Contents lists available atScienceDirect
Food Research International
journal homepage:www.elsevier.com/locate/foodres
Changes in polyphenolics during maturation of Java plum (
Syzygium cumini
Lam.)
Lydia Ninan Lestario
a, Luke R. Howard
b,⁎, Cindi Brownmiller
b, Nathan B. Stebbins
b,
Rohana Liyanage
c, Jackson O. Lay
caDepartment of Chemistry, Faculty of Science and Mathematics, Satya Wacana Christian University, 52-60 Diponegoro Street, Salatiga 50711, Central Java, Indonesia bDepartment of Food Science, University of Arkansas, 2650 North Young Avenue, Fayetteville, AR 72704, United States
cUniversity of Arkansas Statewide Mass Spectrometry Facility, 1260 West Maple Street, Fayetteville, AR 72701, United States
A R T I C L E I N F O
Keywords:
Anthocyanins Flavanonols Flavonols Hydrolysable tannins Maturation
Syzygium cumini
A B S T R A C T
Java plum (Syzygium cuminiLam.) is a rich source of polyphenolics with many purported health benefits, but the effect of maturation on polyphenolic content is unknown. Freeze-dried samples of Java plum from seven different maturity stages were analyzed for anthocyanin, flavonol, flavanonol and hydrolysable tannin composition by HPLC. Anthocyanins werefirst detected at the green-pink stage of maturity and increased throughout maturation with the largest increase occurring from the dark purple to black stages of maturation. Levels of gallotannins, ellagitannins,flavonols, gallic acid and ellagic acid were highest at early stages of maturation and decreased as the fruit ripened. For production of antioxidant-rich nutraceutical ingredients, fruit should be harvested immature to obtain extracts rich in hydrolysable tannins andflavonols. The exceptional anthocyanin content of black fruit may prove useful as a source of a natural colorant.
1. Introduction
Syzygium cuminiLamark fruit, known as black plum or java plum is a small edible tropical fruit with a deep purple peel color, white to pink coloredflesh, with one seed inside. The taste is a combination of sweet, sour and astringent. It is usually consumed in Indonesia with salt on the fruit. The leaves, seeds, bark and fruit have long been used in India for the treatment of diabetes (Schossler et al., 2004; Helmstadter, 2008; Kumar et al., 2008; Ayyanar & Subash-Bubu, 2012; Tupe et al., 2015). The health-promoting effects ofSyzygium cuminiextracts are thought to due to various biological activities. Fruit extracts have anti-oxidative activity (Benherial & Arumughan, 2007; Hassimotto, Genovese, & Lajolo, 2005; Faria, Marques, & Mercadante, 2011; Aqil, Jeyabalan, Munagala, Singh, & Gupta, 2016); anti-inflammatory properties (Pavan Kumar, Prashad, Rao, Reddy, & Abhinay, 2010), antibacterial proper-ties (Kaneria, Baravalia, Vaghasiya, & Chanda, 2009), anti-proliferative activities against human lung (Aqil, Gupta, Munagala, Jeyabalan, & Kausar, 2012) and breast cancer cells (Li et al., 2009; Aqil et al., 2016) and pro-apoptopic effects against human breast cancer cells (Li et al., 2009).
The biological activities of Syzygium cumini extracts have been attributed to the abundant and diverse array of phenolic compounds. The fruit is rich in anthocyanins, primarily the diglucosides of delphinidin, cyanidin, petunidin, peonidin, and malvidin (Brito et al., 2007; Veigas, Narayan, Laxman, & Neelwarne, 2007; Faria et al., 2011; Tavares et al., 2016), flavonols, mostly myricetin derivatives (Faria et al., 2011; Tavares et al., 2016), and flavanonols, flavan-3-ols, proanthocyanidins, ellagitannins and gallotannins (Tavares et al., 2016). Anthocyanins and hydrolysable tannins are reported to be the most abundant phenolics in the fruit followed byflavanonols,flavonols andflavan-3-ols (Tavares et al., 2016).
Similar to other pigmented fruit, the color intensity of Java plum fruit increases as the fruit ripens changing from green-yellow in immature fruit to dark purple to black in fully ripe fruit. However, there are limited reports on composition of anthocyanins and other polyphenolics ofSyzygium cuminiat different stages of fruit maturity.
The objective of this study was to identify and quantify anthocyanins, flavonols, flavanonols, and hydrolysable tannins over seven maturity stages ranging from immature to fully ripe. This information will be useful in development of natural colorants or anti-oxidative food additives.
http://dx.doi.org/10.1016/j.foodres.2017.04.023
Received 2 March 2017; Received in revised form 16 April 2017; Accepted 18 April 2017
⁎Corresponding author at: Department of Food Science, University of Arkansas, 2650 N. Young Ave., Fayetteville, AR 72704, United States.
E-mail address:lukeh@uark.edu(L.R. Howard).
