Evide nc e Ba se d Pra c tic e
Ac ute C a re
Surg e ry a nd
Tra um a
Ed ite d b y
Acute Care Surgery
and Trauma:
Evidence Based Practice
Edited by
Stephen M. Cohn
MD
FACS
First published in the United Kingdom in 2009 by Informa Healthcare, Telephone House, 69-77 Paul Street, London EC2A 4LQ. Informa Healthcare is a trading division of Informa UK Ltd. Registered Office: 37/41 Mortimer Street, London W1T 3JH. Registered in England and Wales number 1072954.
Tel: +44 (0)20 7017 5000 Fax: +44 (0)20 7017 6699
Website: www.informahealthcare.com
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP.
Although every effort has been made to ensure that all owners of copyright material have been acknowledged in this publication, we would be glad to acknowledge in subsequent reprints or editions any omissions brought to our attention.
Although every effort has been made to ensure that drug doses and other information are presented accurately in this publication, the ultimate responsibility rests with the prescribing physician. Neither the publishers nor the authors can be held responsible for errors or for any consequences arising from the use of information contained herein. For detailed prescribing information or instructions on the use of any product or procedure discussed herein, please consult the prescribing information or instructional material issued by the manufacturer.
A CIP record for this book is available from the British Library. Library of Congress Cataloging-in-Publication Data
Data available on application
ISBN-10: 1 420 07513 6 ISBN-13: 978 1 420 07513 7
Distributed in North and South America by Taylor & Francis
6000 Broken Sound Parkway, NW, (Suite 300) Boca Raton, FL 33487, USA
Within Continental USA
Tel: 1 (800) 272 7737; Fax: 1 (800) 374 3401 Outside Continental USA
Tel: (561) 994 0555; Fax: (561) 361 6018 Email: orders@crcpress.com
Book orders in the rest of the world Paul Abrahams
Tel: +44 (0)20 7017 4036 Email: bookorders@informa.com
iii
Contents
Contributors . . . viii
Preface . . . xiv
Foreword . . . xvii
Introduction . . . xxi
Section I – Trauma
Evidence for Injury Prevention Strategies: From Private Practice
1.
to Public Policy . . . 1
Michelle A. Price and Cynthia L.Villarreal
Trauma Systems
2.
. . . 8
S. Morad Hameed and Richard K. Simons
Evidence-Based Review of Trauma
3.
Outcomes . . . 18
Michael M. Badellino, John J. Hong, and Michael D. Pasquale
Evidence-Based Surgery: Military Injury Outcomes
4.
. . . 23
Brian J. Eastridge
Evidence-Based Surgery: Traumatized Airway
5.
. . . 29
Edgar J. Pierre and Amanda Saab
Monitoring of the Trauma Patient
6.
. . . 36
Eugene Y. Fukudome and Marc A. de Moya
Resuscitation of the Trauma Patient
7.
. . . 42
David R. King
Diagnosis of Injury in the Trauma Patient
8.
. . . 46
Pedro G. R. Teixeira and Kenji Inaba
An Evidence-Based Approach to Damage Control Laparotomy
9.
for Trauma . . . 56
Bruce Crookes
Evidence-Based Surgery: Coagulopathy in the Trauma Patient
10.
. . . 65
Joseph J. DuBose and Peter M. Rhee
Traumatic Brain Injury
11.
. . . 72
Ara J. Feinstein and Kenneth D. Stahl
Spine and Spinal Cord Injuries
12.
. . . 80
Facial Injuries
13.
. . . 86
Antonio Jorge V. Forte, Renato da Silva Freitas and Joseph H. Shin
Ocular Trauma: An Evidence-Based
14.
Approach to Evaluation
and Management . . . 92
Heidi I. Becker and M. Kelly Green
Neck Trauma
15.
. . . 97
Marc A. de Moya
Emergency Thoracotomy
16.
. . . 102
Joseph J. DuBose and Mark Gunst
Trauma to the Chest Wall
17.
. . . 105
Joseph J. DuBose and Lydia Lam
Evidence-Based Surgery: Injury to the Thoracic Great Vessels
18.
