suggest that the MAOIs can be used safely with lithium, dival-proex, and carbamazepine, the latter formerly being of some concern because of its TCA-like nature (Ketter et al 1995).
Although there are few doubts about the efficacy of the MAOIs, the side-effects and dietary interaction problems of the MAOIs generally relegate these agents to third- or fourth-line use (Thase et al 1995). Tranylcypromine, phenelzine, and isocarboxazid are irreversible and
nonse-lective inhibitors of both the A and B forms of monoamine oxidase. Inhibition of the A form of the enzyme in the brain is presumed to mediate efficacy (by decreasing degradation of monoamine neurotransmitters), although inhibition of the B form in blood platelets has provided a method for therapeutic drug monitoring (Thase et al 1995). Irreversible inhibition of the A form of the enzyme in the gut results in increased levels of tryamine and other vasoactive amines, which in turn can mediate a paroxymal increase in blood pressure. This can result in a hyperten-sive crisis, a potentially life-threatening event. A low tyramine diet has been known for 25 years to greatly reduce this risk, although dietary noncompliance is an endemic problem, and unexplained hypertensive crises do occur.
Whereas hypertensive crises are relatively uncommon, and largely preventable, the day-to-day nuisance side effects of the MAOIs can be considerable and, for the average patient, are greater than observed with the SSRIs or bupropion. Common side effects include insomnia, excessive daytime sedation, orthostatic hypotension, weight gain, and sexual dysfunction (Thase et al 1995). The irreversible MAOIscannotbe used in close proximity following an SSRI because of the risk of serotonin syndrome (Beasley et al 1993). For fluoxetine, this risk can extend for 28 days or even longer.
Drug development efforts have focused on MAOI compounds that are selective for the type A enzyme. A compound developed early in this process, clorgyline, is a selective but irreversible type A inhibitor. As predicted, clorgyline was found to have antidepressant effects in a small study of treatment-resistant bipolar patients (Potter et al 1982). Subsequent efforts have been directed at reversible inhibitors of monoamine oxidase type A, the so-called RIMAs. A reversible MAOI can be displaced from the enzyme by higher concentrations of a natural substrate, such as tyramine, eliminating the need for a restricted diet. Moclobemide, a RIMA that is available throughout much of the world (but not the United States), does not require dietary restrictions at doses of 300 – 600 mg/day. Moclobemide has an efficacy and overall side effect profile comparable to the SSRIs (Lotufo-Neto et al 1999), although sexual dysfunction is less likely with moclobemide (Kennedy et al 2000). Using a pooled data set, Angst and Stabl (1992) reported that moclobemide and TCAs were equally effective in bipolar depression, al-though switch rates were not reported, and it is not clear whether or not patients were taking mood stabilizers. Despite these strengths, moclobemide is used much less commonly than the SSRIs and there is wide-spread con-cern that it is not as effective as the irreversible, nonse-lective MAOIs (Lotufo-Neto et al 1999; Thase and Nolen 2000). Of note, Vink et al (1994) found that 16 of 28 Figure 2. Response across 6 weeks of double-blind treatment of
(57%) tranylcypromine responders relapsed within 2 months of being switched to moclobemide, an observation that is strongly suggestive of a less potent therapeutic effect. Higher than normal doses of moclobemide (900 – 1200 mg/day) may offer some greater therapeutic benefit for selected patients (Angst et al 1995; Lotufo-Neto et al 1999), although higher doses may convey a greater risk of side effects, and there is some concern about the need for dietary restrictions at such higher doses.
Mood Stabilizers
The mood stabilizers, particularly lithium, divalproex, and carbamazepine, can be viewed as first-line treatments for bipolar I depression for several good reasons. First, virtu-ally all patients with bipolar I disorder will need to be placed on a mood stabilizer anyway for subsequent pro-phylaxis. Second, these agents, when prescribed as mono-therapies, have acute-phase antidepressant response rates of 30%–50% (Sachs 1996; Srisurapanont et al 1995). Third, there is virtually no risk of accelerated cycling or treatment-emergent mania associated with their use. For these reasons, initial treatment of bipolar depression with a mood stabilizer is featured prominently in recent practice guidelines (American Psychiatric Association 1994; Frances et al 1996).
Lithium
The oldest, simplest, least expensive, and best-studied mood stabilizer, the lithium ion has a track record that extends for more than 30 years as an acute-phase treatment for bipolar depression (e.g., Goodwin et al 1972). A number of placebo-controlled studies have examined the efficacy of lithium salts for bipolar depression and, despite small samples and a number of other methodologic short-comings, these early studies (the last was published in 1978) almost uniformly demonstrated significant thera-peutic effects (Srisurapanont et al 1995). Moreover, when active lithium was replaced by placebo in the studies utilizing a crossover design, an average relapse rate of 52% observed (Goodwin and Jamison 1990), the sine qua non of an effective antidepressant (Prien and Kupfer 1986). Lastly, the capacity of lithium to protect patients from suicide during long-term treatment is well estab-lished (Baldessarini et al 1999).
The early studies employed doses of lithium salts that resulted in plasma levels ranging between 0.6 mEq/L and 1.5 mEq/L, with an average across studies of about 0.9 mEq/L; such blood levels are relatively high by today’s standards (Sachs and Thase 2000b). It is now clear that patients with mixed features and those with a recent history of rapid cycling or substance abuse are
signifi-cantly less likely to benefit from to lithium monotherapy (Dunner et al 1977; Swann et al 1997). Conversely, some patients appear to require higher therapeutic doses. Con-sistent with this observation, Nemeroff et al (in press) found that lithium at plasma levels of 0.8 mEq/L or higher plus placebo was just as effective as the combination of lithium and paroxetine.
