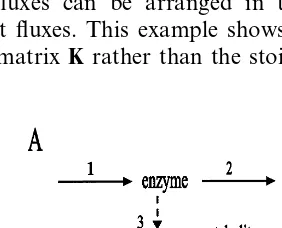Cellular information transfer regarded from a stoichiometry
and control analysis perspective
Stefan Schuster
a,*, Boris N. Kholodenko
b, Hans V. Westerhoff
c,daDepartment of Bioinformatics,Max Delbru¨ck Center for Molecular Medicine,D-13092Berlin-Buch,Germany bDepartment of Pathology,Anatomy and Cell Biology,Thomas Jefferson Uni6ersity,Philadelphia PA19107-6799,USA
cDepartment of Molecular Cell Physiology,Vrije Uni6ersiteit Amsterdam,De Boelelaan1087,
NL-1081HV,Amsterdam,The Netherlands
dE.C.Slater Institute,Uni6ersity of Amsterdam,Plantage Muidergracht12,NL-1018TV Amsterdam,The Netherlands
Abstract
Metabolic control analysis (MCA) allows one to formalize important aspects of information processing in living cells. For example, information processing via multi-level enzyme cascades can be quantified in terms of the response coefficient of a cellular target to a signal. In many situations, control and response coefficients cannot be determined exactly for all enzymes involved, owing to difficulties in ‘observing’ all enzymes experimentally. Here, we review a number of qualitative approaches that were developed to cope with such situations. The usefulness of the concept of null-space of the stoichiometry matrix for analysing the structure of intracellular signaling networks is discussed. It is shown that signal transduction operates very efficiently when the network structure is such that the null-space matrix can be block-diagonalized (which may or may not imply that the network consists of several disconnected parts) and some enzymes have low elasticities to their substrates. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Enzyme cascades; Fructose-2,6-bisphosphate cycle; Metabolic control analysis; Response coefficients; Signal transduction; Stoichiometric analysis
www.elsevier.com/locate/biosystems
1. Introduction
The storage and processing of information is a striking feature of living organisms. To describe and analyze this feature is one of the major challenges in modern biology. Although powerful
theoretical tools for this purpose such as Shan-non’s information theory exist, the specific prop-erties of biological systems such as their network structure and hierarchic and spatial organization cannot completely be covered by these tools so far (Bray, 1995; Fisher et al., 1999). A multitude of intracellular and intercellular signaling pathways and networks have been identified. Part of these pathways is made up of enzyme cascades such as the glutamine synthetase cascade (Jaggi et al., 1997) and the glycogen-phosphorylase/glycogen-* Corresponding author. Tel.:+49-30-94063125; fax:+
49-30-94062834.
E-mail address:[email protected] (S. Schus-ter)
synthase system (Chock et al., 1980). In recent years, the mitogen-activated protein kinase (MAPK) signaling pathway has been intensely investigated (Hsueh and Law, 1999; Bornfeldt and Krebs, 1999). The interconnection of several path-ways has also been studied (Bray, 1995). Al-though these structures have a number of similarities with metabolic pathways and net-works, there is an essential difference in that there is no or only a very small mass flow between the components of a signaling network.
In living cells, information is frequently pro-cessed in hierarchic systems. In gene expression, for example, we can discern the levels of DNA, mRNA, and proteins. Between the levels of such hierarchies, there is flow of information, but again usually no mass flow. In the present paper, we shall analyze what topological and kinetic proper-ties a system should have so that information can be transmitted efficiently without or with only a small concomitant mass flow.
The understanding of the regulatory properties of biochemical systems has been greatly improved by Metabolic Control Analysis (MCA). This is a theoretical framework through which the effect of changes in enzyme activity on the fluxes, concen-trations and other relevant variables characteriz-ing biochemical systems at steady state can be determined (for review see Westerhoff et al., 1995; Heinrich and Schuster, 1996; Fell, 1997). The largest part of the theory so far developed in MCA, concerns systems in which the substances are connected by mass flow (e.g. Westerhoff and Van Dam, 1987; Reder, 1988; Brown et al., 1990; Kholodenko et al., 1998; Schuster and Wester-hoff, 1999). However, also enzyme cascades and other hierarchic systems that are capable of signal transfer have been analyzed (Small and Fell, 1990; Kahn and Westerhoff, 1991; Kholodenko et al., 1997). In the present paper, we will elaborate on the question as to whether information flow can be dealt with by MCA. Moreover, we will study the specific topological properties of cellular sig-naling systems. Special attention will be paid to the fact that knowledge of kinetic parameters of all the enzymes involved in the network is often incomplete.