. . . 115
Mark Cockburn
Evidence-Based Surgery: Cardiac Trauma
19.
. . . 121
Dror Soffer and Edan Sarid
Injury to the Esophagus, Trachea, and Bronchus
20.
. . . 125
Deborah L. Mueller
An Evidence-Based Approach to Spleen Trauma: Management
21.
and Outcomes . . . 131
Anne Saladyga and Robert Benjamin
Injury to the Liver
22.
. . . 138
Alberto Garcia, Maria Fernanda Jimenez and Juan Carlos Puyana
Small Bowel and Colon Injuries
23.
. . . 144
Daniel L. Dent
Diaphragmatic Injuries
24.
. . . 147
Fahim Habib
Pancreatic and Duodenal Trauma
25.
. . . 153
Adrian W. Ong and Elan Jeremitsky
Abdominal Vascular Trauma
26.
. . . 160
Joseph E. Glaser and Alexandra A. MacLean
An Evidence-Based Approach to Pregnant Trauma Patients
27.
. . . 165
Igor Jeroukhimov
Pelvic Fractures
28.
. . . 172
Matthew O. Dolich
An Evidence-Based Approach to Extremity Vascular Trauma
29.
. . . 177
Terence O’Keeffe
Surgery of Upper Extremity
30.
. . . 186
Howard Wang, Patrick Schaner and Sahar David
Lower Extremity Injury
31.
. . . 194
Hany Bahouth and Doron Norman
Limb Salvage for the Mangled Extremity
32.
. . . 199
Gabriel E. Burkhardt and Todd E. Rasmussen
Critical Questions in Support of the Burned Patient
33.
. . . 207
Contents v
Burn Wound Management
34.
. . . 212
Joseph H. Shin, Antonio Jorge V. Forte and Renato Freitas
Inhalation Injury
35.
. . . 218
Leopoldo C. Cancio
Electrical, Cold, and Chemical Injuries
36.
. . . 226
Stephanie A. Savage
Evidence-Based Wound Care Management
37.
. . . 233
David Sahar and Howard Wang
Viperidae Snakebite Envenomation
38.
. . . 240
Steven Granger and Ronald Stewart
Evidence-Based Surgery: War Wounds
39.
. . . 245
Lorne H. Blackbourne
Evidence-Based Surgery: Pediatric Trauma
40.
. . . 250
Gerald Gollin
An Evidence-Based Approach to Geriatric Trauma
41.
. . . 258
Carl I. Schulman
Rural Trauma
42.
. . . 265
Burke Thompson
Reducing Patient Errors in Trauma Care
43.
. . . 268
Kenneth D. Stahl and Susan E. Brien
Section II – Emergency General Surgery
Small Bowel Surgery
44.
. . . 278
Erik J. Teicher, John J. Hong, Michael M. Badellino,
and Michael D. Pasquale
An Evidence-Based Approach to Upper GI Bleed Management
45.
. . . 285
John G. Schneider and Bruce A. Crookes
Peptic Ulcer Disease
46.
. . . 290
Wayne H. Schwesinger
Enterocutaneous Fistula
47.
. . . 299
Peter A. Learn
Paraesophageal Hernia Repair
48.
. . . 303
Omid Noormohammadi, Alicia Logue and Kent R. Van Sickle
Appendicitis
49.
. . . 307
Peter P. Lopez and Amy De Rosa
Lower Gastrointestinal Bleeding
50.
. . . 316
Steven D. Schwaitzberg
Diverticular Disease of the Colon
51.
. . . 322
Brent Izu and Akpofure Peter Ekeh
Large Bowel Obstruction
52.
. . . 327
Jerry Lee Howard, John J. Hong, Michael M. Badellino,
and Michael D. Pasquale
Acute and Chronic Mesenteric Ischemia
Ogilvie’s Syndrome and Colonic Volvulus
54.
. . . 336
Raymond P. Compton
Hemorrhoids
55.
. . . 341
Clarence E. Clark III
Anal Fissure, Fistula, and Abscess
56.
. . . 345
W. Brian Perry
Evidence-Based Surgery: Pilonidal Disease
57.