Approximately 10% of patients started on lithium can-not tolerate its side effects, and longer-term complications include hypothyroidism, interstitial fibrosis and other kid-ney problems, weight gain, and acne and psoriasis. The insidious development of hypothyroidism can be over-looked or misidentified as anergic depression because of the prominence of fatigue and weight gain.
During the decades in which lithium was the only proven treatment for bipolar disorder, a number of lithium resistant cases accumulated, and the availability of multi-ple alternatives is quite welcome. Nevertheless, lithium should not be overlooked as a consequence of the enthu-siasm for newer therapeutic options. Even when alternate strategies are chosen first, lithium can still produce re-sponse rates of 30%–50% when added to an antidepressant as an augmentor (Bauer and Dopfmer 1999).
Divalproex
It is likely that the anticonvulsant divalproex has some antidepressant effects (Petty 1995), although it is far better studied in bipolar disorder as an antimanic (Post 2000). In this context, divalproex appears to be a particularly useful alternative to lithium, either alone or in combination with other appropriate medications, for treatment of rapid-cycling and dysphoric/mixed presentations (Calabrese et al 1993; Frye et al 1996; McElroy et al 1988; Swann et al 1997).
Divalproex has not been proven to be an effective acute-phase monotherapy for bipolar depression; however, there is evidence of g-aminobutyric acid (GABA)-ergic dysfunction associated with depressive disorders (Petty 1995) and some open-label studies have suggested utility in both bipolar and nonbipolar depressions (Davis et al 1996; Lambert 1984).
that extended therapy with divalproex may be associated with an increased risk of polycystic ovaries.
Carbamazepine
Although the use of this agent predates that of divalproex by nearly a decade, it is now generally relegated to third-line use. There is evidence that carbamazepine in doses yielding plasma levels of 5–12 mcg/mL has antide-pressant effects, and several small double-blind, placebo controlled studies suggest modest but statistically signifi-cant symptom reductions (Srisurapanont et al 1995). Post (2000) suggests that carbamazepine response appears to be unrelated to prior outcomes with either lithium or dival-proex. Additive antidepressant effects have been reported when used in combination with lithium; in a long-term study of a difficult group of rapid-cycling patients the combination was more effective than either monotherapy (Denicoff et al 1997). Similar results have recently been reported with the combination of carbamazepine and the selective calcium channel blocker nimodipine in a small series of highly treatment-resistant patients (Pazzaglia et al 1998).
Carbamazepine therapy is associated with side effects such as sedation, tremor, diplopia, and weight gain; rash is the most common serious adverse event (Post 2000).
Lamotrigine
This anticonvulsant has become the most promising new therapy for bipolar depression, with numerous positive open-label reports (Bowden et al 1999) and one published double-blind trial (Calabrese et al 1999). In one larger series of 75 patients, Bowden et al (1999) observed that rapid cyclers responded as well as patients with less frequent episodes. In the double-blind study of lamotrigine monotherapy, 195 unmedicated patients with bipolar I depression were randomly assigned to placebo or either 50 mg/day and 200 mg/day of active drug. Both doses were significantly more effective than placebo (Figure 3).
Al-though the difference between the two dosage groups was not statistically significant, there were trends suggesting a modest advantage for the higher dosage condition. Rates of switching were as follows: placebo, 5%; 50 mg/day, 3%; 200 mg/day, 8%. The efficacy of increasing to higher doses for patients not responding to minimum effective doses needs to be established. Lamotrigine has not yet been shown to have antimanic effects.
Overall, lamotrigine is well tolerated, with the excep-tions of headache (about twice as common as observed during placebo treatment), insomnia, and rash. In our experience, the likelihood of switching may be greater at higher doses, although this observation has not yet been established empirically. Approximately 5%–7% of lam-otrigine-treated patients will need to discontinue the med-ication because of rash. As with carbamazepine, a severe, exfoliative dermatitis can be expected to occur in about three of a 1000 adults (Messenheimer et al 1998). The risk of this potentially lethal reaction is higher in children, people taking other anticonvulsants, and following rapid titration to higher doses. Initiating therapy at 25 mg/day and titrating slowly across 6 – 8 weeks of therapy appears to lessen the risk of rash (Messenheimer et al 1998).
Gabapentin
Despite a lack of evidence of efficacy from double-blind controlled studies in mania (Pande et al, in press), gaba-pentin has become increasingly more widely used as an “add on” for treatment of bipolar disorder (Altshuler et al 1999). This anticonvulsant is used in a relatively wide range of doses (e.g., 600 –3,600 mg/day) and may have collateral benefits for symptoms such as pain and anxiety (Pande et al 1999). There are suggestions in the literature of modest antidepressant effects (Young et al 1997).
Neuroleptics
Like the anticonvulsants, neuroleptics have primarily been studied in mania, with knowledge about efficacy in the
depressed phase of bipolar illness limited to clinical observations and case series. Of course, severe psychotic states of bipolar depression often warrant treatment with neuroleptics on clinical grounds, usually in combination with mood stabilizers and/or antidepressants.
