Differential effect of amino acids on nitrate uptake and reduction
systems in barley roots
Muhammad Aslam, Robert L. Travis *, D. William Rains
Department of Agronomy and Range Science,Uni6ersity of California,One Shields A6enue,Da6is,CA95616-8515, USA Received 29 June 2000; received in revised form 6 August 2000; accepted 6 August 2000
Abstract
This study was conducted to determine whether the inhibition of nitrate reductase activity (NRA; EC 1.6.6.1) in barley (Hordeum 6ulgare L. var. CM-72) roots by the amino acids (glutamic, aspartic, glutamine and asparagine) is a direct effect or
indirect due to inhibition of the NO3−uptake system. Roots of 8-day-old intact seedlings were supplied with the amino acids (I mM) individually either with NO3−(0.1 or 10 mM) or roots were pretreated with the amino acids and then supplied with NO3− only. Nitrate uptake was determined by following NO3−depletion from the uptake solution containing 0.1 mM NO3−. All the amino acids inhibited the increase in NO3− uptake similarly (50 – 60%) when the roots were supplied with 0.1 mM NO3−. Pretreatment with glutamic and aspartic acids was more inhibitory (70 – 80%) than with glutamine and asparagine (30%). The amino acids partially inhibited (35%) the induction of NRA in roots supplied with 0.1 mM NO3−; however, no inhibition occurred at 10 mM NO3−. Likewise, pretreatment with glutamic or aspartic acid inhibited the induction of NRA at 0.1 mM NO3−but not at 10 mM NO3−. In contrast, pretreatment with glutamine or asparagine had no effect on the subsequent induction of NRA, even at 0.1 mM NO3−. The results suggest that, at low NO3−supply, the inhibition of induction of NRA by the amino acids is a result of the lack of substrate availability due to inhibition of the NO3−uptake system. © 2001 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Amino acids; Barley; Nitrate uptake; Nitrate reductase; Roots
www.elsevier.com/locate/plantsci
1. Introduction
Nitrate is the most common form of inorganic N utilized by those higher plants, which lack N2-fixing symbioses [1]. The assimilation of NO3−
into organic N requires the participation of several specific proteins [2]. First NO3−, absorbed by the
plasma membrane-bound transporter, is reduced to NH4+ by nitrate reductase (NR) and nitrite
reductase [3,4]. NH4+ is then incorporated into
amino acids by the glutamine synthetase-glu-tamine-2-oxoglutarate amidotransferase (GS-GOGAT) enzyme system [5], giving rise to glutamine (Gln) and ultimately other amino acids and their metabolites [6]. Free amino acids are the precursors by which N is transferred between cells and organs [7]. Both NO3− uptake and reduction
processes are substrate inducible and are regulated by endogenous metabolites including amino acids [3,4].
A rapid cycling of amino acids between roots and shoots occurs whether NO3− assimilation is
localized mainly in shoots [8 – 10] or roots [11]. Plants that exhibit low rates of NO3− assimilation
in roots export most of the absorbed NO3− to
shoots where it is reduced and incorporated into amino acids. The amino acids from the shoots are
Abbre6iations: Asp, aspartic acid; Asn, asparagine; DON, dia-zoacetyl-DL-norleucine methyl ester; Glu, glutamic acid; Gln, glu-tamine; GOGAT, glutamine-2-oxoglutarate amidotransferase; GS, glutamine synthetase; HPLC, high-performance liquid chromatogra-phy; IHATS, inducible high affinity transport system; LATS, low affinity transport system; NRA, nitrate reductase activity; PPFD, photosynthetic photon flux density; SE, standard error.
* Corresponding author. Tel.: +1-530-7526162; fax: + 1-530-7524361.
E-mail address:[email protected] (R.L. Travis).
then translocated to roots via the phloem [12 – 14]. Thus, plant roots may be exposed internally to elevated levels of amino acids. The pool of amino acids cycling between the root and shoot is consid-ered to serve as a signal for the plant internal N status [9,15,16]. The amino acids, whether accu-mulated in the plant internally or supplied exter-nally, usually down regulate the induction of NO3−
uptake [2,15,17 – 20] and reduction systems [21 – 26].
Regulation of induction of the NO3−uptake and
reduction systems by reduced N compounds has been attributed to feed-back inhibition [26,27]. Since the induction of both of these systems is dependent upon the availability of NO3− in the
tissue [28], the N metabolites may affect the induc-tion processes by affecting the availability of the substrate. Sivasankar et al. [26] observed that Gln and asparagine (Asn) inhibited the induction of NR activity (NRA) in corn roots at both low and high external NO3− supply. They concluded that
inhibition was not the result of altered NO3−
up-take, but was due to the direct effect of these metabolites at the transcription level. In contrast, we recently observed that Gln partially inhibited the induction of NRA in barley roots at lower, but not at higher, exogenous NO3− concentrations (M.