2. Metabolic control analysis in the case of imperfect knowledge of kinetic parameters
One of the major achievements of MCA is the derivation of formulas by which the systemic con-trol properties of a network can be derived from its constituent properties and its structure. In the absence of conservation relations, these formulae can be written in matrix form (Reder, 1988; Hein-rich and Schuster, 1996):
CJ=(dg
J)−1
I−(v (S
N(v (S
−1
N
n
(dgJ), (1)CS= −(dgS)−1(v
(S
N (v (S−1
N(dgJ) (2)
where CJ
, CS
, and N denote the matrices of flux control coefficients and concentration control co-efficients and the stoichiometry matrix. I and #v/#Sstand for the identity matrix and the matrix of non-normalized elasticities, that is, the deriva-tives of reaction rates with respect to concentra-tions. J denotes the vector of steady state fluxes. It fulfills the steady-state equation
N J=0. (3)
If the system involves conservation relations for concentrations, Eqs. (1) and (2) must be slightly modified (Reder, 1988; Heinrich and Schuster, 1996). The elasticities contain information about the kinetic properties of the enzymes involved in the system, while the information about the struc-ture of the biochemical system in terms of how substances are ‘connected’ to each other by reac-tions, is encompassed in the stoichiometry matrix
N.
properties in such situations. In the top-down approach (Brown et al., 1990; Brand, 1996) and modular approach (Westerhoff and Van Dam, 1987; Schuster et al., 1993), enzymes are grouped into blocks so that it is no longer necessary to know all the details within the blocks. The kinetic properties of the blocks and the control properties of the system are described by the overall elastic-ities and control coefficients, respectively. More-over, the modular approach is a suitable tool for describing the control properties of those enzymes that couple exergonic processes to endergonic processes, such as the various ATPases (Schuster and Westerhoff, 1999). Appropriate linear combi-nation of the overall control coefficients gives the coefficients quantifying the control exerted by the enzymes as a whole and the control exerted by the slip.
Kinetic parameters are often difficult to mea-sure for very fast reactions. In this situation, it is often justified to assume these reactions to attain quasi-equilibrium. We were able to show in a general way that quasi-equilibrium enzymes can be eliminated from the analysis of the control properties, allowing the calculation of the control coefficients of the slow reactions even if the ki-netic properties of the fast reactions are unknown (Kholodenko et al., 1998).
3. Topological analysis
A number of interesting conclusions can be derived from knowledge of the stoichiometric structure of a reaction system alone. For example, one can analyze the so-called null-space of the stoichiometry matrix. This is the region in which all flux vectors J in steady-state must be situated (Reder, 1988; Schuster and Schuster, 1991). A set of basis vectors to this space can be compiled as column vectors in a matrix K, which then fulfills the equation
N K=0. (4)
It can now be tested whether this null-space matrix Kcan be block-diagonalized
K=
Á Ã Ã Ã Ã Ã Ä
K1 0 ··· 0
0 K2 ··· 0
· · ·
0 0 ··· Kr
0 0 ··· 0
 à à à à à Å
, (5)
where the null rows of K (if any) have been transferred to the bottom of K. The steady-state flux through the reactions corresponding to such null rows is always zero. The blocks of K in Eq. (5), denoted by Ki, correspond to subsystems of the reaction system, the fluxes of which are com-pletely independent. This means that the fluxes within one subsystem can be changed by suitable parameter changes without affecting the fluxes in the other subsystems.
As any basis in linear algebra is non-unique, neither is the choice of the null-space matrix. A new basis can be formed by linear combination of basis vectors. Such a linear combination can, however, diminish the number of diagonal blocks. In the matrix given in Eq. (5), for example, replac-ing the first column (which then includes part of
K1) by a linear combination of this column and
the first column that includes part of K2 would
lead to a matrix that can be decomposed intor-1
diagonal blocks only. The question arises as to how to find that representation of Kthat can be partitioned into the maximum number of diago-nal blocks. It was proven (Schuster and Schuster, 1991) that such a representation can be obtained by rearranging the rows and columns of the matrix
K=
K%I
, (6)which can be found by the Gaussian elimination method as applied to equation system Eq. (4).
