www.elsevier.com / locate / bres
Research report
Differential modulation of auditory thalamocortical and intracortical
synaptic transmission by cholinergic agonist
1 1
*
Candace Y. Hsieh , Scott J. Cruikshank , Raju Metherate
Department of Neurobiology and Behavior and Center for the Neurobiology of Learning and Memory, University of California, Irvine, CA92697-4550, USA
Accepted 25 July 2000
Abstract
To investigate synaptic mechanisms underlying information processing in auditory cortex, we examined cholinergic modulation of synaptic transmission in a novel slice preparation containing thalamocortical and intracortical inputs to mouse auditory cortex. Extracellular and intracellular recordings were made in cortical layer IV while alternately stimulating thalamocortical afferents (via medial geniculate or downstream subcortical stimulation) and intracortical afferents. Either subcortical or intracortical stimulation elicited a fast, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)-sensitive, monosynaptic EPSP followed by long-duration, polysynaptic activity. The cholinergic agonist carbachol suppressed each of the synaptic potentials to different degrees. At low concentrations (5mM) carbachol strongly reduced (.60%) the polysynaptic slow potentials for both pathways but did not affect the monosynaptic fast potentials. At higher doses (10–50mM), carbachol also reduced the fast potentials, but reduced the intracortically-elicited fast potential significantly more than the thalamocortically-elicited fast potential, which at times was actually enhanced. Atropine (0.5mM) blocked the effects of carbachol, indicating muscarinic receptor involvement. We conclude that muscarinic modulation can strongly suppress intracortical synaptic activity while exerting less suppression, or actually enhancing, thalamocortical inputs. Such differential actions imply that auditory information processing may favor sensory information relayed through the thalamus over ongoing cortical activity during periods of increased acetylcholine (ACh) release. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters and receptors
Topic: Acetylcholine
Keywords: Carbachol; Glutamate; EPSP; Neocortex; Mouse
1. Introduction among these is the projection from the cholinergic basal forebrain, the major source of ACh to the AC (reviewed by Cells of the auditory cortex (AC) receive sensory input [40,62]). The cholinergic system exerts powerful effects on from thalamic relay cells of the medial geniculate nucleus sensory cortex, influencing cellular excitability, cortical
(MG; extrinsic inputs), and from other cells within the state, and receptive field plasticity
cortex (intrinsic inputs) which themselves can receive [5,11,14,22,29,36,38,41,44,50]. In particular, application of either direct or indirect MG input ([8,45,46,55– cholinergic agonists or stimulation of the basal forebrain 57,60,72,75] and reviewed in [73,74]). These two sources can profoundly modulate cortical EPSPs in sensory cortex of input combine to produce sensory evoked responses both in vivo and in vitro [3,6,11,19,31,42,51].
observed in AC. While the influence of ACh on sensory cortical
re-The AC also receives non-sensory inputs that modulate sponses is undisputed, little is known regarding the degree evoked responses (see reviews by [34,35]). Prominent to which it regulates transmission coming from extrinsic (thalamocortical) vs. intrinsic (intracortical) sources. This may be surprising considering the importance of such
*Corresponding author. Tel.: 11-949-824-6141; fax: 1
1-949-824-knowledge for understanding cholinergic regulation of
2447.
sensation and perception. However, the acquisition of
E-mail address: [email protected] (R. Metherate). 1
These authors contributed equally to the work. relevant data has been hampered by several factors,
including the difficulty in conducting appropriate experi- mm) were taken from this area and maintained in a holding ments in vivo and the limited availability of in vitro chamber bubbled with 95% O / 5% CO at room tempera-2 2
preparations with intact thalamocortical connections [1,49]. ture. Recordings in an interface chamber (328C, Haas In one in vitro study which directly tests this issue, Gil et Model, Med. Systems, Greenvale, NY) followed an incu-al. [19] utilized a somatosensory thalamocortical slice to bation period of 1–2 h.
demonstrate that activation of muscarinic ACh receptors suppressed intracortical and thalamocortical EPSPs
simi-2.2. Electrophysiological stimulation and recording larly. In contrast to these findings, muscarinic actions in
hippocampus and piriform cortex result in a strong
sup-To activate extrinsic thalamic afferents and intrinsic pressive effect on intracortical synapses with little effect
cortical processes, electrical stimuli (200 ms, 5–100 mA) on responses to extrinsic inputs [24,26]. An important
were delivered via concentric bipolar electrodes (200 mm question that emerges from these studies is whether the
outer diameter; F. Haer) to MG, subcortical (SC) and contrasting findings reflect functional distinctions between
intracortical (IC) stimulation sites (illustrated in Fig. 1A). neocortex [19] vs. archicortex / paleocortex [24,26], or
Evoked extracellular field potentials and / or intracellular other, possibly methodological, differences. Indirect
evi-(whole-cell patch) synaptic potentials were recorded with dence addressing this issue comes from the recent work of
electrodes placed in cortical layer IV of AC. In some Kimura et al. [30]. They found that ACh suppressed
simultaneous extra- and intracellular recordings, one re-responses to white matter stimulation in upper and lower
cording electrode was placed in lower layer III or upper layers of visual cortex more than in the thalamo-recipient
layer V, and the other in layer IV. Extracellular microelec-middle layers. The authors propose that muscarinic
sup-trodes were pulled on a horizontal puller (P-97, Sutter pression of intracortical synapses is stronger than that of
Instruments Co., Novato, CA) and filled with ACSF. thalamocortical synapses. This would suggest that the
Intracellular patch pipettes (4–6 MV) were pulled with the distinction between neocortex and archicortex / paleocortex
same puller and filled with (in mM): KMeSO 130, NaCl3
(hypothesized above) may not be valid.
10, CaCl2 0.05, Na-GTP 0.5, Mg-ATP 2, HEPES 10, To address this issue, we have utilized a slice
prepara-EGTA 0.16. Neural signals were amplified (CyberAmp tion of the AC that, like the somatosensory thalamocortical
AI-401 and Axoclamp 2B, Axon Instruments, Foster City, slice used by Gil et al. [19], preserves both extrinsic inputs
CA), monitored on a digital oscilloscope (Tektronix, from the thalamus and intrinsic, long-range intracortical
Portland, OR), digitized at 5 kHz and stored on computer connections [43]. With this slice, we directly compare
(Apple PowerMac). Data acquisition was computer con-cholinergic modulation of thalamocortical and intracortical
trolled (AxoData, Axon Instruments) and analyzed off-line synaptic transmission. Portions of this work have appeared
(AxoGraph, Axon Instruments). in abstract form [28].
2.3. Carbachol protocol and receptor pharmacology
2. Material and methods
In most slices, responses were recorded while
alternat-2.1. Preparation of slices ing stimulation between SC and IC sites (interstimulus
interval515 or 30 s, so that each pathway was stimulated All procedures followed the University of California, at 30 or 60 s intervals). Following 10–20 min of stable Irvine, animal use regulation. Slices were taken from 15 to baseline responses, a 2 min bath-applied pulse of carbachol 43-day-old FVB mice and maintained in vitro (described was delivered and the time course of its effect on evoked by Metherate and Cruikshank [43]). Following decapita- responses was monitored for $30 min. The pulse was tion under halothane anesthesia, brains were rapidly re- limited to 2 min to prevent seizure-like activity that can moved (,60 s) and placed in 0–48C artificial cerebrospinal occur with longer application of carbachol. In some slices, fluid (ACSF) containing (in mM): NaCl 125, KCl 2.5, after a complete recovery was observed, the process was KH PO 1.25, NaHCO 25, MgSO 1.2, CaCl 2, dextrose2 4 3 4 2 repeated with additional doses of carbachol. To determine 10; bubbled with 95% O / 5%2 CO .2 Auditory muscarinic receptor involvement, atropine was applied thalamocortical slices were prepared as described previous- continuously beginning 20 min prior to the carbachol ly [43]. Briefly, slice orientation was near-horizontal, with pulse.