2. Material and methods
2.1. Reagents and standards
A mixture of the 3-glucosides of delphinidin, cyanidin, petunidin, peonidin, pelargonidin and malvidin was obtained from Polyphenols Laboratories (Sandnes, Norway). Rutin, gallic acid, ellagic acid, and formic acid were purchased from Sigma Aldrich (St. Louis, MO). HPLC grade methanol, acetonitrile and acetone were obtained from JT Baker Inc. (Phillipsburg, NJ, USA) and formic acid was obtained from Burdick and Jackson (Muskegon, MI, USA).
2.2. Fruit preparation
Syzygium cumini fruit were harvested manually on November 6, 2012 from several trees located at Yogyakarta, Indonesia. On the day of harvest, the trees had similar fruit loads with a full spectrum of fruit from different stages of maturation. After harvest, fruit were separated at seven different stages of maturity based on visual skin color; 1) green-yellow, 2) green-pink, 3) pink, 4) red, 5) light purple, 6) dark purple and 7) black. The color was located predominately in the skin of the fruit, although some fruit had pink to light purple coloration in the pulp. Following color sorting fruit were transported directly to the laboratory. After washing with tap water and manually removing seeds, the edible portions of the fruit at each maturity stage i.e. peel plus pulp, were freeze-dried, pulverized using a coffee grinder and stored at
−20 °C until analysis.
2.3. Polyphenolic extraction
Samples of freeze dried fruit (1 g) from each maturity stage were weighed in 50 mL plastic centrifuge tubes and homogenized for 1 min in 20 mL of extraction solution containing methanol/water/formic acid (60:37:3, v/v/v) using a IKA T18 basic, Ultra Turrax Tissuemizer (Tekmar-Dohrman Corp., Mason, OH). After rinsing the tissuemizer with 5 mL of extraction solution, the homogenates were centrifuged at 2100 xg, 21 °C for 10 min (Allegra, TMX-22R), and filtered through Miracloth (CalBiochem, La Jolla, CA). The extractions were repeated, and thefiltrates were pooled in a 100 mL volumetricflask. The residues were re-extracted with 20 mL of extraction solution containing acet-one/water/acetic acid (AWA) (70:29.5:0.5, v/v/v). All the filtrates were pooled and adjusted to a final volume of 100 mL with AWA solution and stored in 120 mL sealed plastic vials at 4 °C prior to analysis. Extracts (25 mL) were placed in 50 mL plastic vials and dried using a SpeedVac concentrator (Model SC 210A, ThermoSavant, Holbrook, NY). Samples for anthocyanin analysis were re-suspended in 1 mL of 3% formic acid with sonication for 10 min, while samples for analysis offlavonols and other phenolics were re-suspended in 2 mL of 50% methanol with sonication. All samples were passed through 0.45μm PTFE syringe filters (Varian, Inc. Palo Alto, CA) prior to HPLC analysis.
2.4. HPLC and HPLC/ESI-MS analysis of anthocyanins
The HPLC analysis of anthocyanins was conducted using the method of Cho, Howard, Prior, and Clark (2004). Samples (50μL) were analyzed using a Waters HPLC system equipped with a model 600 pump, a model 717 Plus autosampler and a model 996 photodiode array detector. Separation was carried out using a 4.6 mm × 250 mm Symmetry®C18column (Waters Corp, Milford, MA, USA) preceded by a 3.9 mm × 20 mm Symmetry®C18guard column. The mobile phase was a linear gradient of 5% formic acid (A) and methanol (B) from 2% B to 60% B for 60 min at 1 mL/min, then from 60% B to 2% B for 5 min at the sameflow rate. The system was equilibrated for 20 min at the initial gradient prior to each injection. A wavelength of 520 nm was used for peak detection. Individual anthocyanin monoglucosides and
digluco-side derivatives were quantified as Dpd, Cyd, Ptd, Pnd, and Mvd glucoside equivalents using external calibration curves of a mixture of anthocyanin glucosides ranging from 6.25μg/mL to 200μg/mL. Total anthocyanins were calculated as the sum of individual anthocyanin glycosides.
Anthocyanins were identified by HPLC/ESI-MS using conditions described above with the HPLC interfaced to a Bruker Esquire LC/MS ion trap mass spectrometer. Mass spectral data were collected with the Bruker software, which also controlled the instrument and collected the signal at 520 nm. Typical conditions for mass spectral analysis in positive ion electrospray mode for anthocyanins included a capillary voltage of 4000 V, a nebulizing pressure of 30.0 psi, a drying gasflow of 9.0 mL/min and a temperature of 300 °C. Data were collected in full scan mode over a mass range of m/z 50–1000 at 1.0 s per cycle. Characteristic ions were used for peak assignment (Table 1). For compounds where chemical standards were commercially available, retention times were also used to confirm the identification of components.