Aslam, R.L. Travis, and D.W. Rains, unpublished results). At low NO3− concentrations uptake is
mediated by the inducible high affinity transport system (IHATS); whereas at high NO3− most
ab-sorption is via the low affinity transport system (LATS) [29]. While the induction of IHATS is inhibited by N metabolites [2,15,17,20,30], LATS is a constitutive system [29,31] and may not be affected by amino acids. The induction of NRA in both roots [32] and shoots [33] is regulated by NO3− flux. Thus, the inhibition of induction of
NRA by N metabolites in barley roots at lower NO3− supply may be due to decreased NO3−
up-take rather than a direct effect on the reduction system.
In the present study the role of glutamic acid (Glu), aspartic acid (Asp), Gln and Asn in the induction of the NO3− uptake system and of NRA
in barley roots was investigated. These amino acids were selected because in many plant species they are exported from the leaves to the roots via the phloem in considerable amounts [34 – 37]. The results show that while these amino acids directly inhibited enhancement of the activity of NO3−
uptake system, independent of NO3− availability,
inhibition of the induction of NRA is due to a decrease in NO3− uptake resulting in decreased
NO3− availability.
2. Materials and methods
2.1. Plant culture
Barley (Hordeum 6ulgare L., var. CM-72) seeds
were germinated and grown hydroponically in 0.2 mM CaSO4 in the dark as described previously
[38]. After 6 days, seedlings were transferred into N-free, one-quarter strength Hoagland’s solution [39] and placed in a growth chamber (Western Environmental, Napa, CA, USA) under continu-ous light at a photosynthetic photon flux density (PPFD) of 400 mmol m−2 s−1, 25°C and 60 – 65%
relative humidity. Incandescent bulbs (Sylvania 90A 19/TS/8M/SS, 90 W) and cool white fluores-cent tubes (Sylvania F96T12 VHO/CW, Danvers, MA, USA) supplied light in a ratio of 5:4. The seedlings were either maintained in N-free solution (uninduced seedlings) or were induced with NO3−
in the absence or presence of amino acids as described below.
2.2. Induction of NO3− uptake and reduction systems
Induction is defined as an increase in enzyme activity above the initial endogenous level. For induction about 50 seedlings were placed in 51 of one-quarter strength Hoagland’s solution contain-ing 0.1, 1.0 or 10 mM NO3− and 0 or 1.0 mM
amino acids (Glu, Asp, Gln, Asn) or
diazoacetyl-DL-norleucine methyl ester (DON), an inhibitor of
GOGAT, as indicated in the figure legends and tables. NO3− from the induction solution
contain-ing 0.1 mM NO3− was not allowed to deplete
below 0.07 mM. In some experiments the seedlings were pretreated with the amino acids for 6 h before induction with NO3−.
2.3. Measurement of NO3− uptake
Net NO3− uptake rates were determined by
fol-lowing depletion of NO3− from the uptake
(PPFD 700 mmol m−2 s−1), 25°C, and 50 – 60%
relative humidity. The growth chamber was part of a fully automated high-performance liquid chromatography (HPLC) system fabricated by Goyal and Huffaker [40]. Uptake was initiated by placing 15 seedlings in a 100 ml glass beaker (50 mm id, 80 mm height) containing 60 ml of the uptake solution. The beaker was fitted with a stainless steel screen about 10 mm above the bot-tom and a magnetic bar was placed below the screen. The roots were held above the screen. The beaker was placed on a magnetic stirrer for rapid mixing of the solution. The roots were rinsed for 5 – 10 s in N-free solution containing 0.2 mM CaSO4, before placing them in the uptake
solu-tions. The uptake solutions contained 2 mM MES (pH 6.0), 0.5 mM CaSO4 and 0.1 (IHATS range)
or 1.0 (LATS range) mM NO3−. The uptake
solu-tions were aerated during the measurements. The first sample was withdrawn by the automated sam-pling system about 2 min after the seedlings were transferred into the uptake solutions. Thereafter, the system automatically removed 0.5 ml aliquots at 1.5 min intervals for 12 min for NO3−
determi-nation by the HPLC system. Samples taken from the uptake solutions containing 1.0 mM NO3−
required dilution prior to analysis. Those samples were removed manually every 6 min for 30 min. Cumulative uptake was computed from NO3−
de-pletion and solution volume data and uptake rates were calculated by linear regression analysis of the uptake curves as described by Goyal and Huffaker [40].
2.4. Measurement of total NRA
2.4.1. Preparation of extracts
Roots (1.5 – 2 g) were homogenized with 4 ml of extraction buffer g−1 in a chilled mortar and
pestle with acid-washed sand. The extraction buffer consisted of 0.05 mM Tris – HCl (pH 8.5), 1 mM DTT, 10 mM flavin adenine dinucleotide, 1 mM Na2MoO4, 1 mM EDTA, and 10 mM
leu-peptin [41]. The homogenates were centrifuged at 30 000 g for 15 min, and the supernatants were used for the measurement of NRA, NO3− and
NH4+.