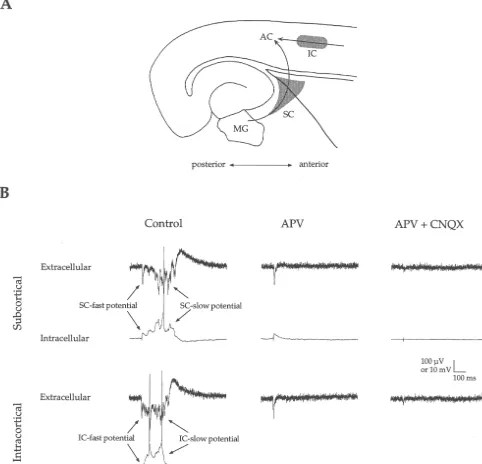
Fig. 1. Auditory cortex responses to subcortical and intracortical stimulation. (A) Schematic illustration of stimulation sites (grey areas) along thalamocortical (SC) and intracortical (IC) fiber pathways (arrows) to AC. Note that for Experiment 2, stimuli were also delivered in the medial geniculate (MG). (B) Stimulation of SC or IC pathways evoked fast and slow potentials in simultaneous intracellular and extracellular recordings from cortical layer IV. APV (50mM) reduced the slow potentials and subsequent application of CNQX (10mM) reduced the remaining fast potentials.
from Research Biochemicals Inc. (Natick, MA). All drugs 2.4. Data analysis were dissolved in ACSF from frozen stock solutions and
perfused at a rate of 1.0–1.6 ml / min. CNQX stock Evoked intracellular and extracellular responses con-solution included dimethyl sulfoxide (DMSO, final con- sisted of ‘‘fast’’ and ‘‘slow’’ potentials. Fast potentials had
centration 0.2%). a peak latency,10 ms and peak amplitude$1 mV or 35
In a second set of experiments, we examined the effects mV (intra- and extracellular, respectively). Slow potentials of carbachol on layer IV responses evoked by direct MG had onset latencies.10 ms and long durations (typically stimulation while alternating either SC or IC stimulation. .100 ms).
Other aspects of the ‘‘carbachol protocol’’ were the same For intracellular fast potentials, onset latency was
point where the membrane potential deviated from the Effective stimulation required that the stimulating elec-resting potential. Amplitude was measured at the first peak trode be placed specifically in the subcortical region (e.g., arrows in Fig. 3C and D). For extracellular fast anterior to the hippocampus (SC grey area in Fig. 1A; potentials, onset latency could not be measured because mean distance, measured in a straight line between record-the stimulus artifact often lasted into record-the beginning of record-the ing and stimulating electrodes, was 1148644 mm) that response. Amplitude was measured at the maximum peak contains the fibers of the auditory thalamocortical pathway of the field potential. For intracellular and extracellular [12,43,60,73]. Stimulation of the hippocampus itself or of slow potentials, onset latency was measured at the point the striatum at positions greater than about 1 mm anterior where the slow potential deviated from baseline (e.g., Fig. to the hippocampus elicited weak or no response. Thus, SC 1B, Subcortical Extracellular Control). In cases where the stimulation in the present study likely activated potential did not recover to baseline after the fast potential thalamocortical fibers (and possibly corticofugal axons, but (e.g., Fig. 1B, Intracortical Extracellular Control), onset see below and Discussion).
latency was measured at the inflection point after the fast For experiment 1, intracellular data derive from 15 potential. The slow potential’s duration was measured slices and extracellular data derive from 35 slices. In from the onset to the point at which the potential returned intracellular recordings, SC stimulation elicited a fast to baseline. Magnitude was measured as the area under that EPSP followed by a slow, long-lasting depolarization (Fig. curve. Variability is expressed as61 Standard Error of the 1B, intracellular control trace in response to subcortical mean. Statistical comparisons are unpaired t-tests except stimulation). Simultaneous extracellular recordings
re-where noted. vealed corresponding fast and slow negative potentials
(Fig. 1B, extracellular control trace in response to subcorti-cal stimulation). Extracellular negativities generally
corres-3. Results ponded to intracellular depolarizations in terms of latency, shape, and duration (cf. intracellular and extracellular The results are divided into two sets of experiments. The traces in Fig. 1B), implying a common cellular basis. initial experiments were designed to separately activate Although the fast intracellular EPSP to SC stimulation extrinsic (subcortical) and intrinsic (intracortical) inputs often consisted of 2 or 3 depolarizing peaks, our aim was leading to AC. We placed one stimulating electrode to examine thalamocortical, monosynaptic responses; subcortically, within the downstream part of the auditory therefore, for quantitative analysis, we measured the first thalamocortical pathway, and a second stimulating elec- peak with the shortest latency and refer to this as the trode within the middle layers of the cortex lateral to the SC-fast potential. The SC-fast potential had a consistent recording electrode (Fig. 1A). Recordings were made in onset latency (3.160.3 ms), initial slope (1.760.3 mV/ ms), layer IV of AC at the site of the maximal field response to peak latency (6.960.7 ms) and amplitude (4.260.8 mV) subcortical stimulation and the effects of carbachol were and produces a dominant current sink in layers III / IV [43]. examined. The results of these first experiments comprise A qualitatively similar potential in AC occurs in response the majority of the dataset in this manuscript. They to stimulation of the MG itself ([12,43]; and see Experi-revealed, among other things, a strong differential ment 2 below). These characteristics, along with pharma-cholineric modulation of responses to subcortical vs. cological data presented below, suggest that the SC-fast intracortical inputs to AC. We hypothesize that this dif- potential is a thalamocortical EPSP. Following the SC-fast ferential effect could be due to differences between EPSP was a slower, long-lasting depolarization with fast thalamocortical synapses (activated by subcortical stimula- fluctuations and spikes. This will be referred to as the tion) and intracortical synapses (activated by intracortical SC-slow potential (indicated by arrows in Fig. 1B). It had stimulation). However, it is possible that the subcortical a more variable latency to onset (33.863.1 ms), duration stimulus may have activated non-thalamic afferents, so we (501.6638.7 ms) and magnitude (2331.86438.0 mV?ms). conducted a second, more limited study, in which the This variability was evident in a given slice from trial to auditory thalamus was stimulated directly, thus decreasing trial, as well as between slices. The temporal characteris-the likelihood of activating non-thalamic cortical afferents. tics and variability of the slow potential indicate that it is The cholinergic modulation of the resulting thalamocorti- polysynaptic (see also [43]).