Over the past few years the novel or atypical antipsy-chotics have gained increasing favor over the older neu-roleptics for treatment of bipolar disorder because of fewer problems with extrapyramidal symptoms, a presumedly lower risk of tardive dyskinesia, and preliminary evidence of antimanic or mood stabilizing effects (Ghaemi and Sachs 1997; Keck et al 1998; Suppes et al 1999; Tohen et al 1999). Olanzapine and risperidone are preferred over clozapine because of tolerability and safety concerns; there is less experience to date with quetiapine. Although these agents should not be thought of as primary treat-ments for bipolar depression, their utility in combination with mood stabilizers and antidepressants cannot be over-looked for more treatment-resistant patients (Ghaemi et al 1998; Suppes et al 1999). Weight gain and sedation constitute the major dose-limiting side effects.
Other Adjunctive Options
As noted earlier, lithium salts have important antidepres-sant effects and can be used to augment an inadequate antidepressant response to divalproex, carbamazepine, or lamotrigine. The study by Young et al (2000) reviewed earlier found that the combination of divalproex and lithium was as effective as a mood stabilizer plus parox-etine combination, although less well tolerated. The next most important adjunctive therapeutic option for treatment of bipolar depression is thyroid hormone (Sachs 1996). Thyroid hormones can be prescribed to correct hypothy-roidism, to treat more subtle states of relative thyroid hypofunction, or as adjuncts for a euthyroid antidepressant nonresponder. Clinicians often pick L-thyroxine (T4) for
the former indication and L-triiodothyronine (T3; 25–50
mcg/day), for the latter purpose; however, the theoretic rationale for such a distinction is controversial (Joffe and Singer 1990). In studies of nonbipolar depression, adjunc-tive thyroid treatment typically results in 30%–50% re-sponse rates and is generally well tolerated (Aronson et al 1996). Higher doses of thyroid hormone are sometimes useful in cases of rapid cycling (Bauer and Whybrow 1990). Long-term, high-dose thyroid therapy may be associated with an increased risk of osteoporosis and, therefore, should be used with caution.
Other strategies used to augment antidepressants in-clude buspirone (a 5-HT1A partial agonist), pindolol (a
5-HT1A antagonist), and various types of catecholamine
agonists, including psychostimulants and direct dopamine agonists (Sachs 1996; Thase and Rush 1997). For bipolar
depressed patients, such approaches usually result in patients taking three or even more psychotropic medica-tions. Despite the current enthusiasm for use of various antidepressant combinations, such unproven strategies should not be used to the exclusion of time-honored strategies such as MAOIs.
Other Treatment Approaches
These strategies can be loosely divided into “somatic” and “nonsomatic,” although it must be recognized that psycho-therapies also may affect brain function either directly or indirectly (Thase 2000). The somatic interventions include electroconvulsive therapy (ECT), phototherapy, and sleep deprivation. Nonsomatic interventions include individual psychotherapies, such as cognitive behavior therapy (Basco and Rush 1996) and interpersonal and social rhythm therapy (Frank et al 1994), psychoeducationally oriented group therapies (Bauer et al 1998), and family-focused therapies (Miklowitz and Goldstein 1997).
Electroconvulsive Therapy
Electroconvulsive therapy is a highly effective treatment of bipolar depression that is typically used after failure of one or more antidepressant trials (Prudic et al 1990; Sachs 1996). This is because ECT is both less acceptable and substantially more expensive than pharmacotherapy.
Many experts favor use of bilateral ECT for treatment of bipolar depressive states (Sachs 1996), perhaps reflect-ing the more common use of bilateral ECT for more resistant depressive states (American Psychiatric Associ-ation 1994); however, another potential advantage of bilateral electrode placement could be broader coverage of mixed episodes as well as symptomatic treatment of switches into mania (e.g., Small et al 1988). It remains to be seen if higher energy forms of unilateral (nondominant) ECT (i.e.,.3 times the seizure threshold) for treatment of bipolar patients offer comparable efficacy, with fewer cognitive side effects than bilateral electrode placement (Sackeim et al 1993).
Recently, rapid transcranial magnetic stimulation (rTMS) has been developed as an alternative to ECT, offering advantages such as minimal cognitive effects and no need for general anesthesia (George et al 1997). This treatment does not appear to be as effective as ECT for antidepressant-resistant states, however, and the indica-tions of rTMS for bipolar depressive states remains to be determined (Nahas et al 1999).
Phototherapy
predominant number of depressive episodes in the fall or winter and hypomania or mania in the spring or summer (Goodwin and Jamison 1990). Phototherapy with full-spectrum bright white lights (i.e., 2,000 –10,000 lux for 30
min to 2 hours per day) thus provides a pragmatic and conceptually based alternative to pharmacotherapy (Ter-man and Ter(Ter-man 1999). As there are no large, well-controlled studies of phototherapy of patients with bipolar Figure 4. Suggested sequential strategies for pharmacotherapy of bipolar depression. TFT, thyroid function test; T4,L-thyroxine; SSRI,
depression taking mood stabilizers, its use thus must be considered empiric. Although phototherapy is generally well tolerated, sleep disruption and switches into mania or hypomania have been reported. The cost of a light box is not trivial (i.e., $200 –$500) and the amount of time required to implement the treatment can be considerable.
Sleep Deprivation
Therapeutic sleep deprivation presumably treats bipolar depressive states by shifting or disrupting biologic rhythms. It is well known, for example, that some patients will switch from severe depression into euthymia or hypomania after a single night of total sleep deprivation (Wehr et al 1987). Unfortunately, the clinical benefits of total sleep deprivation are almost always short lived, as depression relapses after only a few hours of sleep. A schedule of partial sleep deprivation, typically limiting sleep to about 4 hours early in the night (e.g., 10:00 PM–2:00 AM), may have more sustained benefits. This approach is seldom utilized by practitioners, however, and many depressed people have difficulties implementing partial sleep deprivation schedules outside of specialty clinic settings.
