Cyclic Voltammetry of Ion Transfer for Phenylpropanolamine Hydrochloride at
Water|Nitrobenzene Interface
Irdhawati Irdhawati,
a,b* Hirosuke Tatsumi,
cIndra Noviandri,
bBuchari Buchari
band Slamet Ibrahim
da
Department of Chemistry, Faculty of Mathematic and Natural Sciences, Udayana University, Jl. By Pass Ngurah Rai Bali 80361, Indonesia
b
Department of Chemistry, Faculty of Mathematic and Natural Sciences, Bandung Institute of Technology, Jl. Ganesha No. 10, Bandung 40132, Indonesia
c
Department of Chemistry, Faculty of Science, Shinshu University, 3-1-1 Asahi Matsumoto 390-8621, Japan d
School of Pharmacy, Bandung Institute of Technology, Jl. Ganesha No. 10, Bandung 40132, Indonesia
(Received November 12, 2010; Accepted March 10, 2011; Published Online August 19, 2011; DOI: 10.1002/jccs.201000580)
The transfer on phenylpropanolamine ion, PPAH+, has been studied at the Interface between Two Immis-cible Solutions (ITIES). The polarizable potential range was determined by cyclic voltammetry at the in-terface between an aqueous solution of lithium chloride (LiCl) and a nitrobenzene (NB) solution of elec-trolyte tetrabutylammonium tetraphenylborate (TBATPB). The half-wave potential of ion transfer for phenylpropanolamine accross the water|NB interface was found 465.3 mV. The peak separation, the diffu-sion coefficient, and the standard ion transfer potential of PPAH+were observed to be 59.1 mV, 1.7´10-6 cm2/s, and 104.6 mV, respectively. The temperature of experiment was kept constantly at 25±1oC using water flow thermostate.
Keywords: Phenylpropanolamine; Ion transfer; Diffusion coefficient; Liquid|liquid interface.
INTRODUCTION
Phenylpropanolamine hydrochloride [(±) norephed-rine hydrochloride, (±)-2-amino-1-phenylpropan-1-ol hy-drochloride, or PPAH+is employed frequently as a bron-chial decongestant, and administrated orally for the symp-tomatic relief of nasal congestion. It has been used for the reduction of appetite and to monitor urinary incontinente. It also finds its use as a bronchodilator and bronchial de-congestant in asthma.1The chemical structure is shown in Fig. 1.
The electrifield interface is an important aspect of all heterogeneous chemical system and in a variety of physico-chemical phenomena. It is a vital feature of many biochem-ical systems as well as of other less complex system, for
ex-ample, colloids, gels, artificial membranes, and metal-elec-trolyte interfaces. Also, the electrochemical reaction at ITIES represents an essential aspect of various practical ap-plications in chemistry including electroanalysis, phase-transfer catalysis, ion extraction, electrocatalysis, and pur-sued experimentally the idea that liquid|liquid interface could serve as a model for a half of a simplified biological membrane. The similarity with a biological membrane sur-face has been noted by Du Bois-Reymond who then sug-gested that the surfaces of biological systems have proper-ties similar to those of an electrode of a galvanic cell. The success of these studies derives from several factors but es-pecially from the smoothness, the unreactivity, and there-fore the reproducibility of this interface.2-4
Reaction in which electrons are transferred from one phase to another are of electrochemical nature, because a charge particle, the electron, is transferred by an applied electric field. However, it would not be reasonable to con-fine electrochemistry to electron transfer only. There is no difference in principle when other charged species, i.e.