cal responses in AC was then compared with that found for The extracellular SC-fast potential had a mean latency to the other inputs. Each of the two sets of experiments will peak of 6.960.3 ms, similar to that of the intracellular
be discussed in turn. EPSP. The mean amplitude was 159.1612.9 mV, and both
amplitude and latency displayed trial to trial consistency. 3.1. Experiment 1: subcortical vs. intracortical In contrast, the extracellular SC-slow potential had a
responses and modulation variable onset (25.363.9 ms), duration (223.7623.8 ms)
and magnitude (area513,21061390 mV ms). This vari-3.1.1. Responses to subcortical stimulation ability was observed within slices from trial-to-trial, as
Subcortical stimulation within the auditory thalamocorti- well as between slices.
differ-ent stimulus intensities, indicating differdiffer-ent thresholds for 10–25 mA) elicited only the fast potential while higher generation. In 26 / 35 slices (74%) where SC stimulation intensities elicited both fast and slow potentials. In some elicited extracellular fast and slow potentials, low stimulus slices, still higher intensities caused a reduction of the intensities (e.g., 10–25mA) elicited the fast potential alone IC-slow potential, possibly due to the recruitment of whereas higher intensities elicited both potentials. In the intracortical inhibition (see Discussion).
remaining nine of 35 (26%) slices, SC stimuli at intensities Pharmacological manipulations indicated glutamate re-up to 100 mA elicited only fast potentials. ceptor involvement in the generation of IC responses, just Pharmacological manipulations revealed that glutamate as for SC responses. IC-slow potentials were almost receptors contribute to the generation of SC-fast and slow completely blocked by APV (50 mM), and subsequent potentials. Bath application of APV (50 mM), an NMDA application of CNQX (20 mM) greatly reduced the fast receptor antagonist, reduced the slow potential nearly potentials (Fig. 1B). Together, the physiological and completely while having little effect on the fast potential pharmacological data indicated that similar mechanisms (Fig. 1B, Subcortical APV). Subsequent application of underlie SC and IC potentials.
CNQX (10 mM), an AMPA / KA receptor antagonist,
reduced the fast potential (Fig. 1B, Subcortical APV1 3.1.3. Subcortical and intracortical pathway CNQX). These data suggest that generation of the slow independence
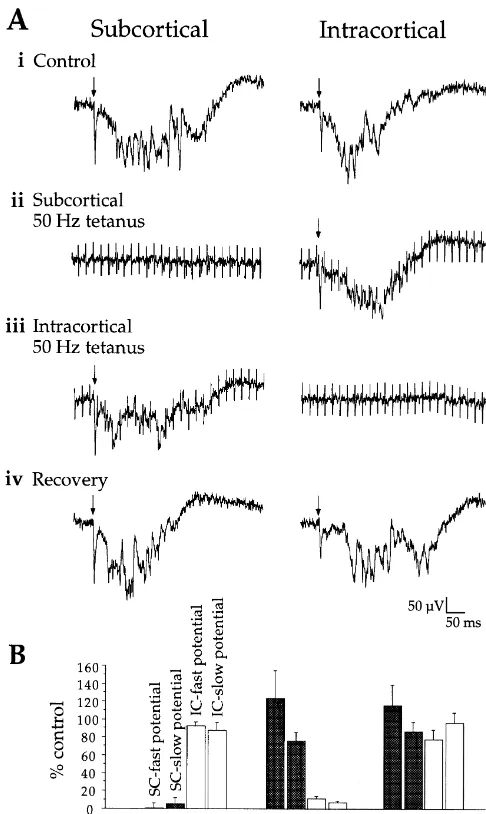
potential involves NMDA receptor activity and generation Because the responses elicited by stimulation of SC and of the fast potential involves AMPA / KA receptor activity. IC sites had similar characteristics, it was necessary to Note, however, that our previous study [43] demonstrated demonstrate that SC and IC stimulation activated distinct that CNQX alone can also completely reduce the slow afferent pathways. To do this, we used a tetanus protocol potential. Thus, both NMDA and AMPA / KA receptors to fatigue one pathway and then determined the response likely contribute to generating the slow potential (see to stimulation of the other pathway (Fig. 2). For the slice
Discussion). in Fig. 2A, both SC and IC stimuli initially elicited
extracellular fast and slow potentials (Fig. 2A (i)). Re-3.1.2. Responses to intracortical stimulation sponses to SC stimulation were then fatigued with a 50 Hz To activate IC afferents, a second stimulating electrode tetanus (Fig. 2A (ii)). During the SC tetanus, a single was placed in the middle cortical layers up to 1 mm (mean stimulus pulse delivered to the IC pathway elicited re-distance 620635 mm) lateral to the recording electrode sponses similar to control (Fig. 2A (ii); stimulus artifacts (Fig. 1A). Stimulation above or below the middle layers from the tetanus appear in both traces). Fifteen seconds generally elicited weaker responses, suggesting that in- after the SC tetanus, both SC and IC responses recovered tracortical stimuli activated fibers that project horizontally (not shown). The protocol was then reversed: the IC
within the middle layers. pathway was fatigued with tetanic stimulation, and a single
The basic physiological and pharmacological properties pulse delivered to the SC pathway elicited responses of the responses elicited by stimulation of the IC pathway similar to control (Fig. 2A (iii)). Fifteen seconds after the were similar to those described for the SC pathway. In IC tetanus, both SC and IC responses had recovered (Fig. intracellular recordings, IC stimulation evoked a fast EPSP 2A (iv)). Fig. 2B shows the group effect of this procedure followed by a slow, long-duration depolarization (Fig. 1B, on the amplitudes of the fast and slow potentials (SC Intracortical). As with SC stimulation, the early part of the tetanus n54; IC tetanus n53). Because the tetanus had response included up to three short latency depolarizing little effect on responses to stimulation of the non-tetanized peaks but again, our quantitative analysis involved only the pathway, we conclude that the SC and IC pathways are first peak, which we call the IC-fast potential. The IC-fast largely independent, at least with respect to the mono-potential had a consistent onset latency (4.060.3 ms), synaptic fast potentials (this conclusion cannot be extended slope (1.760.4 mV/ ms), peak latency (8.361.3 ms), to the slow potentials because of their polysynaptic nature, amplitude (3.260.4 mV), and shape, suggesting a mono- see Discussion). Given this demonstration, we could now synaptic response. In contrast, the IC-slow potential that determine the sensitivity of IC- and SC-evoked responses followed (arrows in Fig. 1B) had a more variable onset to cholinergic modulation.
latency (28.263.2 ms), duration (454.0630.0 ms) and
magnitude (2312.86487.3 mV ms). This variability was 3.1.4. Effects of cholinergic agonist on intracellular seen from trial to trail and between cells, indicating a potentials
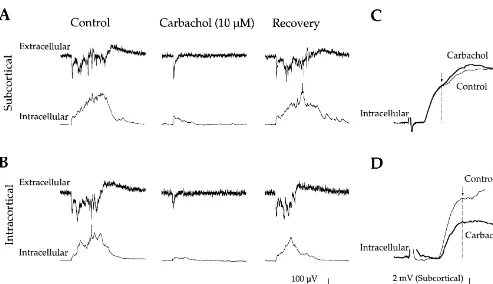
polysynaptic response. In intracellular recordings, 10–50mM carbachol
to make these comparisons for different doses of carbach-ol, we recorded extracellular potentials while alternating SC and IC stimulation, and applied carbachol at con-centrations ranging from 0.5 to 50mM.