2.4.2. NRA assay
Enzyme activity was assayed in vitro by follow-ing the reduction of NO3−– NO2−. The assay
mix-ture contained 50 mmol potassium phosphate
buffer (pH 7.5), 20mmol KNO3, 0.8mmol NADH,
and 0.2 ml of the extract in a final volume of 1.8 ml. The assays were conducted at 28°C for 15 min. Adding 0.1 ml of 1 M zinc acetate terminated the reaction. Excess NADH was oxidized by phena-zine methosulfate. The NO2−that was formed was
determined colorimetrically as described below.
2.5. NO3−, NO2− and NH4+ determination
NO3− was determined by measuring A210 after
separation by HPLC on a Partisil-10 SAX (Phe-nomenex, Torrance, CA) anion exchange column [42]. NO2−was determined by measuring A540after
color development for 15 min with a 1:1 mixture of 1% (w/v) sulfanilamide in 1.5 M HCl and 0.02% (w/v) n-naphthylethylenediamine dihydrochloride. NH4+ was determined using a continuous flow
system in which the samples reacted with 0.45 M KOH to form NH3. The NH3 thus generated
passed through a Teflon membrane and redis-solved in H2O. The electrical conductivity of the
H2O was then measured [43].
The experiments were repeated 2 – 3 times and the results of representative experiments are re-ported. The data are means9SE of three repli-cates. All results are reported on the basis of root fresh weight.
3. Results
3.1. Effect on NO3− uptake system
Both Glu and Asp, and their respective amines Gln and Asn, partially inhibited increase in the IHATS activity when they were supplied with 0.1 mM NO3− in the growth medium (Fig. 1). After 6
h of exposure to NO3−, the IHATS was inhibited
by 55 – 65%. Both amino acids inhibited increase of IHATS to a greater extent than did their amines (Fig. 1). In contrast, when the seedlings were supplied with 1.0 mM NO3− and uptake rates
were determined at 1.0 mM NO3−, Glu and Asp
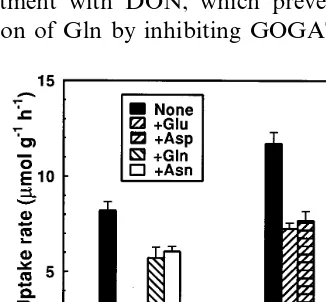
Fig. 1. Effect of amino acids on the inhibition of IHATS and LATS in roots of intact seedlings supplied with NO3−for 6 h. Seedlings were grown hydroponically in N-free solution for 6 days in continuous darkness followed by 24 h in continuous light. The seedlings were then transferred into the solutions containing 0.1 or 1.0 mM NO3− and 1.0 mM amino acid. NO3−uptake rates were determined after 6 h as described in Materials and methods. The uptake solutions contained 0.1 (IHATS) or 1.0 (LATS) mM NO3. Vertical lines above the bars represent +SE.
Fig. 3. Effect of pretreatment with Gln and/or DON on the inhibition of IHATS in roots of intact seedlings supplied with 0.1 mM NO3−for 6 h. Experimental procedure was the same as described in Fig. 2, except that the seedlings were pre-treated with 1.0 mM Gln with or without 0.25 mM DON for 6 h prior to supplying with NO3−. In ‘none’ treatment seedlings were not pretreated. Vertical lines above the bars are +SE.
inhibited the increase in the activity of IHATS and further increased the inhibitory effect of the Gln pretreatment (Fig. 3).
The inhibitory effect of the Glu or Gln plus DON pretreatment on the enhancement of IHATS activity may be due to a decreased concentration of the inducer (NO3−) in the roots. To clarify this
seedlings pretreated with Glu were supplied with 10 mM NO3−. At this high concentration, NO3−
uptake is facilitated by LATS [45]. Under these conditions Glu still inhibited the increase in the NO3− uptake rates (Fig. 4A), even though the
roots accumulated more NO3− than those exposed
to 0.1 mM NO3− in the absence of Glu (Fig. 4B).
3.2. Effect on NRA
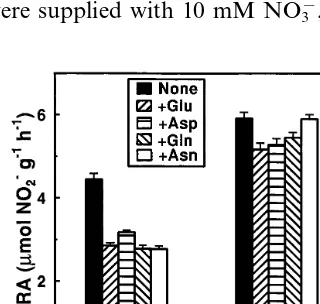
All four amino acids partially inhibited the in-crease of NRA at 0.1 mM NO3− (Fig. 5). The
extent of inhibition was similar in each case. Inhi-bition did not occur when the concentration of NO3− in the external solutions was increased to 10
mM even if the concentration of the amino acids was increased to 10 mM (M. Aslam, R.L. Travis, and D.W. Rains, unpublished results). Pretreat-ment with amino acids resulted in varying re-sponses. Glu was more effective in inhibiting the increase of NRA than was Asp; whereas no inhibi-tion occurred in roots pretreated with Gln or Asn, even at 0.1 mM NO3− (Fig. 6). However, when
IHATS, followed by Asp and Gln and Asn (Fig. 2). The response to Gln and Asn was similar. LATS activity was inhibited only by about 30% by pretreatment with Glu or Asp (Fig. 2). Inhibition of the LATS activity in roots supplied with the amino acids at 1.0 mM NO3− was similar to that
of IHATS in untreated seedlings which were sup-plied with 0.1 mM NO3− (Fig. 1 and Fig. 2).