* Corresponding author. Tel: +62-361-701954 Ext. 235; Fax: +62-361-703137; E-mail: [email protected] Fig. 1. Chemical structure of phenylpropanolamine
ions, are transferred under the action of an electric field. When an ion has a high chemical affinity toward a certain phase, it will not cross the interface until the electrochemi-cal potential are equal. This will create a potential differ-ence the two phases, which counterbalances the chemical affinity. It is also possible to force ions deliberately from one phase into the other when a potential difference is ap-plied across the interface. Imagine that two immiscible liq-uid phases are filled into a tube, so that they build up a com-mon interface in the middle (Fig. 2).5
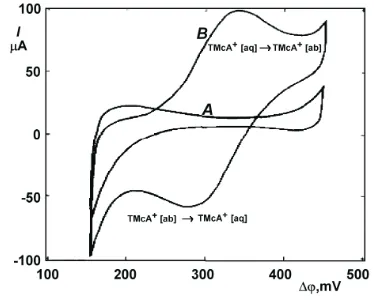
When each of the two liquids contains an electrolyte, which is dissociated (this needs dipolar liquids), and two inert metal electrodes are inserted into the two liquids, it is possible to apply a potential difference across the liquid| liquid interface. For exact measurements one will further introduce into each liquid a reference electrode to control the potential of each of the metal electrode separately. Upon application of a voltage between the two working electrodes, a current may flow. At the two metal electrodes unknown faradaic reactions will occur (electron transfer reaction). However, the overall current has also to cross the liquid|liquid interface. Since the electrolyte solutions on both side are ion conductors only, passage of current can occur only when ions are tranferred from one liquid to the other. The ion transfer at the interface is the rate determin-ing process of the entire current flow. Indeed, it is possible to record a cyclic voltammogram which shows current peaks due to the transfer of, e.g., an anion from water to nitrobenzene and back (Fig. 3). The mid point potential of such cyclic voltammograms also has thermodynamic mean-ing.5
A voltammogram obtained for typical supporting
electrolyte is shown in Fig. 3 (A). The usual supporting electrolytes are lithium chloride (LiCl) solution in the aqueous phase and tetrabutylammonium tetraphenylborate (TBATPB) in the nitrobenzene (NB) phase. Because LiCl is a hydrophilic salt, Li+and Cl-ions will remain confined mostly in the aqueous phase. Similarly, TBATPB dissoci-ates in NB, but its respective ions TBA+and TPB-remain almost exclusively in the NB phase. Thus, an interface be-tween two ionically conductive, but immiscible phases, is formed. Such an interface, as long as it does not charge car-riers, can be polarized to a desired potential value and be-haves as an ideally polarizable interface. The sign conven-tion on ITIES is such that the aqueous phase is most posi-tive at the right extreme of the voltammogram. The onset of the currents limiting the supporting electrolyte working range are due to the supporting electrolyte ions transport into phases in which they are normally absent. The current of the right extreme is caused by crossing of TPB-from NB to water, and Li+in the opposite direction.6
The left limit is caused by Cl-crossing from water into NB and TBA+cation crossing in the reverse direction. In the middle of the potential window only a charging cur-rent corresponding to the double-layer region is observed. This range of potentials in which no significant transport of supporting electrolyte takes place is suitable for studies of semihydrophobic ions, such as tetramethylammonium cat-ion (TMA+) in the following sample. Curve B in Fig. 3
re-Fig. 2. Experimental arrangement for measuring the transfer of ions between two immiscible liquid electrolyte solutions.5
sult from addition of 0.47 mM of TMA+in aqueous phase. If the aqueous phase is made more positive by scanning to the right, a transport of the TMA+cation into NB will oc-cur. Upon reversing the scan at the right extreme, the trans-port of TMA+from NB back to water is observed. The trans-port across the interface is by all indications diffusion con-trolled and therefore the voltammetric curves and equa-tions describing them are similar to those for transport of oxidizable product away from the electrode. Therefore, the voltammograms for both process have similar features, e.g. a separation of the positive and negative peak potential by 58/n mV for a reversible process.6
Numerous methods have been developed for phenyl-propanolamine analysis, including voltammetry,7 potenti-ometry,8,9chromatography,10,11electrophoresis,12 conduct-imetry,13and spectrophotometry methods.14Dvorak et al. reported selective complexation of biogenic amines by macrocyclic polyethers at a liquid|liquid interface, includ-ing phenylpropanolamine (norephedrine). Thermodynam-ics, transport and selectivity in the phase transfer formation of complexes betweenb-phenylethylammonium ions in water and dibenzo-18-crown-6 in NB were observed at 298oK. The result for norephedrine analysis showed the mid point potential and diffusion coefficient were obtained 125 mV and 8´106cm2/s, respectively.7
In this work we employed a voltammetric technique to investigate the mid point or half-wave potential, diffu-sion coefficient, and ion transfer of phenylpropanolamine ion across the water|NB interface.