3.1.5. Dose-dependent effects of carbachol
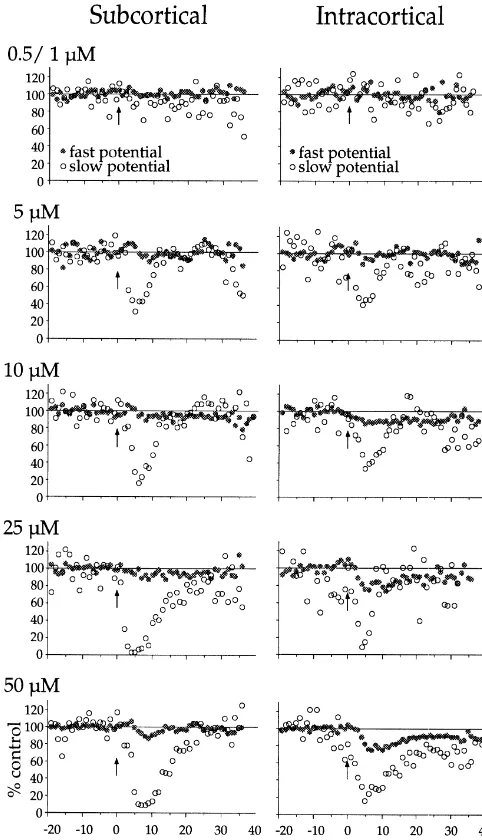
We determined the effects of 0.5, 1, 5, 10, 25 and 50 mM carbachol on extracellular responses to SC and IC stimuli interleaved at 15–30 s intervals. In 11 / 35 slices, more than one dose was applied to the same slice. Fig. 4 depicts the time course and magnitude of carbachol-in-duced effects at each dose and on each of the four synaptic responses (i.e., on SC- and IC-elicited fast and slow potentials). A differential effect on fast vs. slow potentials was first apparent at 5 mM, which reduced the SC- and IC-slow potentials 70.266.9% and 63.768.1%, respective-ly (P,0.001) but did not significantrespective-ly affect the fast potentials (P.0.05). Higher concentrations of carbachol ($10 mM) reduced both slow and fast potentials but the slow potentials were always affected more strongly (P, 0.05, paired t-test). In addition, reduction of the fast potential took longer to develop than that of the slow potential. This was quantified for the 50mM effects dose; the maximal reduction of the slow potential occurred 3.0560.58 min before that of the fast potential (P,0.001, paired t-test; these differences are not apparent in Fig. 4 because of the variable onset latency of carbachol’s effects across slices). Thus, carbachol reduced the slow potentials at a lower dose, to a greater degree, and more rapidly, than it reduced the fast potentials (Figs. 4 and 5).
Differential cholinergic modulation of the extracellular SC and IC fast potentials (Figs. 4 and 5) was similar to that observed in intracellular experiments (Fig. 3C and D). At 10 mM, carbachol reduced both fast potentials
sig-Fig. 2. Independence of subcortical and intracortical pathways. (A) (i) nificantly (P,0.05), but reduced the IC-fast potential to a Single stimuli (at arrows) elicited control SC- and IC-evoked potentials.
greater degree than the SC-fast potential (SC reduction5
(ii) During 50 Hz stimulation to fatigue SC-elicited potentials, a single
6.662.0%; IC reduction515.163.7%; P,0.05, paired
t-pulse delivered to the IC pathway elicited robust potentials. (iii)
Follow-ing recovery from the tetanus (not shown), 50 Hz stimulation then test). The differential reduction of fast potentials was
fatigued IC-elicited potentials, and a single pulse delivered to the SC enhanced at higher carbachol concentrations (Fig. 5). pathway elicited fast and slow potentials similar to control. (iv) Recovery
Since the reduction of synaptic potentials can occur as a
of all potentials after tetanic stimulation. (B) Tetanic stimulation of SC
result of membrane depolarization (which would reduce
(n54 slices) or IC (n53 slices) pathways strongly reduced responses to
excitatory driving force), we determined the effects of
single pulse stimulation of the tetanized pathway (P’s,0.01) but not the
non-tetanized pathway (P’s.0.1). carbachol on membrane potential for two doses. At 10 mM, there was a negligible depolarization of the mem-brane potential (mean51.461.4 mV; n56; P.0.3). In tests, but individual dose effects are shown in Table 1). contrast, 50 mM carbachol significantly depolarized the Second, between pathways, carbachol reduced the IC-fast membrane potential (mean54.360.9 mV; n58; P,0.01). potential more than the SC-fast potential (P,0.02; see However, the carbachol-induced suppression of the synap-Table 1). Both of these differential effects are illustrated in tic responses remained during repolarization of the mem-the examples shown in Fig. 3. Carbachol had no effect on brane potential back to baseline via intracellular current EPSP onset latencies in either pathway (P’s.0.3; mean injection (n53; data not shown).
Table 1
Effects of carbachol on intracellular potentials (% decrease1S.E.)
Subcortical Intracortical
Fast Slow Fast Slow
potential potential potential potential
Amplitude Slope Magnitude Amplitude Slope Magnitude
10mM 15.466.8 12.865.8 94.862.3 36.1613.1 33.3613.0 89.262.7
(n56) (n56) (n56) (n54) (n54) (n53)
50mM 14.765.5 18.567.7 97.961.2 34.069.2 34.1614.9 92.767.2
(n55) (n55) (n55) (n54) (n54) (n53)
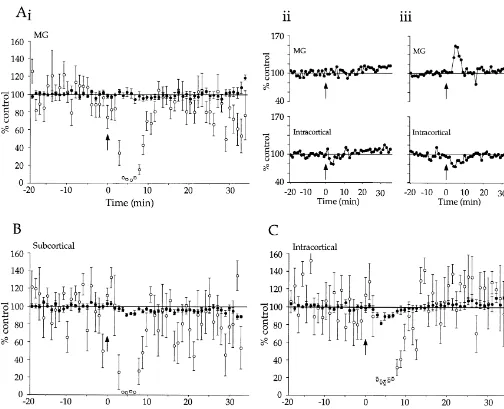
synaptic responses (Fig. 5), indicating the involvement of nated population of thalamocortical synapses. To do this, muscarinic ACh receptors in carbachol’s actions. we developed a fully intact thalamocortical preparation in which direct MG stimulation produced robust cortical 3.2. Experiment 2: cholinergic modulation of responses [12,43]. In such intact slices, we compared the
thalamocortical responses effects of 25mM carbachol on cortical responses evoked
by MG stimulation, alternating with SC or IC stimulation Because the subcortical stimulating electrodes in the (MG vs. SC, n58; MG vs. IC, n57).
above experiments were placed in the auditory Results of these experiments are shown in Fig. 6. As thalamocortical pathway rather than in the MG itself, they before, the slow potentials were suppressed more than the may have also activated cortical afferents originating fast potentials for all pathways (P’s,0.0001). Also, as outside the auditory thalamus. Stimulating the MG directly before, both the IC- and SC-evoked fast potentials were would increase the likelihood of activating an uncontami- suppressed by carbachol (P’s,0.01) and this suppression
Fig. 5. Group effects of carbachol on fast and slow potentials, derived from Fig. 4 data. Values are means of carbachol’s maximal effects (average of 2 min) on fast potential amplitudes and slow potential magnitudes. Atropine, 0.5 mM, (n53) blocked all effects of 50 mM carbachol.
sponses in either full or partial thalamocortical
prepara-Fig. 4. Dose dependence and time course of carbachol’s actions on fast tions. These two cholinergic enhancements contributed to
and slow potentials. Fast potential amplitudes (solid circles) and slow the failure of the average MG data to exhibit significant potential magnitudes (open circles) were normalized to mean of 20 suppression (Fig. 6A (i); P50.90). Examples of an responses preceding carbachol (2 min pulse starting at arrow) at
con-individual enhancement and a more typical effect are
centrations of 0.5 / 1, 5, 10, 25, or 50 mM. (Neither 0.5 nor 1 mM
shown in Fig. 6A (ii) and (iii).
carbachol had any effect; these data were therefore combined.) For all concentrations, SC fast potential n57–12, SC slow potential n55–9, IC fast potential n54–14, IC slow potential n53–6.