Pretreatment with DON, which prevents the as-similation of Gln by inhibiting GOGAT [44], also
Fig. 4. Effect of pretreatment with Glu (1.0 mM) on the inhibition of IHATS (A) and NO3−accumulation (B) in roots of intact seedlings supplied with 0.1 or 10 mM NO3−for 6 h. Experimental procedure was the same as described in Fig. 2. except that the seedlings were supplied with 0.1 or 10 mM NO3−. One hour prior to the uptake measurements the seedlings, supplied with 10 mM NO3−, were transferred into solutions containing 0.1 mM NO3− to remove excess NO3− form the cell wall and free space as well as to minimize efflux. Vertical lines above the bars are +SE.
Fig. 6. Effect of pretreatment with amino acids on the inhibi-tion of NRA in roots of intact seedlings supplied with 0.1 (IHATS range) or 10 (LATS range) mM NO3− for 6 h. Experimental procedure was the same as described in Fig. 2. NRA was assayed as described in Materials and methods. Vertical lines above the bars are +SE.
NRA was not inhibited (Fig. 6). Even the inhibi-tion caused by pretreatment with Gln in the pres-ence of DON was alleviated at 10 mM NO3−(Fig.
7).
3.3. Effect on NO3− and NH4+ accumulation
The amino acids used in this study had little effect on NO3− accumulation in roots after the 6 h
induction period (Table 1). However, about four times more NO3− accumulated in roots supplied
with 10 mM NO3−than in those supplied with 0.1
mM NO3−. On the other hand, NH4+accumulation
was affected by amino acid treatment. About two DON was included in the pretreatment media, Gln
significantly inhibited the increase in NRA (Fig. 7). Pretreatment with DON alone also inhibited the increase in NRA (Fig. 7). When pretreated roots were supplied with 10 mM NO3−, increase in
Fig. 5. Effect of amino acids on the inhibition of NRA in roots of intact seedlings supplied with 0.1 (IHATS range) or 10 (LATS range) mM NO3−. Seedlings were grown hydropon-ically in N-free solution for 6 days in continuous darkness followed by 24 h in continuous light. The seedlings were then transferred to the nutrient solutions containing 0.1 or 10 mM NO3−and 0 or 1.0 mM amino acids in one-quarter strength Hoagland’s solution and placed in continuous light. After 6 h NRA was assayed as described in Materials and methods. Vertical lines above the bars are +SE.
Table 1
Effect of amino acids (1 mM) on the concentrations of NO3− and NH4+in roots of intact seedlings supplied with 0.1 or 10 mM NO3−for 6 ha
None 39.10 0.95 1.1590.02
90.05
Asn 10.95 40.48 8.36 9.2390.21
90.23 9.0.87 90.17
aFor experimental procedure see Fig. 1.
bEach value represents the mean9SE.
Table 3
Concentrations of NO3−and NH4+in roots of intact seedlings pretreated with Gln in the presence or absence of 0.25 mM DON for 16 h and then supplied with 0.1 or 10 mM NO3−for
aEach value represents the mean9SE.
or absence of Gln, decreased NO3− accumulation
at 0.1 mM NO3− but had little effect on NH4+
accumulation (Table 3).
4. Discussion
4.1. Effect on NO3− uptake system
When NO3− is supplied to the roots of barley
seedlings the IHATS activity, after a brief lag period, increases rapidly for 8 – 10 h [28,30]. The amino acids used in this study, whether supplied externally with NO3− or as a pretreatment,
par-tially inhibited this increase in the IHATS activity (Fig. 1 and Fig. 2). Glu and Asp were more effective inhibitors than were Gln or Asn, espe-times more NH4+ accumulated in roots supplied
with Gln or Asn as compared to Glu or Asp (Table 1). The accumulation of NH4+ was similar
both at low and high external NO3−concentrations
(Table 1).
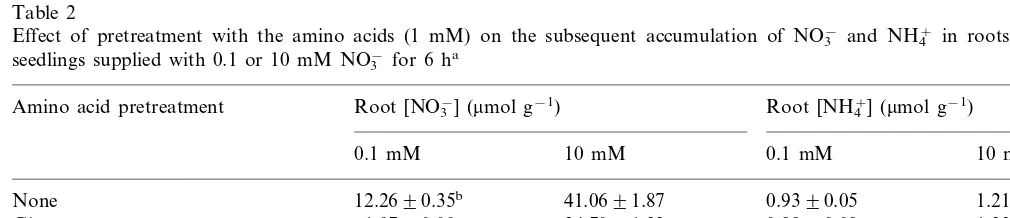
NO3− accumulation was considerably reduced
when roots were pretreated with the amino acids and then supplied with 0.1 mM NO3− (Table 2).