2.5. HPLC and HPLC/ESI-MS analysis offlavonols,flavanonols and hydrolysable tannins
Flavonols,flavanonols, and hydrolysable tannins were analyzed on the same Waters HPLC system described above according to the method ofHager, Howard, and Prior (2010). Separation was performed using a Phenomenex Aqua 5μm C18(250 × 4.6 mm) column (Torrance, CA). The mobile phase ran at aflow rate of 1 mL/min was a linear gradient of 2% acetic acid (A) and acetic acid:acetonitrile (50:50) (B) from 10% B to 55% B for 50 min, then from 55% B to 100% B for 10 min, then from 100% B to 10% B for 5 min. Detection wavelengths used were 254 nm for ellagic acid, 280 nm for gallotannins and galloyl-HHDP-glucose and 360 nm forflavonols andflavanonols. Individualflavonols andflavanonols and gallotannins were quantified as rutin and gallic acid equivalents, respectively using external calibration curves of authentic standards ranging from 6.25μg/mL to 200μg/mL. The ellagitannins, G-2HHDP-glc, 2G-HHDP-glc and ellagic acid were quan-tified using external calibration curves of ellagic acid ranging 6.25μg/ mL to 200μg/mL.
Flavonols,flavanonols and hydrolysable tannins were identified by HPLC/ESI-MS using the same HPLC conditions described. Mass spectral analysis was performed in negative ion electrospray mode and signals were collected at 280 or 360 nm. Characteristic ions were used for peak assignment (Table 1). For compounds where chemical standards were commercially available, retention times were also used to confirm the identification of components.
2.6. Statistical analysis
The effect of maturation on anthocyanin,flavonol,flavanonol and hydrolysable tannin contents were determined by one-way analysis of variance (ANOVA) using JMP 8.0 (Cary, NC). Differences between means (n = 3) were determined by Student'st-test. (α= 0.05).
3. Results and discussion
3.1. Anthocyanin composition of Syzygium cumini fruit
et al., 2016). Delphinidin was the only anthocyanin identified in the form of 3-O-glucoside. Four additional anthocyanins cyanidin-3-O -glucoside, petunidin-3-O-glucoside; malvidin-3-O-glucoside, and del-phinidin-acetyl-O-diglucoside previously reported byFaria et al. (2011) andTavares et al. (2016)inSyzygium cuminifruit were not detected in our samples. The discrepancy between our results and those ofFaria et al. (2011)andTavares et al. (2016)may be due to varietal difference, variation in environmental growing conditions, or differences in extraction protocol.
3.2. Changes in anthocyanin composition during maturation
The anthocyanin composition of Syzygium cumini fruit extracts during maturation is presented inTable 2. As expected no anthocyanins were detected at the green-yellow of maturity, but low levels of cyanidin-3,5-O-diglucoside, delphindin-3,5-O-diglucoside, malvidin-3,5-O-diglucoside and peonidin-3,5-O-diglucoside were detectable at the green-pink stage of maturity. Levels of the two minor anthocyanins inSyzygium cuminifruit, delphinidin-3-O-glucoside and peonidin-3,5-O -diglucoside were not detectable until the light purple stage of maturity. The anthocyanin content increased sharply when the fruit ripened to dark purple. These results confirm those ofPatel and Rao (2014)who reported a large increase in total anthocyanins inSyzygium cuminias fruit fully ripened. Anthocyanin content has also been reported to increase sharply in fully ripe fruit such asVacciniumspecies (Prior et al., 1998), blackberries (Siriwoharn, Wrolstad, Finn, & Pereira, 2004) and lychee (Rivera-Lopez, Ordorica-Falomir, & Wesche-Ebeling, 1999). Throughout fruit maturation, delphinidin-3,5-O-diglucoside was the predominant anthocyanin accounting for 37–48% of total anthocya-nins, followed by petunidin-3,5-O-diglucoside (29–33%) and malvidin-3,5-O-diglucoside (19–27%), while cyanidin-3,5-O-diglucoside (3%), delphinidin-3-O-glucoside (2–3%) and peonidin-3,5-O-diglucoside (1–2%) were minor constituents. A similar distribution of anthocyanins inSyzygium cuminifruit was reported byFaria et al. (2011)andTavares et al. (2016).
The total anthocyanin content of fully ripe black fruit in this research, 1318.4 mg/100 g DW was higher than a previously reported value of 777 mg/100 g DW (Brito et al., 2007). This difference may be due to the advanced stage of ripened fruit (black) analyzed in our study and is consistent with a previous study that reported over-ripe black-berries to have much higher levels of total anthocyanins than ripe berries (Siriwoharn et al., 2004).