Psychotherapy
Both patients and psychiatrists give psychotherapy high marks when used in combination with appropriate phar-macotherapy for bipolar depression (American Psychiatric Association 1994; Lish et al 1994; Sharma et al 1997). The explanation for such popularity, given a dearth of evidence of efficacy, probably reflects the numerous interpersonal difficulties in day-to-day life faced by many people with bipolar disorder and the desire to be treated as a “whole person.”
Given evidence that modern symptom-focused forms of psychotherapy have beneficial effects comparable to anti-depressants for ambulatory patients with nonbipolar de-pressions, one might speculate that bipolar depression could be treated with cognitive behavior therapy or inter-personal therapy with 40%– 60% response rates across 8 to 12 weeks. Moreover, there should be minimal risk of treatment-emergent mania in addition to sparing of med-ication side effects. Although reasonable, these specula-tions remain just that, and it may take a number of years before the indications for and limitations of psychosocial treatments of bipolar depression are clarified. Several trials are underway, however, and preliminary data using these approaches are reviewed in the accompanying arti-cles by Frank et al (2000) and Miklowitz et al (2000).
Conclusions
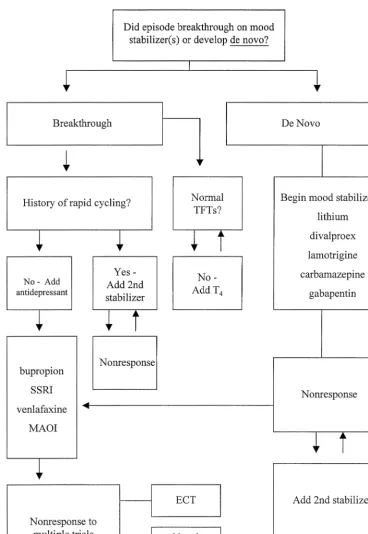
The profound suffering, disability, and mortality associ-ated with the depressed phase of bipolar disorder warrants a careful and systematic approach to therapeutics. The strategies recommended herein for treatment of bipolar I depression are largely consistent with recent expert-de-rived consensus guidelines and emphasize a “mood stabi-lizer first” approach to therapeutics (Figure 4). The via-bility of this approach is enhanced by the recent introduction of a number of pharmacologically distinct alternate mood stabilizers, although much work remains to be done to establish the efficacy of and indications for agents such as lamotrigine, gabapentin, and topiramate. Nonpharmacologic strategies, including phototherapy and various psychotherapies, provide yet other alternatives to enhance therapy with mood stabilizers.
Although this approach may strike some as a variant of pharmacologic Calvinism, it must be recognized that the empiric basis for use of antidepressant medications in bipolar depression is woefully inadequate. As the antide-pressant era enters its fifth decade, no antideantide-pressant medication has been established to be effective in bipolar depression by at least two positive, placebo-controlled clinical trials. For many compounds, not a single well-controlled trial has been conducted with bipolar patients. When antidepressants must be used in bipolar depression, bupropion and the SSRIs receive highest ranking and venlafaxine, MAOIs, and ECT are preferred for patients who do not respond to initial strategies. The addition of antipsychotic medications, particularly the newer “atypi-cal” agents and ECT also provide important alternatives for bipolar patients with psychotic depressions.
Preparation of this report was supported in part by Grants Nos. 1N01 MH-80001-01 and MH-30915 (Mental Health Intervention Research Center).
Aspects of this work were presented at the conference “Bipolar Disorder: From Pre-Clinical to Clinical, Facing the New Millennium,” January 19 –21, 2000, Scottsdale, Arizona. The conference was spon-sored by the Society of Biological Psychiatry through an unrestricted educational grant provided by Eli Lilly and Company.
References
Akiskal HS (1996): The prevalent clinical spectrum of bipolar disorders: Beyond DSM-IV.J Clin Psychopharmacol16:4S– 14S.
depres-sive illness: Phenomenologic, familial, and pharmacologic predictors.J Affect Disord5:115–128.
Altshuler LL, Keck PE Jr, McElroy SL, Suppes T, Brown ES, Denicoff K, et al (1999): Gabapentin in the acute treatment of refractory bipolar disorder.Bipolar Disord1:61– 65. Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A,
Ackerman L (1995): Antidepressant-induced manic and cy-clic acceleration: A controversy revisited. Am J Psychiatry
152:1130 –1138.
American Psychiatric Association (1994): Practice guideline for the treatment of patients with bipolar disorder.Am J Psychi-atry151:1–35.
Amsterdam JD, Garcia-Espana F, Fawcett J, Quitkin FM, Reim-herr FW, Rosenbaum JF, et al (1998a): Efficacy and safety of fluoxetine in treating bipolar II major depressive episode.
J Clin Psychopharmacol18:435– 440.
Amsterdam JD, Hooper MB, Amchin J (1998b): Once- versus twice-daily venlafaxine therapy in major depression: A ran-domized double-blind study.J Clin Psychiatry59:236 –240. Angst J, Amrein R, Stabl M (1995): Moclobemide and tricyclic antidepressants in severe depression: Meta-analysis and pro-spective studies.J Clin Psychiatry15:16S–23S.
Angst J, Stable M (1992): Efficacy of moclobemide in different patient groups: A meta-analysis of studies. Psychopharma-cology106:S109 –S113.