Important examples of matricesKwith a block-diagonal structure are provided by the ubiquitous hierarchic systems in biology. Fig. 1A depicts such a system, for which the null-space matrix can be chosen so as to involve two diagonal blocks
K=
Á Ã Ã Ã Ä
1 0 1 0 0 1 0 1
 à à à Å
. (7)
In addition to intermediary metabolism and protein metabolism, more levels such as those of DNA and mRNA can be included. If reactions 1 (enzyme synthesis) and 2 (enzyme degradation) are both activated in such a way that the enzyme level remains constant, the flux through the upper level increases without any change in the flux through the lower level. Conversely, by changing the corresponding parameters in reactions 3 or 4 or both, the flux through the lower level can be changed without any change in the upper level.
Interestingly, the property of matrix K to be block-diagonal is retained even if the system is depicted on a more detailed level, as in Fig. 1B. In this representation, the stoichiometry matrix can no longer be block-diagonalized. The substances in Fig. 1B cannot be grouped in such a way that the concentration changes in each group are due to distinct chemical reactions, whereas the steady state fluxes can be arranged in two groups of distinct fluxes. This example shows that the null-space matrixKrather than the stoichiometry
ma-trix Nis the more accurate mathematical concept to analyze the subsystem structure.
4. Zero flux control
Another approach that is helpful in the situa-tion where kinetic parameters are incompletely known concerns the decision which enzymes can, or cannot at all, control which fluxes irrespective of the values of kinetic parameters. The situation where some flux cannot be controlled by some reaction may arise if
1. some reactions are irreversible,
2. some enzymes are saturated with their substrates,
3. some enzymes are very fast (quasi-equilibrium enzymes),
4. the topology of the network is such that some subsystems are independent of each other with respect to fluxes.
In the case of incomplete knowledge of kinetic parameters, often qualitative knowledge of whether or not a reaction rate depends on some concentration is available. This knowledge (which is, for example, given in most cases on the Boehringer chart, and nowadays in the several metabolic databases in the WWW) can be com-piled in a qualitative elasticity matrix,
oji
qual
=
0, if (6j/(Si=0 for any
admissible concentration vector
x, otherwise. (8)
From the knowledge of this matrix and the stoichiometry matrix, one can deduce which fluxes are unsusceptible to control by which enzymes (Schuster and Schuster, 1992; Heinrich and Schus-ter, 1996). In the above-mentioned case (d), the block-diagonal structure of the null-space matrix plays a central role. For example, in the system shown in Fig. 1A, reactions 3 and 4 do not exert any control on the flux through reactions 1 and 2. Such a situation has been called ‘dictatorial con-trol’ (Westerhoff et al., 1990). It can also occur in enzyme cascades (Fig. 2) if there is no feedback from a lower level to a higher level in the cascade (Kahn and Westerhoff, 1991). However, the con-trol need not be dictatorial in these situations: If
Í Á
Ä
Fig. 2. Multi-level enzyme cascade. Abbreviations: S, signal;Ei
andEi%, active and inactive forms, respectively, of the enzyme
at leveli;TandT%, active and inactive forms, respectively, of the target.
This matrix cannot be block-diagonalized, while the corresponding null-space matrix can. The latter has formally the same form as given in Eq. (7).
By the formalism developed in Schuster and Schuster (1992), it can be shown that reactions 3 and 4 would not exert any control on the flux through reactions 1 and 2 (which is, in fact, the glycolytic flux) if fructose-2,6-bisphosphate (F2,6P2) were no effector of enzymes 1 and 2.
However, F2,6P2is known to be a potent activator
of 6-phosphofructokinase in many cell types and to play an important role in the regulation of glycol-ysis (Yuan et al., 1990; Lefebvre et al., 1996). This will be analyzed in more detail at the end of the following section.
In a mutant where activation by F2,6P2is absent,
reactions 1 and 2 do exert a dictatorial control on the F2,6P2cycle irrespective of the values of kinetic
parameters. Note that the feature of flux control insusceptibility is asymmetric; it may happen that a subsystem 1 is able to control a subsystem 2 while the latter cannot control the former.