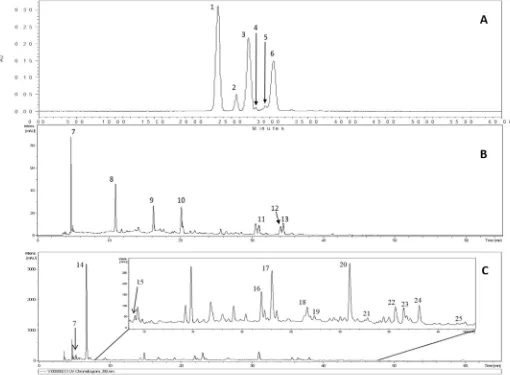
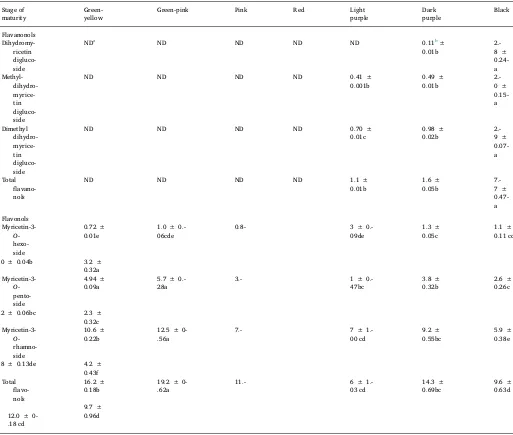
3.3. Flavonol andflavanonol composition of Syzygium cumini fruit
Threeflavanonols detected inSyzygium cuminifruit were dihydro-myricetin diglucoside (m/z643/481), methyl-dihydromyricetin diglu-coside (m/z 657/495), dimethyl-dihydromyricetin diglucoside (m/z 671/509), additionally, three flavonols myricetin-3-O-hexoside (m/z 479/316), myricetin-3-O-pentoside (m/z449/316), and myricetin-3-O -rhamnoside (m/z463/316) were detected (Fig. 1B). These compounds were previously reported byFaria et al. (2011)in addition to myricetin aglycone, which was not detected in our samples.Tavares et al. (2016) identified nineflavonols in skin and pulp extracts ofSyzygium cumini fruit, besides the three flavonols identified in our study they also detected myricetin-3-O-glucuronide, myricetin-3-O-galactoside, larici-trin-3-O-galactoside, laricitrin-3-O-glucoside, syringetin-3-O -galacto-side, and syringetin-3-O-glucoside. They also reported that most of theflavonols present in the fruit were located in the skin.
3.4. Changes inflavonol andflavanonol composition during maturation
The changes in the concentrations of the threeflavonols and three flavanonols detected during maturation are presented in Table 3. Concentrations of the compounds varied in response to maturation. Theflavanonols dihydromyricetin diglucoside,
methyl-dihydromyrice-tin diglucoside, and dimethyl-dihydromyricemethyl-dihydromyrice-tin diglucoside were not detectable until later stages of maturation (light to dark-purple), but concentrations increased markedly as the fruit fully ripened, with the highest concentration at the black stage of maturation. For the flavonols, myricetin-3-O-hexoside was present at low concentration in immature fruit (green-yellow to light-purple), but increased in concen-tration as the fruit fully ripened, with the highest concenconcen-tration present at the black stage of maturation. Conversely, myricetin-3-O-pentoside and myricetin-3-O-rhamnoside were present at the highest concentra-tion in immature fruit (green-yellow to green-pink) and concentraconcentra-tions declined as the fruit fully ripened. Due to thefluctuation in individual Table 1
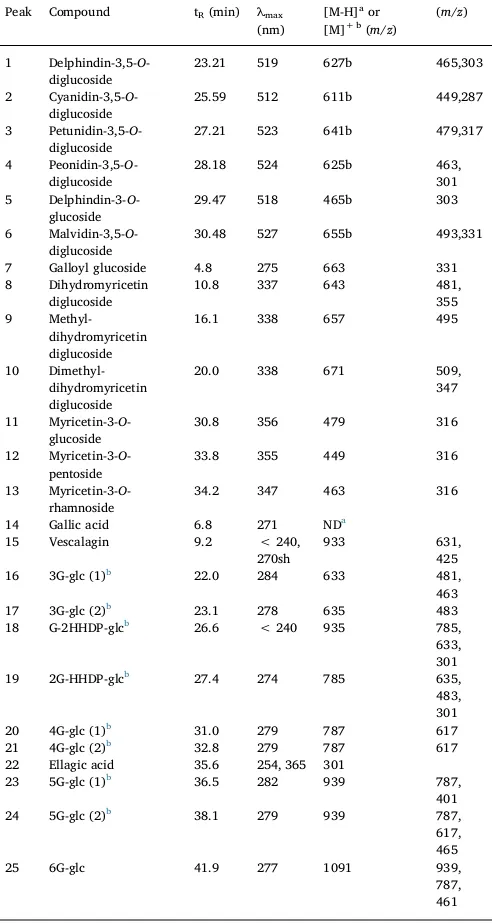
Chromatographic and spectroscopic properties obtained by HPLC-DAD-MS of phenolic compounds fromSyzygium cumini.