. . . 348
Matthew J. Eckert, Joel E. Goldberg and Scott R. Steele
Rectal Prolapse: Evidence-Based Outcomes
58.
. . . 356
Scott R. Steele and Joel E. Goldberg
Evidence-Based Practice: Acute Cholecystitis
59.
. . . 368
Juliane Bingener
Acute Cholangitis
60.
. . . 374
Adrian W. Ong and Charles F. Cobb
Acute Pancreatitis
61.
. . . 382
Stephen W. Behrman
Pancreatic Pseudo-cysts
62.
. . . 390
Olga N. Tucker and Raul J. Rosenthal
Liver Abscess
63.
. . . 397
Andreas G. Tzakis and Pararas Nikolaos
Diagnosis and Treatment of Variceal Hemorrhage due to Cirrhosis
64.
. . . 406
Robert M. Esterl Jr., Greg A. Abrahamian and K. Vincent Speeg
Gangrene of the Foot
65.
. . . 415
Maureen K. Sheehan
Acute Arterial Embolus
66.
. . . 419
Ryan T. Hagino
Ruptured Abdominal Aortic Aneurysm
67.
. . . 423
Boulos Toursarkissian
Acute Aortic Dissection
68.
. . . 427
V. Seenu Reddy
Deep Venous Thrombosis
69.
. . . 433
Paula K. Shireman
Pulmonary Embolism
70.
. . . 438
George C. Velmahos
Necrotizing Soft Tissue Infections
71.
. . . 443
Mark D. Sawyer
Incarcerated Hernias
72.
. . . 447
Steven Schwaitzberg
Surgical Endocrine Emergencies
73.
. . . 451
Christopher Busken, Rebecca Coefield, Robert Kelly and Steven Brower
Section III – Surgical Critical Care Problems
Evidence-Based Surgery: Bacteremia
Contents vii
Prevention of Central Venous Catheter Infections
75.
. . . 463
J. Matthias Walz and Stephen O. Heard
Ventilator-Associated Pneumonia
76.
. . . 467
Aaron M. Fields
Management of Acute Myocardial Infarction and Cardiogenic Shock
77.
. . . 473
Antonio Hernandez
Perioperative Arrhythmias
78.
. . . 480
Bipin K. Ravindran and Mohan N. Viswanathan
Feeds and Feeding Surgical Patients
79.
. . . 486
Jayson D. Aydelotte
Evidence-Based Surgery: Acute Lung Injury/Acute Respiratory
80.
Distress Syndrome . . . 490
Juan J. Blondet and Greg J. Beilman
Acute Renal Failure
81.
. . . 497
Teofilo Lama
Hyperglycemia
82.
. . . 503
Balachundhar Subramaniam and Alan Lisbon
Abdominal Compartment Syndrome
83.
. . . 509
James C. Doherty
Agitation and Delirium in the ICU
84.
. . . 514
Robert Chen
Malignant Hypertension: An Evidence-based Surgery Review
85.