4. Discussion
was greater for the IC than the SC pathway (Fig. 6B and
C; P,0.05). More importantly, the effect of carbachol on We have examined cholinergic modulation of responses MG-evoked fast responses was not significantly different evoked by stimulation of extrinsic (thalamocortical) and from that on SC-evoked responses (Fig. 6A (i) and B; intrinsic (intracortical) inputs to auditory cortex. For both
Fig. 6. Effects of carbachol on intact thalamocortical slice responses to MG, SC and IC stimulation. (A) (i), (B) and (C) Group effects of 25mM carbachol on MG-, SC- and IC-evoked potentials. Fast potential amplitudes (solid circles) and slow potential magnitudes (open circles) were normalized to mean of 20 responses preceding carbachol (2 min pulse starting at arrow) and are presented6S.E. MG fast and slow potentials n512–15; SC fast and slow potentials n55–8; IC fast and slow potentials n57. (A) (ii) An individual example of a typical carbachol effect on MG- vs. IC-evoked fast potentials. (A) (iii) An individual example of carbachol-induced enhancement of MG-evoked fast potentials vs. suppression of IC-evoked fast potentials.
inves-tigators using the somatosensory thalamocortical prepara- the AC to a state of readiness for subsequent sensory tion also recognize this problem and argue that the events.
contribution of corticothalamic activity is minimal [1]
because thalamocortical fibers reputedly have a lower 4.3. Differential suppression of SC /MG- vs. IC-evoked activation threshold than corticothalamic fibers [1,16,17]. monosynaptic potentials
Recently, we have confirmed this notion using direct tests
in the auditory thalamocortical slice, demonstrating that The reduction by carbachol of the IC fast potential to a the threshold for orthodromic activation of the layer IV greater degree than the SC / MG fast potential could field response is about five times lower than the antidromic possibly result from: (1) differential distribution of mus-threshold of layer V and VI neurons [58]. carinic receptors, (2) differential recruitment of inhibition, To evoke intracortical responses with minimal direct and (3) differential enhancement via nicotinic receptors. activation of thalamic afferents we stimulated horizontal We will discuss each possibility in turn.
(300–950 mm) intracortical pathways. This evoked fast Activation of presynaptic muscarinic ACh receptors and slow potentials in layer IV of AC. Again, the onset (mAChRs) can reduce EPSPs by reducing release of latency of the fast potential was short and consistent. This glutamate [27,63,64,68]. Thus, differential density of pre-latency did not change with carbachol application, sug- synaptic mAChRs on SC and IC terminals could differen-gesting that it is monosynaptic. tially reduce fast potentials (discussed by [30]). Sahin et al. We established the independence of the SC and IC [61] combined autoradiography with excitotoxic lesions of pathways by demonstrating that complete fatigue of one thalamic (ventrobasal) or cortical neurons to support such a pathway left responses to stimulation of the remaining differential distribution. In their study, thalamic lesions pathway relatively unaffected. Thus, at least the mono- produced no reduction in cortical mAChR binding, sug-synaptic SC and IC potentials are independent. However, gesting limited presynaptic mAChRs on thalamic afferents. we have not addressed the independence of the polysynap- In contrast, excitotoxic cortical lesions produced a tic slow potentials. In fact, it is possible that the slow dramatic reduction of mAChR binding in layers I, III, IV potentials evoked by stimulation at subcortical and in- and VI, indicating abundant mAChRs on elements intrinsic tracortical sites are similar phenomena with overlapping to the cortex. Other studies, including work at the ultra-circuitry. They both had long latencies and variable structural level, have located mAChRs presynaptically on durations and magnitudes, suggesting they are polysynap- some thalamic afferents [48,70,71]. Thus, presynaptic tic in nature. In addition, Metherate and Cruikshank [43] mAChRs are found at both thalamocortical and intracorti-showed that the SC-evoked slow potential propagated very cal synapses, but may be more prevalent at intracortical slowly through the AC, implying that it is generated synapses. Such a distribution could underlie the preferen-intracortically. Further details on the nature and mecha- tial muscarinic suppression of intracortical fast potentials nisms underlying generation of the slow potential have in the present study.
been published [43]. Differential recruitment of inhibition by thalamocortical
and intracortical inputs also could contribute to preferential suppression of IC potentials. In our slice preparation, 4.2. Differential cholinergic suppression of fast vs. slow thalamocortical inputs activate disynaptic GABAergic
potentials IPSPs only weakly [43], whereas IC stimulation activated
IPSPs more strongly (unpublished observation). The Carbachol produced nearly complete suppression of the stronger IPSPs elicited by IC stimulation may underlie the slow potential while leaving the fast potential relatively greater reduction of IC fast potentials. Since an overlap-unaffected (at a dose of 5 mM) or suppressed to a much ping IC-elicited EPSP–IPSP would have a more negative lesser degree ($10 mM). While carbachol is a more reversal potential than the thalamocortical EPSP (without effective agonist than ACh, due to its resistance to an overlapping IPSP), it seems possible that the combined hydrolysis, it is assumed that the differential effects potential would be reduced to a greater degree by carbach-described here would also occur with the endogenous ol-induced membrane depolarization. However, the data do transmitter, and therefore are functionally relevant. The not support this possibility. Not only could carbachol suppression of the slow potential occurred quite abruptly, produce differential suppression without membrane depo-whereas reduction of the fast potentials occurred gradually larization (effects of 10mM carbachol), but even at higher over many trials. A possible explanation for this differen- carbachol doses that depolarized the membrane several tial effect is that the fast potential acts to ‘‘trigger’’ the millivolts, differential suppression of EPSPs persisted slow potential, and that muscarinic suppression of the fast during repolarization of the potential to its original level potential below the trigger threshold will abruptly prevent with intracellular current injection. Thus, a shift in synaptic the appearance of the slow potential. Cholinergic suppres- reversal potential due to IPSPs does not underlie the sion of polysynaptic responses could effectively shorten differential suppression of fast potentials.