The lowest amount of NO3− accumulated in roots
pretreated with Glu; whereas roots pretreated with Asp, Gln or Asn, accumulated about one-half the amount of NO3− of that in untreated roots. The
amino acid pretreatment decreased NO3−
accumu-lation only slightly at 10 mM NO3−, and
accumu-lation was affected similarly by all amino acids (Table 2). Pretreatment with DON, in the presence
Table 2
Effect of pretreatment with the amino acids (1 mM) on the subsequent accumulation of NO3− and NH4+ in roots of intact seedlings supplied with 0.1 or 10 mM NO3−for 6 ha
aFor experimental procedure see Fig. 2. The seedlings were pretreated with the amino acids for 16 h and then supplied with
NO3−.
cially when roots were pretreated with the amino acids. Vidmar et al. [20] reported similar results from a study comparing the effect of these amino acids on NO3− influx in barley roots. Likewise,
Henriksen and Spanswick [17] reported that pre-treatment with Glu was more effective than Gln in inhibiting increase in the activity of NO3− uptake
system in barley roots. Vidmar et al. [20] reported that when the GOGAT pathway was inhibited, Gln accumulation increased in barley roots. In our experiments, when further assimilation of Gln was prevented by inhibiting the GOGAT pathway, Gln and Glu were equally effective in inhibiting the increase in the activity of the NO3− uptake system,
even when Gln was absent from the external solu-tion (Fig. 2 and Fig. 3). Lee et al. [46] also observed that NO3− uptake by corn roots was
suppressed by all treatments, including DON, that increased the intracellular concentrations of Gln and Asn. These amino acids reportedly reduce the expression ofH6NRT2 transcript in the roots and
that expression is inhibited more by Glu and Asp than by Gln and Asn [20]. LATS, on the other hand, was less severely affected by the amino acid treatments (Fig. 1 and Fig. 2).
Since the exogenously applied amino acids are absorbed and/or metabolized differently, it is difficult to determine which metabolite is the puta-tive inhibitor of the NO3− transport system.
Re-cently, Vidmar et al. [20] exposed barley roots to each of these amino acids then determined their internal concentration. Glu and Gln concentra-tions in the roots increased when they were incu-bated in any of the amino acids, but that increase was greatest when the roots were incubated in Glu or Gln. In all cases the accumulation of Glu was 50 – 70% higher than that of Gln. Nevertheless, based on the observation that azaserine, an in-hibitor of GOGAT, decreased the activity of NO3−
transport system by 95% while decreasing root Glu and increasing Gln levels, Vidmar et al. [20] suggested that Gln is responsible for down regulat-ing expression of the NO3− transport system.
Pretreatment with Glu or Gln plus DON was most effective in inhibiting the increase in the activity of the NO3− uptake system in barley roots
(Fig. 2 and Fig. 3). Since these pretreatments resulted in a decrease in root NO3− concentration
(Table 2 and Table 3) it is possible that increase in the NO3− uptake system activity may be inhibited
by a lack of adequate substrate, NO3−. To test this
possibility roots pretreated with Glu were incu-bated in 0.1 or 10 mM NO3−for 6 h. This resulted
in a two-fold greater NO3− concentration in roots
incubated in 10 mM NO3− relative to 0.1 mM
NO3− (Fig. 4B); however, there was little increase
in the activity of NO3− uptake system in the
for-mer roots (Fig. 4A). This indicates that the in-hibitory effect on NO3− uptake by these amino
acids was not due to a lack NO3−.
Whether the amino acids down regulate the IHATS activity directly or indirectly through their assimilation products is speculative. Henriksen and Spanswick [17] suggested that in roots, even supplied with the amino acids, NH4+ might be the
regulatory metabolite. This would require amino acid deamination followed by the accumulation of NH4+. Our results do not support this
interpreta-tion. In our experiments more NH4+ accumulated
in roots incubated in Gln or Asn (Table 1), but the increase in the activity of NO3− uptake system was
inhibited less than in roots incubated in Glu or Asp (Fig. 1). Also, as noted above, pretreatment with Gln plus DON decreased the IHATS activity more than did Gln alone (Fig. 3), yet the accumu-lation of NH4+ was similar in both treatments
(Table 3). Furthermore, while pretreatment with Glu or Asp inhibited the increase in the activity of NO3− uptake system (Fig. 2), pretreatment with
NH4+ did not [30]. In addition, in an earlier paper
we reported that L-methionine sulfoximine, an
in-hibitor of GS, increased the intracellular concen-tration of NH4+, but had little effect on the NO3−
uptake system [30]. Finally, NH4+ decreased the
NO3−uptake system activity only if it was supplied
with NO3−, and that inhibition was independent of
accumulated NH4+[30,47]. The results suggest that
these amino acids act in barley as they do in soybean [15] by exerting a negative feedback effect on the NO3− uptake system and further suggest
that amino acids transported from shoots to roots control NO3− uptake by plants.