RESULTS AND DISCUSSION
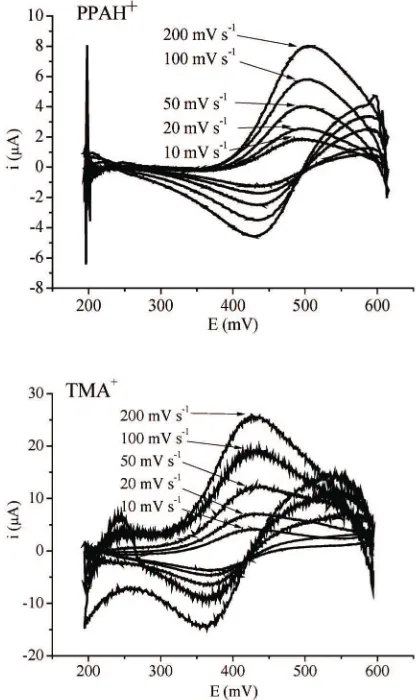
The cyclic voltammograms for ion transfer across the interface between two immiscible electrolyte solution have the shape of a peak known from the classical voltammetry of a simple electron transfer at a planar electrode.16Fig. 4 below shows the anodic and cathodic waves of PPAH+and TMA+0.4 mM at the 0.1 M LiCl (W)|0.1 M TBATPB (NB) from each of five voltammograms taken at 200; 100; 50; 20; and 10 mV s-1.
The voltammograms are characterized by the anodic and cathodic peak potentials, Epaand Epcrespectively; the peak potential difference, DEp, between the anodic and cathodic peaks; the midpoint potential, Epmdefined by (Epa + Epc)/2; and the peak current, Ip. From the Fig. 4, the val-ues of Epa, Epc, and Epmwere observed and shown in the
Ta-ble 1.
The wave of the transfer of PPAH+and TMA+showed the characteristic of the reversible reaction. The peak sep-aration,DEp, at 0.4 mM was obtained to be 59.1 mV and 59.9 mV for PPAH+and TMA+, respectively, closed to the theoretical value ofDEp= 59.9 mV/n, where n is the num-ber of electrons involved in the redox couple. In this case, n is clearly +1, so it can be confirmed that PPAH+ and TMA+are monovalent cations. The pH of PPAH+ contain-ing 0.1 M LiCl was 6.98, and the pKa value is 9.44. The pH of solution was lower than pKa. It showed that PPAH+ ion were in protonated form. In addition, the reversible half-wave potential being giving by E1/2revfor a reversible redox couple, that can be easily found from an experimen-tal voltammogram because it is equal to the mid point po-tential.17
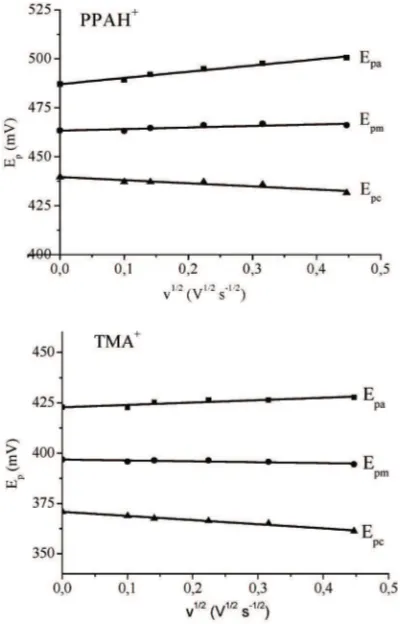
Plots of the square root of scan rate againts peak po-tentials gave straigh lines, and were found to be linear in the scan rate range from 200 – 10 mV s-1. The anodic peak potential increased and shifted to more positive potential and the cathodic peak potential shifted to more negative potential with increasing of scan rate. The plots are shown in Fig. 5.