ef-fects by exciting GABAergic interneurons [36,50]. If Because the latter did not recover to baseline levels, they cholinoceptive GABAergic interneurons inhibit IC-elicited were excluded from the analysis (see Material and meth-responses preferentially, then such actions could contribute ods). However, as with the two cases that did recover, the to differential suppression of fast potentials. Gil et al. [19] onset of facilitation was time locked to carbachol infusion, found that muscarinic actions suppressed thalamocortical the slow potentials evoked by MG stimulation were and intracortical EPSPs equally. However, Gil et al. [19] suppressed normally, and responses along the control continuously infused the region surrounding the recording pathways within the same slices (e.g., SC or IC) behaved electrode with a GABAA receptor antagonist to explicitly normally (e.g., Fig. 6A (iii)). This supports the possibility reduce the influence of differentially evoked inhibition that the increases were caused by specific cholinergic [18]. While this manipulation isolated muscarinic actions, modulation of the thalamocortical pathway and not a it may have masked interactions that produced differential general change in state or health of the slice.
effects in the present study. Other recent studies indicate Obviously the increases represent a minority of the total, that cholinergic modification of GABAergic function may and the majority of MG-evoked responses were either produce complex effects in neural circuits [2,39,52,76]. suppressed or not affected by carbachol, similar to SC-Clearly, further studies are needed to resolve the role of evoked responses. Thus, it is likely that the SC and MG inhibition in muscarinic suppression of thalamocortical and stimuli activated a largely overlapping population of
intracortical pathways. thalamocortical synapses. It is possible that the small
Finally, activation of presynaptic nicotinic ACh recep- number of enhanced responses to MG stimulation may tors (nAChRs) can enhance cortical EPSPs by increasing result from carbachol-induced increased excitability of release of glutamate [3,19–21,53,69]. In the somatosensory some MG soma [13,37,47]. This in turn may have en-system, Gil et al. [19] observed that nicotinic agonists hanced the presynaptic input to the cortex during carbachol selectively enhanced thalamocortical EPSPs. Such actions infusion for some slices in Experiment 2. In contrast, since could combine with general muscarinic suppression of axons of the MG cells would not be excited by carbachol, EPSPs to produce the differential suppression by carbachol no cholinergic enhancement would be expected for SC (a mixed cholinergic agonist) in the present study. How- stimuli. The transient and sustained increases in MG-ever, two lines of evidence argue against this possibility. evoked response are of great interest, especially in contrast First, bath application of agonist can lead to rapid de- to the suppression of intracortical synapses, and warrant sensitization of nAChRs [10,65,77] that precludes nicotine- further study.
induced enhancement of EPSPs [3]. In support of this, bath
application of nicotine to auditory thalamocortical slices 4.5. Functional implications and relevance to previous from the rat produced no consistent effect on layer IV field in vitro and in vivo studies
responses to SC, IC, or intracortical ‘‘on-beam’’
stimula-tion (n518, nicotine concentrastimula-tion 0.5–20 mM, slices Studies of cortical neurons in vivo have generally found obtained from animals aged 8–18 days postnatal; un- that muscarinic actions increase responsiveness to sensory published observations). Second, whereas atropine blocked stimuli [15,42,44,54,67], whereas, in striking contrast, in the suppression produced by carbachol, it did not reveal vitro studies of cortical neurons have generally found enhancement of the SC-evoked response, as would be suppression of EPSPs [2,4,27,64,69]. We propose two expected if nAChRs were simultaneously enhancing explanations for these conflicting findings, based partly on thalamocortical EPSPs (see also [30]). Thus, it is unlikely the present work. First, sensory stimuli in vivo activate that nAChRs contribute to the differential actions of cortical neurons via thalamocortical inputs, whereas
elec-carbachol in the present study. trical stimuli in vitro generally activate intracortical
synapses (most cortical slice preparations either do not
4.4. MG vs. SC modulation contain thalamocortical connections or do not allow for
selective stimulation of thalamocortical inputs). Given the
Although the effects of carbachol on the SC- and MG- present results on preferential suppression of intracortical evoked responses were not statistically different from each EPSPs, it is likely that in vitro studies typically demon-other, some potentially important observations were noted. strate strong suppression of EPSPs due to their dependence First, unlike the SC pathway, the average fast response on intracortical stimulation. The relatively weak suppres-evoked by MG stimulation was not suppressed signifi- sion, or outright enhancement of thalamocortical EPSPs in cantly by carbachol. As stated in the Results, a large the present study more closely resembles in vivo findings. contribution to this lack of suppression came from 2 / 15 A second factor is that neurons recorded in vivo have slices that exhibited increases in MG-evoked response; more depolarized membrane potentials than neurons in such increases never occurred for the SC- (or IC-) evoked vitro, and as a result may have activated voltage-dependent
1
responses. It may also be noteworthy that two additional K currents. Muscarinic blockade of voltage-dependent
1
post-voltage-sensitive K1 current in a vertebrate neurone, Nature 283
synaptic actions are less pronounced in vitro because of
(1980) 673–676.
more hyperpolarized membrane potentials. The lack of
[8] V. Caviness Jr., D.O. Frost, Tangential organization of thalamic
postsynaptic muscarinic actions could leave presynaptic projections to the neocortex in the mouse, J. Comp. Neurol. 194 actions relatively unopposed, resulting in net reduction of (1980) 335–367.
EPSPs. Thus, in the present study, thalamocortical EPSPs [9] A.E. Cole, R.A. Nicoll, Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells, Science 221 (1983) 1299–
are generally only ‘‘enhanced’’ relative to the stronger
1301.
suppression of intracortical EPSPs, whereas an analogous
[10] S. Couturier, D. Bertrand, J.M. Matter, M.C. Hernandez, S.
Ber-in vivo study could reveal greater EPSP enhancement due trand, N. Millar, S. Valera, T. Barkas, M. Ballivet, A neuronal to increased postsynaptic excitability (cf. [42]). A similar nicotinic acetylcholine receptor subunit (alpha 7) is developmentally proposal stems from the work of Hasselmo and colleagues regulated and forms a homo-oligomeric channel blocked by
alpha-BTX, Neuron 5 (1990) 847–856.
in piriform cortex [24,26,30]. Differential presynaptic
[11] C.L. Cox, R. Metherate, J.H. Ashe, Modulation of cellular
suppression, combined with increased postsynaptic
ex-citability in neocortex: muscarinic receptor and second
messenger-citability, would presumably result in an enhanced ability mediated actions of acetylcholine, Synapse 16 (1994) 123–136. for extrinsic inputs to generate spikes while decreasing the [12] S.J. Cruikshank, H.J. Rose, R. Metherate, In vitro physiological effectiveness of intrinsic inputs [25,52]. comparison of MGV inputs to layer 3 / 4 of auditory cortex vs.
nonlemniscal inputs to layer 1, Soc. Neurosci. Abstr. (1999).
Thus, the present results support the concept that
[13] R. Curro Dossi, D. Pare, M. Steriade, Short-lasting nicotinic and
cholinergic modulation serves to favor responses to
exter-long-lasting muscarinic depolarizing responses of thalamocortical
nal stimuli over ongoing cortical activity. Such selective
neurons to stimulation of mesopontine cholinergic nuclei, J.