Peak Compound tR(min) λmax (nm)
[M-H]aor [M]+ b(m/z)
(m/z)
1 Delphindin-3,5-O -diglucoside
23.21 519 627b 465,303
2 Cyanidin-3,5-O -diglucoside
25.59 512 611b 449,287
3 Petunidin-3,5-O -diglucoside
27.21 523 641b 479,317
4 Peonidin-3,5-O -diglucoside
28.18 524 625b 463, 301 5 Delphindin-3-O
-glucoside
29.47 518 465b 303
6 Malvidin-3,5-O -diglucoside
30.48 527 655b 493,331
7 Galloyl glucoside 4.8 275 663 331 8 Dihydromyricetin
diglucoside
10.8 337 643 481, 355 9
Methyl-dihydromyricetin diglucoside
16.1 338 657 495
10 Dimethyl-dihydromyricetin diglucoside
20.0 338 671 509, 347
11 Myricetin-3-O -glucoside
30.8 356 479 316
12 Myricetin-3-O -pentoside
33.8 355 449 316
13 Myricetin-3-O -rhamnoside
34.2 347 463 316
14 Gallic acid 6.8 271 NDa 15 Vescalagin 9.2 < 240,
270sh
933 631,
425 16 3G-glc (1)b 22.0 284 633 481,
463 17 3G-glc (2)b 23.1 278 635 483 18 G-2HHDP-glcb 26.6 < 240 935 785,
633, 301 19 2G-HHDP-glcb 27.4 274 785 635,
483, 301 20 4G-glc (1)b 31.0 279 787 617 21 4G-glc (2)b 32.8 279 787 617 22 Ellagic acid 35.6 254, 365 301
23 5G-glc (1)b 36.5 282 939 787, 401 24 5G-glc (2)b 38.1 279 939 787,
617, 465
25 6G-glc 41.9 277 1091 939,
787, 461
aIdenti
fication was confirmed with an authentic standard.
b3G-glc (1) = trigalloyl glucose (isomer 1), 3G-glc (2) = trigalloyl glucose (isomer 2),
flavonols during maturation, fruit at the green-pink stage of maturation had the highest concentration of totalflavonols, while fruit at the light-purple and black stages of maturation had the lowest concentrations. In studies involving other fruit, total flavonol content was reported to decline during maturation of pomegranate (Fawole, Opara, & Theron, 2013) and blueberries (Castrejon, Eichholz, Rohn, Kroh & Huyskens-Keil, 2008), but levels remained stable during maturation of
strawber-ries (Kosar, Kafkas, Paydas, & Baer, 2004).
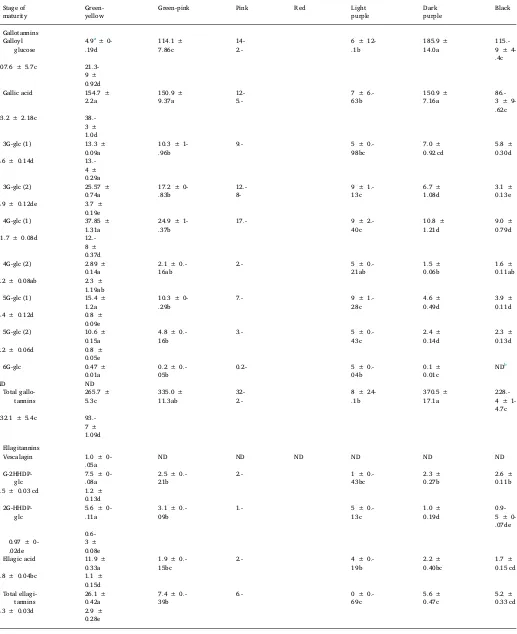
3.5. Hydrolysable tannin composition of Syzygium cumini fruit
Eight gallotannins were identified inSyzygium cuminifruit (Fig. 1C); galloyl glucose (m/z331), two isomers each of trigalloyl glucose (m/z 635), tetragalloyl glucose (m/z 787), and pentagalloyl glucose (m/z
Table 2
Anthocyanin composition (mg/100 g FW) ofSyzygium cuminifruit at different maturity stages.