Aronson R, Offman HJ, Joffe RT, Naylor D (1996): Triiodothy-ronine augmentation in the treatment of refractory depres-sion—A meta-analysis.Arch Gen Psychiatry53:842– 848. Ascher JA, Cole JO, Colin J-N, Feighner JP, Ferris RM, Fibiger
JC, et al (1995): Bupropion: A review of its mechanism of antidepressant activity.J Clin Psychiatry56:395– 401. Baldessarini RJ, Tondo L, Hennen J (1999): Effects of lithium
treatment and its discontinuation on suicidal behaviour in bipolar manic-depressive disorders.J Clin Psychiatry60:77– 84.
Basco MR, Rush AJ (1996).Cognitive-Behavioral Therapy for Bipolar Disorder.New York: Guilford.
Bauer MS, Calabrese JC, Dunner DL, Post R, Whybrow PC, Gyulai L, et al (1994): Multisite data reanalysis of the validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV.Am J Psychiatry151:506 –515.
Bauer M, Dopfmer S (1999): Lithium augmentation in treatment-resistant depression: Meta-analysis of placebo-controlled studies.J Clin Psychopharmacol19:427– 434.
Bauer MS, McBride L, Chase C, Sachs G, Shea N (1998): Manual-based group psychotherapy for bipolar disorder: A feasibility study.J Clin Psychiatry59:449 – 455.
Bauer MS, Whybrow PC (1990): Rapid cycling bipolar affective disorder II. Treatment of refractory rapid cycling with high-dose levothyroxine: A preliminary study.Arch Gen Psychia-try47:435– 440.
Beasley CM, Masica DN, Heiligenstein JH, Wheadon DE, Zerbe RL (1993): Possible monoamine oxidase inhibitor-serotonin reuptake inhibitor interaction: Fluoxetine clinical data and preclinical findings.J Clin Psychopharmacol13:312–320. Bowden CL (1999): “A two-illness model of bipolar
disor-der”— by RT Joffe, LT Young, and GM MacQueen: A commentary.Bipolar Disord1:31–35.
Bowden CL, Calabrese JR, McElroy SL, Rhodes LJ, Keck PE Jr,
Cookson J, et al (1999): The efficacy of lamotrigine in rapid cycling and non-rapid cycling patients with bipolar disorder.
Biol Psychiatry45:953–958.
Bowden CL, Janicak PG, Orsulak P, Swann AC, Davis JM, Calabrese JR, et al (1996): Relation of serum valproate concentration to response in mania. Am J Psychiatry 153: 765–770.
Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monoaghan E, Rudd GD (1999): A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depres-sion. Lamictal 602 Study Group.J Clin Psychiatry60:79 – 88. Calabrese JR, Woysheville MJ, Kasimond SE, Rapport DJ (1993): Predictors of valproate response in bipolar rapid cycling.J Clin Psychopharmacol13:280 –283.
Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH (1985): Somatic symptoms in primary affective disorder.Arch Gen Psychiatry42:1098 –1104.
Clerc GE, Ruimy P, Verdeau-Paille`s J (1994): A double-blind comparison of venlafaxine and fluoxetine in patients hospi-talized for major depression and melancholia. The Venlafax-ine French Inpatient Study Group.Int Clin Psychopharmacol
9:139 –143.
Cohn JB, Collins G, Ashbrook E, Wernicke JF (1989): A comparison of fluoxetine, imipramine, and placebo in patients with bipolar depressive disorder. Int Clin Psychopharmacol
4:313–322.
Coryell W, Andreason NC, Endicott J, Keller M (1987): The significance of past mania or hypomania in the course and outcome of major depression.Am J Psychiatry144:309 –315. Danish University Antidepressant Group (1986): Citalopram: Clinical effect profile in comparison with clomipramine. A controlled multicenter study. Psychopharmacology90:131– 138.
Danish University Antidepressant Group (1990): Paroxetine: A selective serotonin reuptake inhibitor showing better toler-ance but weaker antidepressant effect than clomipramine in a controlled multicenter study.J Affect Disord18:289 –299. Danish University Antidepressant Group (1993): Moclobemide:
A reversible MAO-A-inhibitor showing weaker antidepres-sant effect than clomipramine in a controlled multicenter study.J Affect Disord28:105–116.
Davis LL, Kabel D, Patel D, Chaote AD, Foslien-Nash C, Gurguis GNM, et al (1996): Valproate as an antidepressant in major depressive disorder. Psychopharmacol Bull 32:647– 652.
Denicoff KD, Smith-Jackson EE, Disney ER, Ali SO, Leverich GS, Post RM (1997): Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar dis-order.J Clin Psychiatry58:470 – 478.
Dierick M, Ravizza L, Realini R, Martin A (1996): A double-blind comparison of venlafaxine and fluoxetine for treatment of major depression in outpatients. Prog Neuropsychophar-macol Biol Psychiatry20:57–71.
Dunner DL (1993): A review of the diagnostic status of “Bipolar II” for the DSM-IV; work group on mood disorders. Depres-sion1:2–10.
(1997): Emergence of adverse events following discontinua-tion of treatment with extended-release venlafaxine. Am J Psychiatry154:1760 –1762.
Feighner JP, Gardner EA, Johnston A, Batey SR, Khayrallah MA, Ascher JA, Lineberry CG (1991): Double-blind com-parison of bupropion and fluoxetine in depressed outpatients.
J Clin Psychiatry52:329 –335.
Fogelson DL, Bystritsky A, Pasnau R (1992): Bupropion in the treatment of bipolar disorders: The same old story? J Clin Psychiatry53:443– 446.
Frances A, Docherty JP, Kahn DA (1996): The expert consensus guideline series. Treatment of bipolar disorder. J Clin Psy-chiatry57:1– 88.