If not only information is available about which elasticities are zero and which are not, but also the signs of the elasticities are known, qualitative conclusions about the sign pattern of control coeffi-cients can be drawn (Lundy and Sen, 1995). Gen-erally, however, only the signs of a subset of the control coefficients can be determined in this situ-ation.
5. Control of signal transduction
Consider a multi-level enzyme cascade as shown in Fig. 2. An important quantity is here the response coefficient of the cellular target,T, to the signal, S:
RS T
=d lnT
d lnS. (10)
The signal may be a growth factor, hormone, cytokine or neurotransmitter.
When a single cascade module is considered ‘in isolation’, the response of a signaling protein in this module (Ei) to the immediately preceding module (Ei−1) is quantified as the intrinsic response
coeffi-cient: Fig. 3. Scheme of the fructose-2,6-bisphosphate cycle.
En-zymes: E1, phosphoglucoisomerase (EC 5.3.1.9); E2,
6-phos-phofructokinase (EC 2.7.1.11); E3, fructose-2,6-bisphosphate
2-phosphatase (EC 3.1.3.46); E4, 6-phosphofructo-2-kinase
(EC 2.7.1.105). Metabolites: F6P, fructose-6-phosphate; F2,6P2, fructose-2,6-bisphosphate.
the concentrations of enzyme-enzyme complexes forming in the catalytic cycles (similar to the complex ES in Fig. 1B) are so high that they cannot be neglected (enzyme sequestration), a lower level enzyme can control a higher level cycle (Schuster and Schuster, 1992).
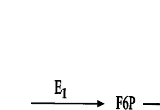
In hierarchical systems without feedback, dicta-torial control is intuitively clear. The situation is more complex in certain systems that are stoichio-metrically connected. Consider the example of the fructose-2,6-bisphosphate cycle (Fig. 3), which is a frequently present futile cycle (Yuan et al., 1990; Lefebvre et al., 1996). The stoichiometry matrix to this scheme reads
N=
1 −1 1 −1ri−1
For linear signal transduction pathways without feedback, it has been shown (Kholodenko et al., 1997) that the response coefficients of each cas-cade level multiply to give the total response of a target to a signal:
RS
Here, it is assumed that the sequestration of molecules at each level by the enzymes at the successive level can be neglected (Kholodenko et al., 1992). In the cases of branched pathways in the cascade and of pathways with feedback, ana-lytical formulas with a more complex structure can be derived (Kholodenko et al., 1997).
From Eq. (12), it can be seen that merely having more cascade levels greatly enhances the sensitivity of the target. As the elasticity coeffi-cient can be considered as a sort of reaction order, it can be seen that when each elasticity is larger than one, high overall reaction orders and, hence, a strong cooperativity, can be obtained (Brown et al., 1997).
The question arises as to how the local response depends on the kinetics of its own level. Let us consider a simple kinase/phosphatase cycle, where the phosphorylated form Ei is the active form, and the dephosphorylated form Ei% is inactive. Other forms of covalent enzyme modification such as acylation, adenylylation, or uridylylation can be described similarly. Kinase and phos-phatase reactions can often be assumed nearly irreversible, so that the kinase depends only on its substrate (Ei%) (and is catalysed by Ei−1) and the
phosphatase depends only on the form Ei. Thus, only the two elasticities oE
i’
kin and
oEi
phos are needed.
The following formula can be derived (Small and Fell, 1990; Kholodenko et al., 1997):
ri−1 When both protein kinase and phosphatase follow Michaelis – Menten kinetics (and are far from equilibrium and product insensitive), the elastic-ities read
Here KmKin and KmPhos are the respective Michaelis constants. If the kinase and phos-phatase are nearly saturated with their substrates (i.e. with the enzymes on the next lower level), the elasticities are very low. This implies that the local response coefficient is very high. Therefore, even a monocyclic cascade can constitute a highly effec-tive on/off switching device (Szedlacsek et al., 1992; Acerenza, 1996). This phenomenon is called ‘zero-order ultrasensitivity’ (Goldbeter and Koshland, 1984).
We will now show that similar considerations apply to stoichiometrically connected systems the null-space matrix of which can be block-diagonal-ized. In particular, we will again consider the F2,6P2 cycle (Fig. 3). Let S1 and S2 denote the
concentrations of F6P and F2,6P2. Furthermore, A is to denote the level of an effector of 6-phos-phofructo-2-kinase (E4). As most kinase and
phosphatase reactions, steps 3 and 4 in Fig. 3 can be assumed to be nearly irreversible. We shall also assume them to be product insensitive. Accord-ingly, the elasticities o31 ando42 can be neglected.