. . . 523
David S. Owens and Marshall A. Corson
Appendix . . . 533
viii
1
Contributors
Greg A. Abrahamian MD
Assistant Professor of Surgery, Department of Surgery Transplant Center, University of Texas Health Science Center at San Antonio, Texas, USA
Omid Noormohammadi, Alicia Logue MD University of Texas Health Science Center San Antonio, Texas, USA
Joshua B. Alley MD
Major USAF MC, Staff General Surgeon Wilford Hall Medical Center, Lackland AFB Texas. Clinical Assistant Professor, Department of Surgery, University of Texas Health Science Center San Antonio, Texas, USA
Jayson D. Aydelotte MD
Director of Trauma, Department of Surgery Walter Reed Army Medical Center
Washington, DC, USA
Michael M. Badellino MD Associate Professor of Surgery Penn State College of Medicine
Program Director, General Surgery Residency Lehigh Valley Health Network
Allentown, Pennsylvania, USA
Hany Bahouth MD BSC
General and Trauma Surgeon, Director Acute Care Surgery, Rambam Health Campus Haifa, Israel
Heidi I. Becker MD Assistant Professor
Department of Ophthalmology, University of Texas Health Science Center at San Antonio
San Antonio, Texas, USA
Stephen W. Behrman MD FACS
Associate Professor of Surgery, Department of Surgery The University of Tennessee Health Science-Center Memphis, Tennessee, USA
Greg J. Beilman MD FACS
Professor of Surgery, Department of Surgery University of Minnesota
Minneapolis, Minnesota, USA
Robert Benjamin MD FACS Chief of Trauma Services
William Beaumont Army Medical Center Department of Surgery
El Paso, Texas, USA
Bethesda MD
Department of Surgery
Madigan Army Medical Center, Tacoma Fort Lewis, Washington, USA
Juliane Bingener MD
Department of Surgery, Mayo Clinic Rochester, Minnesota, USA
COL Lorne H. Blackbourne MD FACS U.S. Army Institute of Surgical Research Fort Sam Houston
San Antonio, Texas, USA
Juan J. Blondet MD
Postdoctoral Fellow, Department of Surgery University of Minnesota
Minneapolis, Minnesota, USA
Col. W. Brian Perry MD USAF MC
Wilford Hall Medical Center, Lackland AFB San Antonio, Texas, USA
Susan E. Brien MD MEd CSPQ FRCSC CPE
Associate Director, Professional Affairs, Royal College of Physicians and Surgeons of Canada
Ottawa, Ontario, Canada
Steven Brower MD
Division of General Surgery Memorial Health Medical Center Savannah, Georgia, USA
Gabriel E. Burkhardt MD Capt USAF MC
Vascular Surgery Resident, Wilford Hall United States Air Force Medical Center, Lackland Air Force Base The University of Texas Health Science Center at San Antonio, San Antonio, Texas, USA
Contributors ix
Leopoldo C. Cancio MD FACS
Colonel, Medical Corps, U.S. Army, U.S., Army Institute of Surgical Research, Fort Sam Houston
San Antonio, Texas, USA
Robert Chen MD FRCPC
Attending Anaesthetist and Intensivist Assistant Professor
St. Michael’s Hospital, Department of Anaesthesia University of Toronto,
Toronto, Canada, USA
Clarence E. Clark III MD Chief Resident, General Surgery Department of Surgery
University of Texas Health Science Center San Antonio and Wilford Hall Medical Center
San Antonio, Texas, USA
Charles F. Cobb MD
Associate Professor of Surgery, Drexel University College of Medicine, Allegheny General Hospital Department of Surgery
Pittsburgh, Pennsylvania, USA
Mark Cockburn MD
New Rochelle, New York, USA
Rebecca Coefi eld MD Division of General Surgery Memorial Health Medical Center Savannah, Georgia, USA
Raymond P. Compton MD FACS Paris Surgical Specialists,
Chief of Surgery, Henry County Medical Center Paris, Tennessee, USA
Marshall A. Corson MD Associate Professor of Medicine
Head, Section of Cardiology Medical Center Division of Cardiology
University of Washington Medical Center Seattle, Washington, USA
Bruce A. Crookes MD FACS
Assistant Professor of Surgery, Division of Trauma Burns and Critical Care, Department of Surgery University of Vermont College of Medicine Burlington, Vermont, USA
Marc A. de Moya MD FACS Assistant Professor of Surgery Harvard Medical School
Division of Trauma, Emergency Surgery
and Surgical Critical Care, Massachusetts General Hospital Boston, Massachusetts, USA
Amy P. De Rosa DO Resident Surgeon
MCRMC: Department of General Surgery Michigan State University
East Larsing, Michigan, USA
Daniel L. Dent MD