Stage of maturity Green-yellow Green-pink Pink Red Light purple Dark purple Black
Cyanidin-3,5-O-diglucoside ND1 0.76f2± 0.03 (3%)3 1.22e± 0.03 (3%) 2.58d± 0.22 (3%) 6.7c± 0.2 (3%) 12.8b± 0.56 (3%) 35.6a± 0.69 (3%) Delphinidin-3,5-O
-diglucoside
ND 13.8f± 0.58 (48%) 20.5e± 0.14 (42%)
35.4d± 1.50 (37%)
104.5c± 1.33 (39%)
199.8b± 5.17 (43%)
583.5a± 12.9 (44%) Delphinidin-3-O-glucoside ND ND ND ND 8.6c± 1.17
(3%)
11.0b± 0.19 (2%) 30.8a± 0.82 (2%)
Malvidin-3,5-O-diglucoside ND 5.4f± 0.12 (19%) 11.7e± 0.23 (24%)
25.9d± 1.01 (27%)
62.8c± 7.32 (24%)
98.7b± 1.76 (21%)
268.3a± 3.80 (20%) Petunidin-3.5-O
-diglucoside
ND 8.5f± 0.35 (30%) 15.4e± 0.07 (32%)
30.9d± 1.37 (33%)
80.9c± 2.19 (30%)
139.5b± 3.59 (30%)
385.6a± 6.60 (29%) Peonidin-3,5-O-diglucoside ND ND ND ND 4.1c± 0.14
(2%)
5.9b± 0.05 (1%) 14.6a± 0.38 (1%)
Total anthocyanins ND 28.5f± 1.08 48.8e± 0.09 94.8d± 4.09 267.6c± 11.9 467.7b± 11.2 1318.4 ± 24.7
1Non-detectable.
2Mean values ± standard error of the mean (n = 3). Values within columns with di
fferent letters are significantly different (P < 0.05). 3Values within parentheses represent percentage of total anthocyanins.
939), and hexagalloyl glucose (m/z 1091) in addition to gallic acid. Additionally, three ellagitannins, galloyl-diHHDP-glucose (m/z 935), digalloyl-HHDP-glucose (m/z785) and vescalagin (m/z933) as well as free ellagic acid (m/z301) were identified in the fruit extracts. These compounds were previously identified inSyzygium cuminiskin and pulp extracts using HPLC-DAD-ESI—MS/MS (Tavares et al., 2016) in addi-tion to a number of other gallotannins and ellagitannins that were not detected in our samples. According toTavares et al. (2016)most of the gallotannins and ellagitannins are present in much higher amounts in the skin as opposed to the pulp.
3.6. Changes in hydrolysable tannin composition during maturation
The hydrolysable tannin content ofSyzygium cuminifruit from seven different stages of maturity are presented in Table 4. All of the hydrolysable tannins (gallo- and ellagitannins) were present at the highest concentration in immature (green-yellow) fruit, and their
concentrations declined precipitously as the fruit ripened. An exception was galloyl glucose, which was present at low concentration in green-yellow fruit, but increased with further ripening reaching a maximum concentration at the red stage, followed by a major decline as the fruit fully ripened. Changes in concentration of the phenolic acid gallic acid during maturation also varied from the hydrolysable tannins as concentration remained high until the red stage of maturity and then declined markedly as the fruit fully ripened from red to black. Hexagalloyl glucose (6G-glu) was not detectable from the light purple to black stages of maturity and vescalagin was only detected in the green-yellow stage. Total gallotannin content was highest at the red stage of maturation (370.5 mg/100 g DW) due to the abundance of galloyl glucose and gallic acid, but content decreased to 93.7 mg/100 g DW when the fruit fully ripened to the black stage. Total ellagitannin content was highest in immature green-yellow fruit (26.1 mg/100 g DW) and decreased throughout maturation reaching a low of 2.9 mg/ 100 g DW at the fully ripe black stage. The loss of gallotannins during Table 3
Flavanonol andflavonol contents (mg/100 g DW) ofSyzygium cuminifruit at different maturity stages.
Stage of maturity
Green-yellow
Green-pink Pink Red Light
purple
bMean values ± standard error of the mean (n = 3). Values within columns with di
Table 4
Hydrolysable tannin content (mg/100 g DW) ofSyzygium cuminifruit at different maturity stages.
Stage of maturity
Green-yellow
Green-pink Pink Red Light
purple Gallic acid 154.7 ±
2.2a
Vescalagin 1.0 ± 0-.05a Ellagic acid 11.9 ±
0.33a
aMean values ± standard error of the mean (n = 3). Values within columns with di
maturation are consistent with a previous study on Syzygium cumini fruit, where total tannins measured using a casein precipitation method decreased from 669.4 mg/100 g FW in immature fruit (green-purple) to 185.8 mg/100 g FW in ripe mature fruit (Brandão, Sena, TEShiMA, David, & Assis, 2011). Additionally, the gallotannin content of pome-granate juice prepared from immature fruit and mature-green mangoes had 2-fold higher levels of gallotannins than fully ripe fruit (Fawole et al., 2013; Kim, Brecht, & Talcott, 2007). Loss of extractable tannins during fruit ripening is associated with loss of astringency. It is thought that tannins polymerize during ripening and readily bind to proteins and cell wall polysaccharides, which greatly reduces their extractability and astringency (Goldstein & Swain, 1963). The loss of ellagic acid during maturation agrees with previous studies reporting loss of ellagic acid in many strawberry genotypes during berry maturation (Kosar et al., 2004; Gasperroti et al., 2013).