At steady state, we have
61(S1)=62(S1,S2), 63(S2)=64(S1,A). (15)
As this equation must still hold if the value of A
is changed, we can write, by the chain rule of differentiation
After normalization, these equations can be writ-ten as
S2 denote the response
RA
S1= o22p4A
o32(o11−o21)−o41o22
,
RA
S2= p4A(o11−o21)
o32(o11−o21)−o41o22
. (18)
These equations can also be derived from the block summation and block connectivity theo-rems established by Kahn and Westerhoff (1991). It has been shown that these theorems hold even if the system is stoichiometrically connected, pro-vided that the null-space matrix and link matrix (which expresses the conservation relations in the system) can be block-diagonalized (Heinrich and Schuster, 1996).
It can be seen from Eq. (18) that an extremely high response of F2,6P2(S2) can be achieved if the
two elasticities o32 and o41 are very low, that is, if
enzymes 3 and 4 are nearly saturated with their substrates. The effect of activating 6-phospho-fructo-2-kinase on the glycolytic flux, J2, can be quantified by a flux response coefficient,
RA J2=
o21RA
S1+
o22RA
S21= o21o22p4A
o32(o11−o21)−o41o22
.
(19)
Again, this effect is very high in the case of saturation of enzymes 3 and 4. Accordingly, the phenomenon of zero-order ultra-sensitivity can occur even in systems that do not have a cascade structure. An important difference to the cyclic systems studied earlier (Goldbeter and Koshland, 1984; Small and Fell, 1990; Kholodenko et al., 1997) is that one substance involved in the cycle (F6P in our case) is subject to turnover, so that the total amount of substance in the cycle is not conserved.
6. Discussion
Metabolic control analysis (MCA) helps us for-malize important aspects of signal transfer in living cells by providing us with clear-cut concepts (elasticity and control coefficients) to quantify the effect of a change in enzyme activity (triggered by a certain flow of information) on metabolite con-centrations, fluxes and other relevant variables. In particular, MCA allows one to quantify
informa-tion processing (e.g. via protein kinases and phos-phatases) in terms of the response coefficient of a cellular target to a signal (Kholodenko et al., 1997; Brown et al., 1997). For linear signal trans-duction pathways without feedback, the response coefficients of each cascade level multiply to give the total response of a target to a signal. There-fore, merely having more cascade levels enhances the sensitivity of the target towards agents that may act at the various levels. It has turned out that for the quantitative description of signal transfer processes, the concept of response coeffi-cient plays a special role (in comparison to the concept of flux control coefficient). This may be due to the fact that mass flow is limited in cascade systems.
Here, we have stressed the importance and usefulness of the concept of null-space of the stoichiometry matrix for analysing the structure of intracellular signaling networks. This concept had been used earlier in MCA (Reder, 1988; Kahn and Westerhoff, 1991) and has also turned out to be important in metabolic engineering (Schilling et al., 1999).
Enzyme cascades are prominent examples of systems in which information processing can suit-ably be performed because subsystems interact with each other without any mass flow between them. This absence of mass flow is economic in that it avoids loss of material. Mathematically, this feature is reflected by the block-diagonal structure of the null-space matrix. Moreover, we have focused on the case in which each enzyme catalysing a conversion in the next lower level is nearly saturated with its protein substrate, that is, with one of the enzyme forms on that level. This amounts to the assumption that the enzyme con-centrations increase downstream the cascade, as is often observed (Goldbeter and Koshland, 1984).
special structure allowing high response coeffi-cients is often linked with a limited control of subsystems on each other (Schuster and Schuster, 1992). These paradoxical, yet probably comple-mentary features are worth being studied in more detail.
As the F2,6P2 cycle considered above
demon-strates, a block-diagonal null-space matrix occurs not only in stoichiometrically disconnected sys-tems. We have here shown that also in connected networks, arbitrarily high response coefficients can be achieved if both product insensitivity and saturation occur in the system. This may be an explanation to the design of the F2,6P2 cycle,
which is at first sight surprising because F2,6P2
forms a sort of dead end in the network. Such a subsystem structure of a metabolic network ap-pears to be highly appropriate for regulation.