Neuro-processing of thalamocortical inputs during periods of physiol. 65 (1991) 393–406.
increased ACh release (e.g., during behavioral arousal or [14] J.P. Donaghue, K.L. Carroll, Cholinergic modulation of sensory responses in rat primary somatic sensory cortex, Brain Res. 408
attention), could underlie the widely hypothesized function
(1987) 367–371.
of ACh to increase the ‘‘signal-to-noise’’ ratio of sensory
[15] J.M. Edeline, B. Hars, C. Maho, E. Hennevin, Transient and
responses over ongoing cortical activity (reviewed in
prolonged facilitation of tone-evoked responses induced by basal
[23,66]). forebrain stimulations in the rat auditory cortex, Exp. Brain Res. 97
(1994) 373–386.
[16] D. Ferster, S. Lindstrom, An intracellular analysis of geniculo-cortical connectivity in area 17 of the cat, J. Physiol. (Lond.) 342
Acknowledgements (1983) 181–215.
[17] D. Ferster, S. Lindstrom, Synaptic excitation of neurones in area 17 of the cat by intracortical axon collaterals of cortico-geniculate cells,
Thanks to Dr. V. B. Aramakis for helpful discussions and
J. Physiol. (Lond.) 367 (1985) 233–252.
comments on the manuscript. This work was supported by [18] Z. Gil, Y. Amitai, Properties of convergent thalamocortical and the NSF (IBN 9510904), NIDCD (DC02967), University intracortical synaptic potentials in single neurons of neocortex, J. of California Tobacco-Related Disease Research Program Neurosci. 16 (1996) 6567–6578.
[19] Z. Gil, B.W. Connors, Y. Amitai, Differential regulation of
neocorti-(8RT-0059) and an NIMH training grant (MH14599).
cal synapses by neuromodulators and activity, Neuron 19 (1997) 679–686.
[20] Y. Gioanni, C. Rougeot, P. Clarke, C. Lepouse, A. Theirry, C. Vidal, Nicotinic receptors in the rat prefrontal cortex: increase in glutamate
References release and facilitation of mediodorsal thalamo-cortical
transmis-sion, Eur. J. Neurosci. 11 (1999) 18–30.
[1] A. Agmon, B.W. Connors, Thalamocortical responses of mouse [21] R. Gray, A.S. Rajan, K.A. Radcliffe, M. Yakehiro, J.A. Dani, somatosensory (barrel) cortex in vitro, Neuroscience 41 (1991) Hippocampal synaptic transmission enhanced by low concentrations
365–379. of nicotine, Nature 383 (1996) 713–716.
[2] V.B. Aramakis, A.E. Bandrowski, J.H. Ashe, Muscarinic reduction [22] B. Hars, C. Maho, J.M. Edeline, E. Hennevin, Basal forebrain of GABAergic synaptic potentials results in disinhibition of the stimulation facilitates tone-evoked responses in the auditory cortex AMPA / kainate-mediated EPSP in auditory cortex, Brain Res. 758 of awake rat, Neuroscience 56 (1993) 61–74.
(1997) 107–117. [23] M.E. Hasselmo, Neuromodulation and cortical function: modeling [3] V.B. Aramakis, R. Metherate, Nicotine selectively enhances NMDA the physiological basis of behavior, Behav. Brain Res. 67 (1995)
receptor-mediated synaptic transmission during postnatal develop- 1–27.
ment in sensory neocortex, J. Neurosci. 18 (1998) 8485–8495. [24] M.E. Hasselmo, J.M. Bower, Cholinergic suppression specific to [4] J.M. Auerbach, M. Segal, Muscarinic receptors mediating depres- intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex,
sion and long-term potentiation in rat hippocampus, J. Physiol. 492 J. Neurophysiol. 67 (1992) 1222–1229.
(1996) 479–493. [25] M.E. Hasselmo, J.M. Bower, Acetylcholine and memory, Trends [5] J.S. Bakin, N.M. Weinberger, Induction of a physiological memory Neurosci. 16 (1993) 218–222.
in the cerebral cortex by stimulation of the nucleus basalis, Proc. [26] M.E. Hasselmo, E. Schnell, Laminar selectivity of the cholinergic Natl. Acad. Sci. USA 93 (1996) 11219–11224. suppression of synaptic transmission in rat hippocampal region [6] S. Brocher, A. Artola, W. Singer, Agonists of cholinergic and CA1: computational modeling and brain slice physiology, J.
Neuro-noradrenergic receptors facilitate synergistically the induction of sci. 14 (1994) 3898–3914.
long-term potentiation in slices of rat visual cortex, Brain Res. 573 [27] J. Hounsgaard, Presynaptic inhibitory action of acetylcholine in area
(1992) 27–36. CA1 of the hippocampus, Exp. Neurol. 62 (1978) 787–797.
differentially suppresses auditory thalamocortical and intracortical [51] T. Murakoshi, Cholinergic modulation of synaptic transmission in transmission, Soc. Neurosci. Abstr. (1998). the rat visual cortex in vitro, Vision Res. 35 (1995) 25–35. [29] M.P. Kilgard, M.M. Merzenich, Cortical map reorganization enabled [52] M.M. Patil, M.E. Hasselmo, Modulation of inhibitory synaptic
by nucleus basalis activity, Science 279 (1998) 1714–1718. potentials in the piriform cortex, J. Neurophysiol. 81 (1999) 2103– [30] F. Kimura, M. Fukuda, T. Tsumoto, Acetylcholine suppresses the 2118.
spread of excitation in the visual cortex revealed by optical [53] K.A. Radcliffe, J.A. Dani, Nicotinic stimulation produces multiple recording: possible differential effect depending on the source of forms of increased glutamatergic synaptic transmission, J. Neurosci. input, Eur. J. Neurosci. 11 (1999) 3597–3609. 18 (1998) 7075–7083.
[31] A. Kirkwood, C. Rozas, J. Kirkwood, F. Perez, M.F. Bear, Modula- [54] D.D. Rasmusson, R.W. Dykes, Long-term enhancement of evoked tion of long-term synaptic depression in visual cortex by acetyl- potentials in cat somatosensory cortex produced by co-activation of choline and norepinephrine, J. Neurosci. 19 (1999) 1599–1609. the basal forebrain and cutaneous receptors, Exp. Brain Res. 70 [32] K. Krnjevic, R. Pumain, L. Renaud, The mechanism of excitation by (1988) 276–286.
acetylcholine in the cerebral cortex, J. Physiol. 215 (1971) 247–268. [55] M. Roger, P. Arnault, Anatomical study of the connections of the [33] D.V. Madison, B. Lancaster, R.A. Nicoll, Voltage clamp analysis of primary auditory area in the rat, J. Comp. Neurol. 287 (1989)
cholinergic action in the hippocampus, J. Neurosci. 7 (1987) 733– 339–356.
741. [56] L.M. Romanski, J.E. LeDoux, Information cascade from primary
[34] R.T. Marrocco, E.A. Witte, M.C. Davidson, Arousal systems, Curr. auditory cortex to the amygdala: corticocortical and cor-Opin. Neurobiol. 4 (1994) 166–170. ticoamygdaloid projections of temporal cortex in the rat, Cereb. [35] D.A. McCormick, Neurotransmitter actions in the thalamus and Cortex 3 (1993) 515–532.
cerebral cortex, J. Clin. Neurophysiol. 9 (1992) 212–223. [57] L.M. Romanski, J.E. LeDoux, Organization of rodent auditory [36] D.A. McCormick, D.A. Prince, Mechanisms of action of acetyl- cortex: anterograde transport of PHA-L from MGv to temporal
choline in the guinea-pig cerebral cortex in vitro, J. Physiol. 375 neocortex, Cereb. Cortex 3 (1993) 499–514.