The peak current increases with larger scan rates. Quantitative information regarding analyte concentration can be obtained from the voltammogram using the
Randles-Sevcik equation, if the temperature is assumed to be 25 o
C,16,18
ip= (2.69´105) n3/2v1/2D1/2A C
where n is the number of electrons appearing in the half-re-action for the redox couple, v is the rate at which the poten-tial is swept (V s-1), D is analyte diffusion coefficient (cm2 s-1), A is the electrode area (cm2), C is the analyte concen-tration (mM), directly proportional to the peak current. If the analyte concentration is a known quantity, cyclic vol-tammetry can be used to measure the analyte diffusion co-efficient. The diffusion coefficient measures of how fast the analyte moves through the solution as a result of ran-dom collision with other molecules. This equation predicts that the peak current shoud be proportional to the square root of the scan rate. For the experimental result depicted in Fig. 6, the electrode area, A, was 0.126 cm2and the concen-tration, C, was 0.4 mM.
As expected, the plot of square root of scan rates ver-sus peak current yields a straight line. The Randles-Sevcik equation can be modified to give an expression for the slope of this straight line as follows,
(slope) = (2.69´105) n3/2A D1/2C
where the constant is understood to have unit 2.69´105C mol-1V-1/2.
For the result in Fig. 6, the slope are 17.854 and 62.051 for PPAH+and TMA+, respectively. After careful substitution and unit analysis, the diffusion coefficient, D,
Table 1. The observed values of anodic, cathodic, peak separa-tion, and mid point potentials (mV) from the cyclic voltammogram of PPAH+and TMA+in the different
scan rates
Epa Epc DEp Epm
PPAH+ 494.9 435.8 59.1 465.3 TMA+ 425.7 365.8 59.9 395.7
Fig. 5. Plots of Epa, Epc, and Epmof the transfer of PPAH+and TMA+at W|NB interface againts v1/2at C = 0.4 mM.
obtained a value equal to 2.1´10-5cm2/s for TMA+, and 1.7´10-6cm2/s for PPAH+. The value for the standard ion transfer potential of TMA+was taken from reference 19.
The standard for ion transfer potential of PPAH+was determined by,
By taking the average of mid point potentials were 465.3 mV and 395.7 mV for PPAH+and TMA+, respectively, the standard ion transfer potential of PPAH+ at W|NB was found to be 104.6 mV.
EXPERIMENTAL Chemicals
Phenylpropanolamine hydrochloride, crystalline pow-der, was obtained from Indonesian Medicine and Food Testing Centre, Jakarta. Tetra-n-butylammonium bromide (TBABr, white-nearly white, crystalline powder, 98%) and tetramethylammonium chloride (TMACl, white-nearly white, crystalline powder, 98%) were purchased from Wako as special grade chemicals. Tetra-n-butylammonium chloride (TBACl, solid, crystal coagulation, extra pure re-agent, 95%) and lithium chloride anhydrous (LiCl, solid, crystal aggregated, guaranted reagent, 99%) were pur-chased from Nacalai Tesque Inc., Kyoto, Japan. Kalibor/ sodium tetraphenylborate (NaTPB, white crystalline pow-der, 99.5%) was purchased from Dojindo. Tetrabutylam-monium tetraphenylborate (TBATPB) was precipitated from aqueous solution of TBABr and NaTPB, repeated crystallization by acetone as described elsewhere.15 Nitro-benzene from Merck and distilled water were used for pre-paring solutions in organic and water phases, respectively. All of the chemicals were used as received without further purification.