Frank E, Kupfer DJ, Ehlers CL, Monk TH, Cornes C, Carter S, et al (1994): Interpersonal and social rhythm therapy for bipolar disorder: Integrating interpersonal and behavioral approaches.Behav Ther17:143–149.
Frank E, Swartz HA, Kupfer DJ (2000): Interpersonal and social rhythm therapy: Managing the chaos of bipolar disorder.Biol Psychiatry48:593– 604.
Frye MA, Altshuler LL, Szuba MP, Finch NN, Mintz J (1996): The relationship between antimanic agent for treatment of classic or dysphoric mania and length of hospital stay.J Clin Psychiatry57:17–21.
George MS, Wasserman EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, et al (1997): Mood improvement follow-ing daily left prefrontal repetitive transcranial magnetic stim-ulation in patients with depression: A placebo-controlled crossover trial.Am J Psychiatry154:1752–1756.
Ghaemi SN, Katzow JJ, Desai SP, Goodwin FK (1998): Gaba-pentin treatment of mood disorders: A preliminary study.
J Clin Psychiatry59:426 – 429.
Ghaemi SN, Sachs GS (1997): Long-term risperidone treatment in bipolar disorder: 6-month follow up.Int Clin Psychophar-macol12:333–338.
Goldberg JF, Kocsis JH (1999): Depression in the course of bipolar disorder. In: Goldberg JF, Harrow M, editors.Bipolar Disorders. Clinical Course and Outcome.Washington, DC: American Psychiatric Press, 129 –147.
Golden RN, DeVane CL, Laizure SC, Rudorfer MV, Sherer MA, Potter WZ (1988): Bupropion in depression. II. The role of metabolites in clinical outcome. Arch Gen Psychiatry 45: 145–149.
Goodnick PJ, Dominguez RA, DeVane L, Bowden CL (1998): Bupropion slow-release response in depression: Diagnosis and biochemistry.Biol Psychiatry44:629 – 632.
Goodwin FK, Jamison KR (1990): Manic-Depressive Illness.
New York: Oxford University Press.
Goodwin FK, Murphy DL, Dunner DL, Bunney WE Jr (1972): Lithium response in unipolar versus bipolar depression.Am J Psychiatry129:44 – 47.
Griest JH, Jefferson JW, Kobak KA, Katzelnick DJ, Serlin RC (1995): Efficiency and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. Arch Gen Psy-chiatry52:53– 60.
Harvey AT, Rudolph RL, Preskorn SH (2000): Evidence of the dual mechanisms of action of venlafaxine.Arch Gen Psychi-atry57:503–509.
Haykal RF, Akiskal HS (1990): Bupropion as a promising
approach to rapid cycling bipolar II patients.J Clin Psychi-atry51:450 – 455.
Himmelhoch JM, Detre J, Kupfer DJ, Swartzburg M, Byck R (1972): Treatment of previously intractable depression with tranylcypromine and lithium.J Nerv Ment Dis155:216 –220. Himmelhoch JM, Fuchs CZ, Symons BJ (1982): A double-blind study of tranylcypromine treatment of major anergic depres-sion.J Nerv Ment Dis170:628 – 634.
Himmelhoch JM, Thase ME, Mallinger AG, Houck P (1991): Tranylcypromine versus imipramine in anergic bipolar de-pression.Am J Psychiatry148:910 –916.
Joffe RT, Singer W (1990): A comparison of triiodothyronine and thyroxine in the potentiation of tricyclic antidepressants.
Psychiatry Res32:241–251.
Joffe RT, Young LT, MacQueen GM (1999): A two-illness model of bipolar disorder.Bipolar Disord1:25–30. Johnston JA, Lineberry CG, Ascher JA, Davidson J, Khayrallah
MA, Feighner JP, et al (1991): A 102-center prospective study of seizure in association with bupropion. J Clin Psy-chiatry52:450 – 456.
Kavoussi RJ, Segraves RT, Hughes AR, Ascher JA, Johnston JA (1997): Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients.J Clin Psychi-atry58:532–537.
Keck PE Jr, McElroy SL, Strakowski SM (1998): Anticonvul-sants and antipsychotics in the treatment of bipolar disorder.
J Clin Psychiatry59:74 – 81.
Kennedy SH, Eisfeld BS, Dickens SE, Bacchiochi JR, Bagby RM (2000): Antidepressant-induced sexual dysfunction dur-ing treatment with moclobemide, paroxetine, sertraline, and venlafaxine.J Clin Psychiatry61:276 –281.
Ketter TA, Post RM, Parekh PI, Worthington K (1995): Addition of monoamine oxidase inhibitors to carbamazepine: Prelimi-nary evidence of safety and antidepressant efficacy in treat-ment-resistant depression.J Clin Psychiatry56:471– 475. Koukopoulos A, Koukopoulos A (1999): Agitated depression as
a mixed state and the problem of melancholia.Psychiatr Clin North Am22:547–564.
Kraepelin E (1921): Manic-Depressive Insanity and Paranoia.
Edinburgh: E&S Livingston.
Lambert PA (1984): Acute and prophylactic therapies of patients with affective disorders using valpromide (dipropylacet-amide). In: Emrich HM, Okuma T, Muller AA, editors.
Anticonvulsants in Affective Disorders.Amsterdam: Excerpta Medica, 33–34.
Larsen JK (1991). MAOIs in the treatment of depression. A review.Eur J Psychiatry5:79 – 88.
Leibenluft E (1996): Women with bipolar illness: Clinical and research issues.Am J Psychiatry152:163–173.
Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RMA (1994): The National Depressive and Manic-Depres-sive Association (DMDA) survey of bipolar members. J Affect Disord31:283–294.
Lotufo-Neto F, Trivedi M, Thase ME (1999): Metaanalysis of the reversible inhibitors of monoamine oxidase type A mo-clobemide and brofaromine in the treatment of depression.
Neuropsychopharmacology20:226 –247.
(1999, June): Effectiveness of traditional antidepressants is suboptimal in the depressed phase of bipolar disorder. Poster presented at the annual meeting of the New Clinical Drug Evaluation Unit Program, Boca Raton, FL.
Massana J, Moller H, Burrows GD, Montenegro RM (1999): Reboxetine: A double-blind comparison with fluoxetine in major depressive disorder.Int Clin Psychopharmacol14:73– 80.
McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI (1988): Valproate in the treatment of rapid-cycling bipolar disorder.
J Clin Psychopharmacol8:275–279.
Messenheimer J, Mullens EL, Giorgi L, Young F (1998): Safety review of adult clinical trial experience with lamotrigine.
Drug Saf18:281–296.
Miklowitz DJ, Goldstein MJ (1997):Bipolar Disorder. A Fam-ily-Focused Treatment Approach.New York: Guilford. Miklowitz DJ, Simoneau TL, George EL, Richards JA, Kalbag
A, Sachs-Ericsson N, Suddath R (2000): Family-focused treatment of bipolar disorder: 1-year effects of a psychoedu-cational program in conjunction with pharmacotherapy.Biol Psychiatry48:582–592.
Mitchell P, Parker G, Jamieson K, Wilhelm K, Hickie I, Brodaty H, et al (1992): Are there any differences between bipolar and unipolar melancholia?J Affect Disord25:97–106.
Montgomery SA, Reimitz P-E, Zivkov M (1998): Mirtazapine versus amitriptyline in the long-term treatment of depression: A double-blind placebo-controlled study. Int Clin Psycho-pharmacol13:63–73.
Nahas Z, Molloy MA, Hughes PL, Oliver NC, Arana GW, Risch SC, et al (1999): Repetitive transcranial magnetic stimulation: Perspectives for application in the treatment of bipolar and unipolar disorders.Bipolar Disord2:73– 80.
Nemeroff CB, DeVane CL, Pollock BG (1996): Newer antide-pressants and the cytochrome P450 system.Am J Psychiatry
153:311–320.
Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergl I, et al (in press): A double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression.Am J Psychiatry.
Nolen WA, Van de Putte JJ, Dijken WA, Kamp JS, Blansjaar BA, Kramer HJ, et al (1988): Treatment strategy in depres-sion: I. Nontricyclic and selective reuptake inhibitors in resistant depression: A double-blind partial crossover study on the effects of oxaprotiline and fluvoxamine.Acta Psychi-atr Scand78:668 – 675.
Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G, Gabapentin Bipolar Disorders Study Group (in press): Gaba-pentin in bipolar disorder: A placebo-controlled trial of adjunctive therapy.Bipolar Disord.
Pande AC, Davidson JRT, Jefferson JW, Janney CA, Katzelnick DJ, Weisler RH, et al (1999): Treatment of social phobia with gabapentin: A placebo-controlled study.J Clin Psychophar-macol19:341–348.
Pazzaglia PJ, Post RM, Ketter TA, Callahan AM, Marangell LB, Frye MA, et al (1998): Nimodipine monotherapy and carba-mazepine augmentation in patients with refractory recurrent recurrent affective illness.J Clin Psychopharmacol18:404 – 413.
Peet M (1994): Induction of mania with selective serotonin
re-uptake inhibitors and tricyclic antidepressants.Br J Psy-chiatry164:549 –550.
Perris C (1966): A study of bipolar (manic-depressive) and unipolar recurrent depressive psychoses. Acta Psychiatr Scand42:1–188.
Petty F (1995): GABA and mood disorders: A brief review and hypothesis.J Affect Disord34:275–281.
Poirier M-F, Boyer P (1999): Venlafaxine and paroxetine in treatment-resistant depression. Double-blind, randomised comparison.Br J Psychiatry174:12–16.
Post RM (2000): Mood disorders: Treatment of bipolar disorders. In: Sadock BJ, Sadock VA, editors. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry,7th ed. New York: Williams & Wilkins, 1385–1430.
Potter WZ, Murphy DL, Wehr TA, Linnoila M, Goodwin FK (1982): Clorgyline. A new treatment for patients with refrac-tory rapid-cycling disorder. Arch Gen Psychiatry 39:505– 510.
Preskorn SH (1995): Comparison of the tolerability of bupro-pion, fluoxetine, imipramine, nefazodone, paroxetine, sertra-line, and venlafaxine.J Clin Psychiatry56:12–21.
Prien RF, Kupfer DJ (1986): Continuation drug therapy for major depressive episodes: How long should it be maintained?Am J Psychiatry143:18 –23.
Prudic J, Sackeim H, Devanand DP (1990): Medication resis-tance and clinical response to ECT.Psychiatry Res31:287– 296.
Quitkin FM, Stewart JW, McGrath PJ, Tricamo E, Rabkin JG, Ocepek-Welikson K, et al (1993): Columbia atypical depres-sion. A subgroup of depressives with better resopnse to MAOI than to tricyclic antidepressants or placebo. Br J Psychiatry163:30 –34.
Rush AJ, Armitage R, Gillin JC, Yonkers KA, Winokur A, Moldofsky H, et al (1998): Comparative effects of nefaz-odone and fluoxetine on sleep in outpatients with major depressive disorder.Biol Psychiatry44:3–14.