4. Conclusions
Anthocyanins werefirst detected at the green-pink stage of maturity and increased throughout maturation with the largest increase occur-ring from the dark purple to black stages of maturation. Although present at low concentrations, the levels of threeflavanonols detected also showed the largest increase from the dark purple to black stages of maturation. Levels of gallotannins,flavonols, ellagitannins and ellagic acid were highest at early stages of maturation (green-yellow and green-pink) and decreased as the fruit ripened. For production of nutraceutical ingredients fruit should be harvested immature to obtain extracts rich in gallotannins, ellagitannins, ellagic acid, gallic acid and flavonols. In contrast, fruit should be harvested at the fully ripe black stage to obtain extracts rich in anthocyanins for possible use as a natural colorant.
Appendix A. Abbreviations used
All abbreviations used in this study are listed as follows:
High performance liquid chromatography (HPLC), mass spectro-metry (MS), electrospray ionization (ESI), photodiode array detector (DAD), polytetrafluoroethylene (PTFE), trolox equivalents (TE), trigal-loyl glucose isomer one ((3G-glc (1)), trigaltrigal-loyl glucose isomer two ((3G-glc (2)), tetragalloyl glucose isomer one ((4G-glc (1)), tetragalloyl glucose isomer two ((4G-glc (2)), pentagalloyl glucose isomer one ((5G-glc (1)), pentagalloyl glucose isomer two ((5G-((5G-glc (2)), hexagalloyl glucose (6G-glc), galloyl dihexahydroxydiphenoyl glucose (G-2HHDP-glc), digalloyl dihexahydroxydiphenoyl glucose (2G-HHDP-(G-2HHDP-glc), non-detectiale (ND).
References
Aqil, F., Gupta, A., Munagala, R., Jeyabalan, J., & Kausar, H. (2012). Antioxidant and antiproliferative activities of anthocyanins/ellagitannin-enriched extracts from Syzygium cuminiL. (Jamun the Indian blackberry).Nutrition and Cancer an International Journal,64, 428–438.
Aqil, F., Jeyabalan, J., Munagala, R., Singh, I. P., & Gupta, R. (2016). Prevention of hormonal breast cancer by dietary jamun.Molecular Nutrition & Food Research,60, 1470–1481.
Ayyanar, M., & Subash-Bubu, P. (2012).Syzygium cumini(L.) skeels. A review of its phytochemical constituents and traditional uses.Asian Pacific Journal of Tropical Biomedicine,2, 240–246.
Benherial, P. S., & Arumughan, C. (2007). Chemical composition and in vitro antioxidant studies onSyzygium cuminifruit.Journal of the Science of Food and Agriculture,87, 2560–2569.
Brandão, T. S. D. O., Sena, A. R. D., TEShiMA, E., David, J. M., & Assis, S. A. (2011). Changes in enzymes, phenolic compounds, tannins and vitamin C in various stages of jambolan (Syzgium cuminiLamark) development.Ciência e Tecnologia de Alimentos, 31, 849–855.
Brito, E. S., Araujo, M. C. P., Alves, R. E., Carkeet, C., Clevidence, B. A., & Novotny, J. A. (2007). Anthocyanins present in selected tropical fruits: acerola, jambolao, jussara,
and guajiru.Journal of Agricultural and Food Chemistry,55, 9389–9394. Castrejon, A. D. R., Eichholz, I., Rohn, S., Kroh, L. W., & Huyskens-Keil, S. (2008).
Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosumL.) during fruit maturation and ripening.Food Chemistry,109, 564–572. Cho, M. J., Howard, L. R., Prior, R. L., & Clark, J. R. (2004). Flavonoid glycosides and
antioxidant capacity of various blackberry, blueberry, and red grape genotypes determined by high performance liquid chromatography/mass spectrometry.Journal of the Science of Food and Agriculture,84, 1771–1782.
Faria, A. F., Marques, M. C., & Mercadante, A. Z. (2011). Identification of bioactive compounds from jambolao (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions.Food Chemistry,126, 1571–1578.
Fawole, A. O., Opara, U. L., & Theron, K. I. (2013). Influence of fruit developmental and maturity stages on chemical, phytochemical and antioxidant properties of pomegranate juice.Acta Horticulturae,1007, 461–469.
Gasperroti, M., Masuero, D., Guella, G., Palmieri, L., Martinatti, P., Pojer, E., ... Vrhovsek, U. (2013). Evolution of ellagitannin content and profile during fruit ripening in Fragariaspp.Journal of Agricultural and Food Chemistry,61, 8597–8607.
Goldstein, J. L., & Swain, T. (1963). Changes in tannins in ripening fruit.Phytochemistry, 2, 371–383.