Both hierarchic systems and suitably designed non-hierarchic systems appear to play an impor-tant role in regulation in cellular physiology. Ob-viously, any regulation requires the transfer of information. We believe that elucidation of the relatedness of the concepts of regulation and sig-nal transfer deserves further work. It may be argued that information processing is often linked with amplification of signals and, hence, with high response coefficients. Large effects due to small causes occur in the expression of DNA (the re-placement of just one nucleotide may have large effects), in the action of hormones, enzymes (i.e. catalytic effects) and in other instances of biologi-cal information processing. Amplification is here not a contradiction of the situation that the effect may consist in decreasing (rather than increasing) a certain concentration (e.g. an enzyme level); both the absolute and the relative concentration change may still be much higher than the concen-tration change of the signaling substance initiating the effect.
The cycles constituting enzyme cascades as well as the F2,6P2 cycle are sometimes called futile
cycles because they are driven by a permanent hydrolysis of ATP. Another example of such a futile cycle is provided by the pumping of Ca2+
from the cytosol into the endoplasmic reticulum and the passive outflow that occurs in parallel. This cycle is of crucial importance in the
genera-tion of calcium oscillagenera-tions, which play a major role in intracellular signaling (Goldbeter, 1996). Further examples are the light-activated trans-ducin cycle and the activation/inactivation cycle of the G-protein (Stryer, 1995). However, as these cycles are important in regulation and informa-tion transfer, they are not really futile. The amount of ATP loss can in fact be kept quite low because it is the ratio between the activities of the two reactions in each cycle that is important for steady-state regulation rather than their absolute values. For example, the concentration of F2,6P2
is determined by the ratio of the activities of 6-phosphofructo-2-kinase and fructose-2,6-bis-phosphate 2-phosphatase. The absolute activities are only important for the response time.
If sufficient information about the kinetic prop-erties of the enzymes and non-enzymatic processes involved in signaling networks is available, dy-namic models of these networks can be estab-lished (Bhalla and Iyengar, 1999). Frequently, however, this knowledge is not available or in-complete. Therefore, we have here focused on a topological and control-analytical approach, which does not necessitate complete knowledge of kinetic parameters.
Acknowledgements
Financial support by the DFG (Germany), NWO (The Netherlands) and the NIH Grant AA11689 is gratefully acknowledged.
References
Acerenza, L., 1996. Sensitivity constraints in a chemical/ bio-chemical highly responsive system. BioSystems 39, 109 – 116.
Bhalla, U.S., Iyengar, R., 1999. Emergent properties of net-works of biological signaling pathways. Science 283, 381 – 387.
Bornfeldt, K.E., Krebs, E.G., 1999. Crosstalk between protein kinase A and growth factor receptor signaling pathways in arterial smooth muscle. Cell Signal 11, 465 – 477. Brand, M.D., 1996. Top down metabolic control analysis. J.
Theor. Biol. 182, 351 – 360.
Brown, G.C., Hafner, R.P., Brand, M.D., 1990. A ‘top-down’ approach to the determination of control coefficients in metabolic control theory. Eur. J. Biochem. 188, 321 – 325. Brown, G.C., Hoek, J.B., Kholodenko, B.N., 1997. Why do protein kinase cascades have more than one level? Trends Biochem. Sci. 22, 288.
Chock, P.B., Rhee, S.G., Stadtman, E.R., 1980. Interconvert-ible enzyme cascades in cellular regulation. Annu. Rev. Biochem. 49, 813 – 843.
Fell, D., 1997. Understanding the Control of Metabolism. Portland Press, London.
Fisher, M.J., Paton, R.C., Matsuno, K., 1999. Intracellular signalling proteins as ‘smart’ agents in parallel distributed processes. Biosystems 50, 159 – 171.
Goldbeter, A., 1996. Biochemical Oscillations and Cellular Rhythms. Cambridge University Press, Cambridge. Goldbeter, A., Koshland, D.E. Jr., 1984. Ultrasensitivity in
biochemical systems controlled by covalent modification. Interplay between zero-order and multistep effects. J. Biol. Chem. 259, 14441 – 14447.
Heinrich, R., Schuster, S., 1996. The Regulation of Cellular Systems. Chapman & Hall, New York.
Hsueh, W.A., Law, R.E., 1999. Insulin signaling in the arterial wall. Am. J. Cardiol. 84, 21J – 24J.