The mechanism by which the amino acids down regulate the activity of IHATS is unknown. It has been reported that these amino acids inhibit the expression of H6NRT2 transcript which encodes
for the transporter in the roots [20]; thus blocking the synthesis of mRNA encoding the NO3−
trans-porter. Alternatively, induction of the NO3−
the transporter may be increased. Also, there may be repression of the synthesis of mRNA by the amino acids.
4.2. Effect on NRA
Although NR is regulated by a variety of fac-tors, NO3, which stimulates the expression
(induc-tion) of the NR gene appears to be the primary signal for induction [48,49]. Consequently, metabolites that affect NO3−uptake may also
infl-uence the induction of NRA. As with the uptake system, enhancement of NRA with 0.1 mM NO3−
was partially inhibited by externally supplied amino acids (Fig. 5). Similar results were obtained when the roots were pretreated with Glu and Asp (Fig. 6). Since the amino acids decreased the con-centration of NO3−in the roots (Table 1 and Table
2), it follows that inhibition may be due to lack of adequate NO3−. Accordingly, when the roots were
supplied with 10 mM NO3− the amino acids did
not inhibit the enhancement of NRA (Fig. 5 and Fig. 6). Exposure of barley roots to NH4+ also
inhibited the enhancement of NRA at 0.1 mM but not at 10 mM NO3− [50].
At 0.1 mM NO3− uptake is facilitated by
IHATS; whereas, at 10 mM a significant amount of NO3− is absorbed via LATS [29,51]. While
IHATS is inducible, LATS is constitutive [51] and was little affected by these amino acids (Fig. 1and Fig. 2). This is apparent because NO3− uptake
rates by roots of seedlings supplied with 1.0 mM NO3− in the presence of the amino acids were
similar to those supplied with 0.1 mM NO3− in
their absence (Fig. 1 and Fig. 2). Since IHATS is saturated at 0.1 mM NO3− [31], inhibition of the
enhancement of NRA at low NO3− concentration
may be due to lack of substrate rather than feed-back inhibition. At 10 mM NO3−, uptake by
LATS would ensure adequate substrate for the enhancement of NRA.
Pretreatment with Gln alone did not inhibit NRA even at 0.1 mM NO3− (Fig. 7). However,
when further assimilation of Gln was inhibited by DON, NRA was inhibited in pretreated roots (Fig. 7). Treatment with DON increased the con-centration of Gln in roots [46]. Nevertheless, at 10 mM NO3− NRA was not inhibited (Fig. 7). These
results indicate that the amino acids prevent the increase of NRA by altering NO3− uptake. In
contrast, Radin [21,22] concluded that the effect of
amino acids appeared to be independent of NO3−
uptake by cotton root tips. Recently, Sivasankar et al. [26] reported that Gln and Asn inhibited the enhancement of NRA in corn roots even at 5 mM NO3−. They also concluded that Gln and Asn
inhibited increase of NRA in corn roots directly rather than by altering NO3− uptake. Our results
indicate that the mechanism by which these amino acids inhibit increase of NRA in barley roots may be different than that in corn and cotton roots. In barley roots, as in soybean roots [15], these amino acids apparently exert a negative feedback effect on NO3− uptake system, which in turn inhibits the
increase of NRA.
In summary, the results show that the amino acids inhibited the NO3−-enhanced increase in
IHATS activity, and that Glu and Asp were more effective inhibitors than were Gln and Asn. In contrast, these amino acids have little effect on LATS. The amino acids partially inhibited the increase of NRA in roots where most NO3−uptake
was facilitated via IHATS but had little effect where LATS is operative. Likewise, increase of the NRA was inhibited when roots were pretreated with Glu or Asp and then exposed to 0.1 mM NO3−. The inhibitory effect of the pretreatment
was relieved when roots were supplied with 10 mM NO3−. The results indicate that amino acids
regulate the increase of NRA indirectly by altering substrate availability.
References
[1] H. Marschner, Mineral Nutrition of Higher Plants, Aca-demic Press, London, 1995, pp. 229 – 312.
[2] P.E. Padgett, R.T. Leonard, Free amino acid levels and the regulation of nitrate uptake in maize cell suspension cultures, J. Exp. Bot. 47 (1996) 871 – 883.
[3] E.J. Hewitt, Assimilatory nitrate – nitrite reduction, Annu. Rev. Plant Physiol. 26 (1975) 73 – 100.
[4] M.G. Guerrero, J.M. Vega, M. Losada, The assimila-tory nitrate-reducing system and its regulation, Annu. Rev. Plant Physiol. 32 (1981) 169 – 204.
[5] B.J. Miflin, P.J. Lea, Amino acid metabolism, Annu. Rev. Plant Physiol. 28 (1977) 299 – 329.
[6] P.J. Lea, S.A. Robinson, G.R. Stewart, The enzymology and metabolism of glutamine, glutamate and asparagine, in: B.J. Miflin, P.J. Lea (Eds.), The Biochemistry of Plants, Intermediary Nitrogen Metabolism, vol. 16, Aca-demic Press, New York, 1990, pp. 121 – 158.