(1986) 169–194. [58] H.J. Rose, R. Metherate, Lower threshold and increased probability [37] D.A. McCormick, D.A. Prince, Actions of acetylcholine in the for orthodromic vs. antidromic activation of cortex in an auditory
guinea-pig and cat medial and lateral geniculate nuclei, in vitro, J. thalamocortical slice, Soc. Neurosci. Abstr. 26 (2000) 637. Physiol. (Lond.) 392 (1987) 147–165. [59] E.M. Rouiller, E. Welker, Morphology of corticothalamic terminals [38] T.M. McKenna, J.H. Ashe, N.M. Weinberger, Cholinergic modula- arising from the auditory cortex of the rat: a Phaseolus vulgaris-tion of frequency receptive fields in auditory cortex: I. Frequency- leucoagglutinin (PHA-L) tracing study, Hear Res. 56 (1991) 179– specific effects of muscarinic agonists, Synapse 4 (1989) 30–43. 190.
[39] A.R. McQuiston, D.V. Madison, Muscarinic receptor activity has [60] D.K. Ryugo, H.P. Killackey, Differential telencephalic projections of multiple effects on the resting membrane potentials of CA1 hip- the medial and ventral divisions of the medial geniculate body of the pocampal interneurons, J. Neurosci. 19 (1999) 5693–5702. rat, Brain Res. 82 (1974) 173–177.
[40] M.M. Mesulam, The systems-level organization of cholinergic [61] M. Sahin, W.D. Bowen, J.P. Donoghue, Location of nicotinic and innervation in the human cerebral cortex and its alterations in muscarinic cholinergic and mu-opiate receptors in rat cerebral Alzheimer’s disease, Prog. Brain Res. 109 (1996) 285–297. neocortex: evidence from thalamic and cortical lesions, Brain Res. [41] R. Metherate, J.H. Ashe, Ionic flux contributions to neocortical slow 579 (1992) 135–147.
waves and nucleus basalis-mediated activation: whole-cell record- [62] R. Schliebs, S. Rossner, V. Bigl, Immunolesion by 192IgG-saporin ings in vivo, J. Neurosci. 13 (1993) 5312–5323. of rat basal forebrain cholinergic system: a useful tool to produce [42] R. Metherate, J.H. Ashe, Nucleus basalis stimulation facilitates cortical cholinergic dysfunction, Prog. Brain Res. 109 (1996) 253–
thalamocortical synaptic transmission in the rat auditory cortex, 264.
Synapse 14 (1993) 132–143. [63] M. Segal, Multiple action of acetylcholine at a muscarinic receptor [43] R. Metherate, S.J. Cruikshank, Thalamocortical inputs trigger a studied in the rat hippocampal slice, Brain Res. 246 (1982) 77–87. propogating envelope of gamma-band activity in auditory cortex, in [64] M. Segal, Presynaptic cholinergic inhibition in hippocampal cul-vitro, Exp. Brain Res. 126 (1999) 160–174. tures, Synapse 4 (1989) 305–312.
[44] R. Metherate, N. Tremblay, R.W. Dykes, Transient and prolonged [65] P. Seguela, J. Wadiche, K. Dineley-Miller, J.A. Dani, J.W. Patrick, effects of acetylcholine on responsiveness of cat somatosensory Molecular cloning, functional properties, and distribution of rat cortical neurons, J. Neurophysiol. 59 (1988) 1253–1276. brain alpha 7: a nicotinic cation channel highly permeable to [45] A. Mitani, M. Shimokouchi, Neuronal connections in the primary calcium, J. Neurosci. 13 (1993) 596–604.
auditory cortex: an electrophysiological study in the cat, J. Comp. [66] A.M. Sillito, The cholinergic neuromodulatory system: an evalua-Neurol. 235 (1985) 417–429. tion of its functional roles, Prog. Brain Res. 98 (1993) 371–378. [46] A. Mitani, M. Shimokouchi, K. Itoh, S. Nomura, M. Kudo, N. [67] A.M. Sillito, J.A. Kemp, Cholinergic modulation of the functional
Mizuno, Morphology and laminar organization of electrophysiologi- organization of the cat visual cortex, Brain Res. 289 (1983) 143– cally identified neurons in the primary auditory cortex in the cat, J. 155.
Comp. Neurol. 235 (1985) 430–447. [68] R.J. Valentino, R. Dingledine, Presynaptic inhibitory effect of [47] D.M. Mooney, B. Hu, V.V. Senatorov, Muscarine induces an anomal- acetylcholine in the hippocampus, J. Neurosci. 1 (1981) 784–792.
ous inhibition of synaptic transmission in rat auditory thalamic [69] C. Vidal, J.P. Changeux, Nicotinic and muscarinic modulations of neurons in vitro, J. Pharmacol. Exp. Ther. 275 (1995) 838–844. excitatory synaptic transmission in the rat prefrontal cortex in vitro, [48] L. Mrzljak, A.I. Levey, P.S. Goldman-Rakic, Association of m1 and Neuroscience 56 (1993) 23–32.
m2 muscarinic receptor proteins with asymmetric synapses in the [70] B.A. Vogt, D.L. Burns, Experimental localization of muscarinic primate cerebral cortex: morphological evidence for cholinergic receptor subtypes to cingulate cortical afferents and neurons, J. modulation of excitatory neurotransmission, Proc. Natl. Acad. Sci. Neurosci. 8 (1988) 643–652.
USA 90 (1993) 5194–5198. [71] B.A. Vogt, P.B. Crino, E.L. Jensen, Multiple heteroreceptors on [49] M. Muhlethaler, M. de Curtis, K. Walton, R. Llinas, The isolated limbic thalamic axons: M2 acetylcholine, serotonin 1B, beta 2-and perfused brain of the guinea-pig in vitro, Eur. J. Neurosci. 5 adrenoceptors, mu-opioid, and neurotensin, Synapse 10 (1992) 44–
(1993) 915–926. 53.
[73] F.H. Willard, D.K. Ryugo, Anatomy of the central auditory system, radish peroxidase and autoradiographic methods in the rat medial in: J.F. Willott (Ed.), The Auditory Psychobiology of the Mouse, geniculate body, J. Comp. Neurol. 257 (1987) 282–315.
Charles C. Thomas, Springfield, Illinois, 1983, pp. 201–304. [76] Z. Xiang, J.R. Huguenard, D.A. Prince, Cholinergic switching [74] J.A. Winer, The functional architecture of the medial geniculate within neocortical inhibitory networks, Science 281 (1998) 985–
body and the primary auditory cortex, in: D.B. Webster, A.N. Popper 988.
(Eds.), The Mammalian Auditory Pathway: Neuroanatomy, Spring- [77] Z.W. Zhang, S. Vijayaraghavan, D.K. Berg, Neuronal acetylcholine er-Verlag, New York, 1992, pp. 222–409. receptors that bind alpha-bungarotoxin with high affinity function as [75] J.A. Winer, D.T. Larue, Patterns of reciprocity in auditory ligand-gated ion channels, Neuron 12 (1994) 167–177.