Sachs GS (1996): Treatment-resistant bipolar depression. Psy-chiatr Clin North Am19:215–236.
Sachs GS, Lafer B, Stoll AL, Banov M, Thibault AB, Tohen M, Rosenbaum JF (1994): A double-blind trial of bupropion versus desipramine for bipolar depression.J Clin Psychiatry
55:391–393.
Sachs GS, Printz DJ, Kahn DA, Carpenter D, Docherty JP (2000): The Expert Consensus Guideline Series: Medication treatment of bipolar disorder.Postgrad MedApril: 1–104. Sachs GS, Thase M (2000a): Bipolar disorder therapeutics:
Maintenance treatment.Biol Psychiatry48:573–581. Sachs GS, Thase ME (2000b):Bipolar Disorder. A Systematic
Approach to Treatment.London: Martin Dunitz.
Sackeim HA, Prudic J, Devanand DP, Kiersky JE, Fitzsimons L, Moody BJ, et al (1993): Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy.N Engl J Med328:839 – 846. Salloum IM, Thase ME (in press): Impact of substance abuse on
the course and treatment of bipolar disorder.Bipolar Disord.
Shopsin B (1983): Bupropion’s prophylactic efficacy in bipolar affective illness.J Clin Psychiatry44:163–169.
Simpson SG, DePaulo JR (1991): Fluoxetine treatment of bipolar II depression.J Clin Psychopharmacol11:52–54.
Small JG, Klapper MH, Kellams JJ, Miller MJ, Milstein V, Sharpley PH, et al (1988): Electroconvulsive treatment com-pared with lithium in the management of manic states.Arch Gen Psychiatry45:727–732.
Srisurapanont M, Yatham LN, Zis AP (1995): Treatment of acute bipolar depression: A review of the literature.Can J Psychi-atry40:533–544.
Suppes T, Webb A, Paul B, Carmody T, Kraemer H, Rush AJ (1999): Clinical outcomes in a randomized 1-year trial of clozapine versus treatment as usual for patients with treat-ment-resistant illness and a history of mania.Am J Psychiatry
156:1164 –1169.
Swann AC, Bowden CL, Morris D, Calabrese JR, Petty F, Small J, et al (1997): Depression during mania. Treatment response to lithium or divalproex.Arch Gen Psychiatry54:37– 42. Terman M, Terman JS (1999): Bright light therapy: Side effects
and benefits across the symptom spectrum.J Clin Psychiatry
60:799 – 808.
Thase ME (1997): Do we really need all these new antidepres-sants? Weighing the options. J Pract Psychiatry Behav Health3:3–17.
Thase ME (1998): Effects of venlafaxine on blood pressure: A meta-analysis of original data from 3744 depressed patients.
J Clin Psychiatry59:502–508.
Thase ME (1999): Antidepressant treatment of the depressed patient with insomnia.J Clin Psychiatry60:28 –31. Thase ME (2000): Modulation of biological factors by
psycho-therapeutic interventions in bipolar disorder. In: Soares JC, Gershon S, editors.Basic Mechanisms and Therapeutic Im-plications of Bipolar Disorder. New York: Marcel Dekker, 373–385.
Thase ME, Frank E, Mallinger A, Hamer T, Kupfer DJ (1992a): Treatment of imipramine-resistant recurrent depression: III. Efficacy of monoamine oxidase inhibitors.J Clin Psychiatry
53:5–11.
Thase ME, Mallinger AG, McKnight D, Himmelhoch JM
(1992b): Treatment of imipramine resistant recurrent depres-sion: IV. A double-blind, crossover study of tranylcypromine in anergic bipolar depression.Am J Psychiatry149:195–198. Thase ME, Nolen W (2000): Tricyclic antidepressant and irre-versible monoamine oxidase inhibitors. In: Buckley P, Wad-dington J, editors. Schizophrenia and Mood Disorders: The New Drug Therapies in Clinical Practice. Oxford, UK: Butterworth Heinemann, 85–99.
Thase ME, Rush AJ (1997): When at first you don’t succeed . . . sequential strategies for antidepressant nonresponders.J Clin Psychiatry58(suppl 13):23–29.
Thase ME, Trivedi MH, Rush AJ (1995): MAOIs in the contemporary treatment of depression. Neuropsychopharma-cology12:185–219.
Tohen M, Sanger TM, McElroy SL, Tollefson GD, Chengappa KNR, Daniel DG, et al (1999): Olanzapine versus placebo in the treatment of acute mania.Am J Psychiatry156:702–709. Vink J, Nolen WA, Verbraak M (1994): Is moclobemide bij therapieresistente depressie een alternatief voor tranylcypro-mine? Enige ervaringen.Tijdschr Psychiatrie36:639 – 646. Wehr TA, Sack DA, Rosenthal NE (1987): Sleep reduction as a
final common pathway in the genesis of mania. Am J Psychiatry144:542.
Weissman MM, Prusoff BA, Merikangas KR (1984): Is delu-sional depression related to bipolar disorder?Am J Psychiatry
141:892– 892.
Wright G, Galloway L, Kim J, Dalton M, Miller L, Stern W (1985): Bupropion in the long-term treatment of cyclic mood disorders: Mood stabilizing effects.J Clin Psychiatry46:22– 25.
Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I (2000): Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression.Am J Psychiatry157:124 –126.
Young LT, Robb JC, Patellis-Siotis I, MacDonald C, Joffe RT (1997): Acute treatment of bipolar depression with gabapen-tin.Biol Psychiatry42:851– 853.