[8] B. Touraine, N. Grignon, C. Grignon, Charge balance in NO3−-fed soybean. Estimation of K+and carboxylate recirculation, Plant Physiol. 88 (1988) 605 – 612. [9] H.D. Cooper, D.T. Clarkson, Cycling of amino-nitrogen
and other nutrients between shoots and roots in cereals. A possible mechanism integrating shoot and root in the regulation of nutrient uptake, J. Exp. Bot. 40 (1989) 753 – 762.
[10] C.-M. Larsson, M. Larsson, J.V. Purves, D.T. Clarkson, Translocation and cycling through roots of recently absorbed nitrogen and sulfur in wheat (Triticum aes-ti6um) during vegetative and generative growth, Physiol.
Plant 82 (1991) 345 – 352.
[11] W.G. Keltjens, J.W. Nieuwenhauis, J.A. Nelemans, Ni-trogen retranslocation in plants of maize, lupin and cocklebur, Plant Soil 91 (1986) 323 – 327.
[12] M. Andrews, The partitioning of nitrate assimilation between root and shoot of higher plants, Plant Cell Environ. 9 (1986) 511 – 519.
[13] X.-Z. Li, D.E. Larson, M. Glibetic, A. Oaks, Effect of glutamine on the induction of nitrate reductase, Physiol. Plant 93 (1995) 740 – 744.
[14] A. Geßler, M. Schultze, S. Schrempp, H. Rennenberg, Interaction of phloem-translocated amino compounds with nitrate net uptake by the roots of beech (Fagus syl6atica) seedlings, J. Exp. Bot. 49 (1998) 1529 – 1537.
[15] B. Muller, B. Touraine, Inhibition of NO3− uptake by various phloem-translocated amino acids in soybean seedlings, J. Exp. Bot. 43 (1992) 617 – 623.
[16] P. Tillard, L. Passama, A. Gojon, Are phloem amino acids involved in the shoot to root control of NO3− uptake in Ricinus communis plants?, J. Exp. Bot. 49 (1998) 1371 – 1379.
[17] G.H. Henriksen, R.M. Spanswick, Investigation of the apparent induction of nitrate uptake in barley (Hordeum
6ulgare L.) using NO3−-selective microelectrodes, Plant
Physiol. 103 (1993) 885 – 892.
[18] C.O. Rodgers, A.J. Barneix, The effect of amino acids and amides on the regulation of nitrate uptake by wheat seedlings, J. Plant Nutr. 16 (1993) 337 – 348.
[19] A.J. Barneix, H.F. Causin, The central role of amino acids on nitrogen utilization and plant growth, J. Plant Physiol. 149 (1996) 358 – 362.
[20] J.J. Vidmar, D. Zhuo, M.Y. Siddiqi, J.K. Schjoerring, B. Touraine, A.D.M. Glass, Regulation of high-affinity nitrate transporter genes and high-affinity nitrate influx by nitrogen pools in roots of barley, Plant Physiol. 123 (2000) 307 – 318.
[21] J.W. Radin, Differential regulation of nitrate reductase induction in roots and shoots of cotton plants, Plant Physiol. 55 (1975) 178 – 182.
[22] J.W. Radin, Amino acid interactions in the regulation of nitrate reductase induction in cotton roots tips, Plant Physiol. 60 (1977) 467 – 469.
[23] A. Oaks, M. Aslam, I. Boesel, Ammonium and amino acids as regulators of nitrate reductase in corn roots, Plant Physiol. 59 (1977) 391 – 394.
[24] A. Oaks, I. Stulen, I. Boesel, Influence of amino acids and ammonium on nitrate reduction in corn seedlings, Can. J. Bot. 57 (1979) 1824 – 1829.
[25] M. Vincentz, T. Moureaux, M.-T. Leydecker, H. Vauce-hert, M. Caboche, Regulation of nitrate and nitrite reductase expression in Nicotiana plumbaginifolia leaves by nitrogen and carbon metabolites, Plant J. 3 (1993) 315 – 324.
[26] S. Sivasankar, S. Rothstein, A. Oaks, Regulation of the accumulation and reduction of nitrate by nitrogen and carbon metabolites in maize seedlings, Plant Physiol. 114 (1997) 583 – 589.
[27] B.J. King, M.Y. Siddiqi, T.J. Ruth, R.L. Warner, A.D.M. Glass, Feedback regulation of nitrate influx in barley roots by nitrate, nitrite, and ammonium, Plant Physiol. 102 (1993) 1279 – 1286.
[28] M. Aslam, R.L. Travis, R.C. Huffaker, Comparative induction of nitrate and nitrite uptake and reduction systems by ambient nitrate and nitrite in intact roots of barley (Hordeum6ulgareL.) seedlings, Plant Physiol. 102
(1993) 811 – 819.
[29] M.Y. Siddiqi, A.D.M. Glass, T.J. Ruth, T.W. Rufty, Studies of the uptake of nitrate in barley. I. Kinetics of 13NO
3
−-influx, Plant Physiol. 93 (1990) 1426 – 1432.