Hager, T. J., Howard, L. R., & Prior, R. L. (2010). Processing and storage effects on the ellagitannin composition of processed blackberry products.Journal of Agricultural and Food Chemistry,58, 11749–11754.
Hassimotto, N. M. A., Genovese, M. I., & Lajolo, F. M. (2005). Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps.Journal of Agricultural and Food Chemistry,53, 2928–2935.
Helmstadter, A. (2008).Syzygium cumini(L.) skeels (myrtaceae) against diabetes-125 years of research.Pharmazie,63, 91–101.
Kaneria, M., Baravalia, Y., Vaghasiya, Y., & Chanda, S. (2009). Determination of antibacterial and antioxidant potential of some medicinal plants from Saurashtra Region, India.Indian Journal of Pharmaceutical Sciences,71, 406–412.
Kim, Y., Brecht, J. K., & Talcott, S. T. (2007). Antioxidant phytochemical and fruit quality changes in mango (Mangifera indicaL.) following hot water immersion and controlled atmosphere storage.Food Chemistry,105, 1327–1334.
Kosar, M., Kafkas, E., Paydas, S., & Baer, K. H. C. (2004). Phenolic composition of strawberry genotypes at different maturation stages.Journal of Agricultural and Food Chemistry,52, 1586–1589.
Kumar, A., Ilavarasan, R., Jayachandran, T., Deecaraman, M., Aravindran, P., & Padmanabhan, N. (2008). Anti-diabetic activity ofSyzygium cuminiand its isolated compound against streptozotocin-induced diabetic rats.Indian Journal of Medicinal Plants Research,2, 246–249.
Li, L., Adams, L. S., Chen, S., Killian, C., Ahmed, A., & Seeram, N. P. (2009).Eugenia jambolanaLam. berry extracts inhibit growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells.Journal of Agricultural and Food Chemistry,55, 1084–1096.
Patel, P. R., & Rao, T. V. R. (2014). Growth and ripening in black plum [Syzygium cumini (L.) skeels].International Journal of Fruit Science,14, 147–156.
Pavan Kumar, K., Prashad, P. D., Rao, A. N., Reddy, P. D., & Abhinay, G. (2010). Anti-inflammatory activity ofEugenia jambolanain albino rats.International Journal of Pharma and Bio Sciences,1, 435–438.
Prior, R. L., Cao, G., Martin, A., Sofic, E., Mc Ewen, J., O'Brien, C., ... Mainland, C. M. (1998). Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety ofVacciniumspecies.Journal of Agricultural and Food Chemistry,46, 2686–2693.
Rivera-Lopez, J., Ordorica-Falomir, C., & Wesche-Ebeling, P. (1999). Changes in anthocyanin concentration in lychee (Litchi chinensisSonn.) pericarp during maturation.Food Chemistry,65, 195–200.
Santos, D. T., Cavalcanti, R. N., Rostagno, M. A., Queiroga, C. L., Eberlin, M. N., & Meireles, M. A. A. (2013). Extraction of polyphenols and anthocyanins from the Jambul (Syzygium cumini) fruit peels.Food and Public Health,3, 12–20.
Sari, P., Wijaya, C. H., Sajuthi, D., & Supratman, U. (2012). Colour properties, stability, and free radical scavenging activity of jambolan (Syzygium cumini) fruit anthocyanins in a beverage model system: Natural and co-pigmented anthocyanins.Food Chemistry, 132, 1908–1914.
Schossler, D. R. C., Mazzanti, C. M., Luz, S. C. A., Filappi, A., Prestes, D., Silveira, A. F., & Cecim, M. (2004).Syzygium cuminiand the regeneration of insulin positive cells from the pancreatic duct.Brazilian Journal of Veterinary Research and Animal Science,41, 236–239.
Siriwoharn, T., Wrolstad, R. E., Finn, C. E., & Pereira, C. B. (2004). Influence of cultivar, maturity, and sampling on blackberry (RubusL. hybrids) anthocyanins,
polyphenolics, and antioxidant properties.Journal of Agricultural and Food Chemistry, 52, 8021–8030.
Tavares, I. M. D. C., Lago-Vanzela, E. S., Robello, L. P. G., Ramos, A. M., Gomez-Alonso, S., Garcia-Romero, E., ... Hermosin-Gutierrez, I. (2016). Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini(L.) skeels).Food Research International,82, 1–13.
Tupe, R. S., Kulkarni, A., Adeshara, K. M., Shaikh, S., Shah, N., & Jadhav, A. (2015). Syzygium jambolanandCephalandra indicahomeopathic preparations inhibit albumin glycation and protect erythrocytes: An in vitro study.Homeopathy,104, 197–204. Veigas, J. M., Narayan, M. S., Laxman, P. M., & Neelwarne, B. (2007). Chemical nature,