Jaggi, R., Van Heeswijk, W.C., Westerhoff, H.V., Ollis, D.L., Vasudevan, S.G., 1997. The two opposing activities of adenylyl transferase reside in distinct homologous do-mains, with intramolecular signal transduction. EMBO J. 16, 5562 – 5571.
Kahn, D., Westerhoff, H.V., 1991. Control theory of regula-tory cascades. J. Theor. Biol. 153, 255 – 285.
Kholodenko, B.N., Lyubarev, A.E., Kurganov, B.I., 1992. Control of the metabolic flux in a system with high enzyme concentrations and moiety-conserved cycles. The sum of the flux control coefficients can drop significantly below unity. Eur. J. Biochem. 210, 147 – 153.
Kholodenko, B.N., Hoek, J.B., Westerhoff, H.V., Brown, G.C., 1997. Quantification of information transfer via cel-lular signal transduction pathways. FEBS Lett. 414, 430 – 434.
Kholodenko, B.N., Schuster, S., Garcia, J., Westerhoff, H.V., Cascante, M., 1998. Control analysis of metabolic systems involving quasi-equilibrium reactions. Biochim. Biophys. Acta 1379, 337 – 352.
Lefebvre, V., Me´chin, M.-C., Louckx, M.P., Rider, M.H., Hue, L., 1996. Signaling pathway involved in the activa-tion of heart 6-phosphofructo-2-kinase by insulin. J. Biol. Chem. 271, 22289 – 22292.
Lundy, T., Sen, A.K., 1995. Sign pattern analysis of control coefficients of metabolic pathways. SIAM J. Matrix Anal. Appl. 16, 828 – 842.
Reder, C., 1988. Metabolic control theory: a structural ap-proach. J. Theor. Biol. 135, 175 – 201.
Schilling, C.H., Schuster, S., Palsson, B.O., Heinrich, R., 1999. Metabolic pathway analysis: basic concepts and scientific applications in the post-genomic era. Biotechnol. Prog. 15, 296 – 303.
Schuster, S., Hilgetag, C., 1994. On elementary flux modes in biochemical reaction systems at steady state. J. Biol. Syst. 2, 165 – 182.
Schuster, S., Schuster, R., 1991. Detecting strictly detailed balanced subnetworks in open chemical reaction networks. J. Math. Chem. 6, 17 – 40.
Schuster, S., Schuster, R., 1992. Decomposition of biochemical reaction systems according to flux control insusceptibility. J. Chim. Phys. 89, 1887 – 1910.
Schuster, S., Westerhoff, H.V., 1999. Modular control analysis of slipping enzymes. BioSystems 49, 1 – 15.
Schuster, S., Kahn, D., Westerhoff, H.V., 1993. Modular analysis of the control of complex metabolic pathways. Biophys. Chem. 48, 1 – 17.
Small, J.R., Fell, D.A., 1990. Covalent modification and metabolic control analysis. Modification to the theorems and their application to metabolic systems containing co-valently modifiable enzymes. Eur. J. Biochem. 191, 405 – 411.
Stryer, L., 1995. Biochemistry. Freeman, New York. Szedlacsek, S.E., Ca´rdenas, M.L., Cornish-Bowden, A., 1992.
Response coefficients of interconvertible enzyme cascades towards effectors that act on one or both modifier en-zymes. Eur. J. Biochem. 204, 807 – 813.
Westerhoff, H.V., Van Dam, K., 1987. Thermodynamics and Control of Biological Free-Energy Transduction. Elsevier, Amsterdam.
Westerhoff, H.V., Koster, J.G., Van Workum, M., Rudd, K.E., 1990. On the control of gene expression. In: Cornish-Bowden, A., Ca´rdenas, M.L. (Eds.), Control of Metabolic Processes. Plenum, New York, pp. 399 – 412.
Westerhoff, H.V., Kholodenko, B.N., Cascante, M., Van Dam, K., 1995. Elusive control. J. Bioenerg. Biomembr. 27, 491 – 497.
Yuan, Z., Medina, M.A., Boiteux, A., Mu¨ller, S.C., Hess, B., 1990. The role of fructose 2,6-bisphosphate in glycolytic oscillations in extracts and cells of Saccharomyces cere-6isiae. Eur. J. Biochem. 192, 791 – 795.