[30] M. Aslam, R.L. Travis, D.W. Rains, R.C. Huffaker, Effect of ammonium on the regulation of nitrate and nitrite transport systems in roots of intact barley (Hordeum 6ulgare L.) seedlings, Planta 200 (1996) 58 –
63.
[31] M. Aslam, R.L. Travis, R.C. Huffaker, Comparative kinetics and reciprocal inhibition of nitrate and nitrite uptake in roots of uninduced and induced barley (Hordeum6ulgareL.) seedlings, Plant Physiol. 99 (1992)
1124 – 1133.
[32] C.A. Neyra, R.H. Hageman, Nitrate uptake and induc-tion of nitrate reductase in excised corn roots, Plant Physiol. 56 (1975) 692 – 695.
[33] D.L. Shaner, J.S. Boyer, Nitrate reductase activity in maize (Zea maysL.) leaves. I. Regulation by nitrate flux, Plant Physiol. 58 (1976) 499 – 504.
[34] H. Winter, G. Lohaus, H.W. Heldt, Phloem transport of amino acids in relation to their cytosolic levels in barley leaves, Plant Physiol. 99 (1992) 996 – 1004.
[35] G. Lohaus, M. Burba, H.W. Heldt, Comparison of the contents of sucrose and amino acids in the leaves, phloem sap and taproots of high and low sugar-produc-ing hybrids of sugar beet (Beta6ulgarisL.), J. Exp. Bot.
45 (1994) 1097 – 1101.
[36] K.M.U. Peeters, A.J. Van Laere, Amino acid metabolism associated with N-mobilization from the flag leaf of wheat (Triticum aesti6umL.) during grain
devel-opment, Plant Cell Environ. 17 (1994) 131 – 141. [37] C. Caputo, A.J. Barneix, Export of amino acids to the
phloem in relation to N supply in wheat, Physiol. Plant 101 (1997) 853 – 860.
[38] M. Aslam, R.C. Huffaker, D.W. Rains, K.P. Rao, Influ-ence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings, Plant Physiol. 63 (1979) 1205 – 1209.
[39] D.R. Hoagland, D.I. Arnon, The water-culture method for growing plants without soil, Calif. Agric. Exp. Stn. Circ. 347 (1950) 1 – 39.
kinetics of NO3−, NO2−, and NH4+ transport simulta-neously by intact wheat seedlings, Plant Cell Environ. 9 (1986) 209 – 215.
[41] T.-M. Kuo, R.L. Warner, A. Kleinhofs, In vitro stability of nitrate reductase from barley leaves, Phytochemistry 21 (1982) 531 – 533.
[42] J.R. Thayer, R.C. Huffaker, Determination of nitrate and nitrite by high-pressure liquid chromatography: Comparison with other methods for nitrate determina-tion, Anal. Biochem. 102 (1980) 110 – 119.
[43] R.M. Carlson, Automated separation and conductimet-ric determination of ammonia and dissolved carbon dioxide, Anal. Chem. 50 (1978) 1528 – 1531.
[44] J.M. Buchanan, Formylglycinamide ribonucleotide amido-transferase, in: S. Prusiner, E.R. Stadtman (Eds.), The Enzymes of Glutamine Metabolism, Academic Press, New York, 1973, pp. 387 – 408.
[45] A.D.M. Glass, M.Y. Siddiqi, T.J. Ruth, T.W. Rufty, Studies of the uptake of nitrate in barley. II. Energetics, Plant Physiol. 93 (1990) 1585 – 1589.
[46] R.B. Lee, J.V. Purves, R.G. Ratcliffe, L.R. Saker, Nitro-gen assimilation and the control of ammonium and
nitrate absorption by maize roots, J. Exp. Bot. 43 (1992) 1385 – 1396.
[47] T.W. Rufty, W.A. Jackson, C.D. Raper, Inhibition of nitrate assimilation in roots in the presence of ammo-nium: The moderating influence of potassium, J. Exp. Bot. 33 (1982) 1122 – 1137.
[48] G. Gowri, J.D. Kenis, B. Ingemarsson, M.G. Riden-baugh, W.H. Campbell, Nitrate reductase transcript is expressed in the primary response of maize to environ-mental nitrate, Plant Mol. Biol. 18 (1992) 55 – 64. [49] D.M. Long, A. Oaks, S.J. Rothstein, Regulation of
maize root nitrate reductase mRNA levels, Physiol. Plant 85 (1992) 561 – 566.
[50] M. Aslam, R.L. Travis, D.W. Rains, R.C. Huffaker, Differential effect of ammonium on the induction of nitrate and nitrite reductase activities in roots of barley (Hordeum 6ulgare) seedlings, Physiol. Plant 101 (1997)
612 – 619.
[51] M.Y. Siddiqi, A.D.M. Glass, T.J. Ruth, M. Fernando, Studies of the regulation of nitrate influx by barley seedlings using 13NO
3
−, Plant Physiol. 90 (1989) 806 –
813.