Electrochemical Measurements
The ion transfer of phenylpropanolamine ion at the interface between water and nitrobenzene solutions inves-tigated by cyclic voltammetry. As suppoting electrolytes in voltammetric measurement, 0.1 M LiCl and 0.1 M TBATPB were added in water and NB, respectively. The electrochemical cell can be represented by the following scheme:
Scheme I Electrochemical cell for measurement of
ion transfer potential of PPAH+
where x = 0.1, 0.2, 0.3, 0.4, or 0.5 mM. Voltammetric mea-surements for the ion transfer across water and NB inter-face were performed in a three-electrode potentiostat (BASi Epsilon_EC Ver. 1.60.70_XP) with IR compensa-tion, at temperature 25±1oC. The potential difference was applied between two Ag/AgCl electrodes (working and ref-erence electrodes) and the current between working and counter (Pt wire) electrodes was recorded.
ACKNOWLEDGMENT
We gratefully acknowledge to the Directorate Gen-eral of Higher Education, Ministry of National Education, Republic of Indonesia for the Grant in Sandwich-Like Programme (2009) in Department of Chemistry, Faculty of Science, Shinshu University, Matsumoto, Japan.
REFERENCES
1. Ashutosh, K.Medicinal Chemistry, 3rded.; New Age Inter-national: New Delhi, 2005; Chapter 12, p 316.
2. Girault, H. H. J.; Schiffrin, D. J. In Electroanalytical Chemisty: Electrochemistry at Liquid|Liquid Interface; Bard, A. J., Eds.; Marcel Dekker Inc.: New York, 1989; Vol. 15, p 71.
3. Samec, Z.Pure Appl. Chem.2004,76, 2147-2180.
4. Koryta, J.; Vanysek, P.; Brezina, M.J. Electroanal. Chem.
1976,67, 263.
5. Scholz, F. InElectroanalytical Chemistry; Scholz, F.; Eds.; Springer: Heidelberg, 2010, pp 27-29.
6. Vanysek, P.Electrochim. Acta1995,40(18), 2841.
7. Dvorak, O.; Marecek, V.; Samec, Z.J. Electroanal. Chem.
1991,300, 407.
8. Issa, Y. M.; Khalil, M. M.; Zayed, S. I. M.; Hussein, A. Ara-bian J. Chem.2009,2, 41.
9. Ganjali, M. R.; Hariri, M.; Riahi, S.; Norouzi, P.; Javaheri, M.Intern. J. Electrochem. Sci.2009,4(2), 295.
10. Abbasi, K.; Bhanger, M. I.; Khuhawar, M. Y.J. Chem. Soc. Pakistan2008,30(5), 692.
11. Harsono, T.; Yuwono, M.; Indarayanto, G.J. AOAC Intern.
12. Azhagvuel, S.; Sekar, S.J. Pharm. Biomed. Anal. 2007, 43(3), 873.
13. Issa, Y. M.; Youssef, A. P. A.; Mutair, A. A.Farmaco2005, 60(6-7), 541.
14. Khuhawar, M. Y.; Rind, F. M. A.; Rajper, A.J. Food Drug Anal.2005,13(4), 388.
15. Kakutani, T.; Osakai, T.; Senda, M.Bull. Chem. Soc. Jpn.
1983,56, 991.
16. Samec, Z. InElectrochemistry for Environmental
Protec-tion; Rao, D. B.; Eds.; Discovery Publishing House: New Delhi, 2001; No. 2, p 33.
17. Liu, B.; Mirkin, M. V.J. Anal. Chem.2001,1, 670A. 18. Wang,Anal. Electrochem. 2nded.; Wiley-VCH: New York,
2001; Chapter 2, 28.
19. Osakai, T.Rev. Polarogh.2006,52(1), 3-12.