Associate Professor, Division of Trauma and Emergency Surgery, University of Texas Health Science Center San Antonio, Texas, USA
James C. Doherty MD MPH
Director of Trauma Surgery and Critical Care Advocate Christ Medical Center
Oak Lawn, Illinois
Clinical Assistant Professor of Surgery, University of Illinois College of Medicine, Chicago, Illinois, USA
Matthew O. Dolich MD FACS
Associate Clinical Professor, Department of Surgery University of California, Irvine
Orange, California, USA
Joseph J. DuBose MD
Division of Acute Care Surgery, Trauma and Surgical Critical Care, Wilford Hall Medical Center
Lackland AFB, Texas
Clinical Instructor, Trauma and Surgical Critical Care Division of Trauma and Surgical Critical Care Los Angeles County, University of Southern California Hospital
Los Angeles, California, USA
COL Brian J. Eastridge MD FACS Director, Joint Trauma System
U.S. Army Institute of Surgical Research Fort Sam Houston, San Antonio, Texas, USA
Matthew J. Eckert MD
Department of Surgery, Madigan Army Medical Center Fort Lewis, Washington, USA
Akpofure Peter Ekeh MD Associate Professor Wright State University
Department of Surgery, Boonshoft School of Medicine Dayton, Ohio, USA
Robert M. Esterl Jr. MD
Professor of Surgery, Department of Surgery
Transplant Center, University of Texas Health Science Center at San Antonio, Texas, USA
Ara J. Feinstein MD MPH
Ryder Trauma Center, Jackson Memorial Hospital Division of Trauma and Surgical Critical Care DeWitt Daughtry Family Department of Surgery Miami, Florida, USA
Aaron M. Fields MD Assistant Program Director
Critical Care Fellowship, Staff Intensivist Staff Anesthesiologist, United States Air Force Wilford Hall Medical Center
Lackland AFB, Texas, USA
Antonio Jorge V. Forte MD Resident Plastic Surgery, Section of Plastic Surgery Department of Surgery
Yale University School of Medicine New Haven, Connecticut, USA
Renato Freitas MD PhD
Eugene Y. Fukudome MD Resident, Department of Surgery Massachusetts General Hospital Boston, Massachusetts, USA
Alberto Garcia MD
Assistant Professor of Surgery, Universidad del Valle Chief Emergency Department, Fundacion Valle de Lili Cali, Colombia
Joseph E. Glaser MD
Resident, Department of General Surgery New York Hospital of Queens, Flushing New York, USA
Joel E. Goldberg MD FACS
Assistant Professor of Surgery, Brigham and Women’s Hospital
Boston, Masschusetts, USA
Gerald Gollin MD
Associate Professor of Surgery and Pediatrics Loma Linda University School of Medicine Division of Pediatric Surgery
Loma Linda, California, USA
Steven Granger MD Department of Surgery Intermountain Medical Center Salt Lake City, Utah, USA
M. Kelly Green MD
Resident, Department of Ophthalmology
University of Texas Health Science Center at San Antonio San Antonio, Texas, USA
Mark Gunst MD MPH
Division of Acute Care Surgery Trauma and Surgical Critical Care Wilford Hall Medical Center Lackland AFB, Texas, USA
Fahim Habib MD
Assistant Professor of Surgery
University of Miami, Miller School of Medicine Miami, Florida, USA
Ryan T. Hagino MD FACS
Associate Professor, Division of Vascular Surgery University of Texas Health Science Center San Antonio, Texas, USA
S. Morad Hameed MD MPH FRCSC
Assistant Professor of Surgery and Critical Care Medicine, University of British Columbia Vancouver, Canada
Stephen O. Heard MD
Professor, Departments of Anesthesiology and Surgery University of Massachusetts Medical School
Worcester, Masschusetts, USA
Antonio Hernandez MD Assistant Professor
Director of Cardiothoracic & Transplant Anesthesiology Department of Anesthesiology
University of Texas Health Science Center San Antonio, Texas, USA
John J. Hong MD
Chief, Section of Trauma Research Division of Trauma/Surgical Critical Care Lehigh Valley Health Network
Allentown, Pennsylvania
, USA
Jerry Lee Howard MD
Department of Surgery, Lehigh Valley Health Network Allentown, Pennsylvania, USA
Kenji Inaba MD MSC FRCSC FACS Assistant Professor of Surgery University of Southern California Division of Trauma Surgery and Surgical Critical Care Los Angeles, California, USA
Brent Izu MD Resident in Surgery
Wright State University Department of Surgery Boonshoft School of Medicine
Dayton, Ohio, USA
Elan Jeremitsky MD Division of Trauma Surgery Department of Surgery Allegheny General Hospital Pittsburgh, Pennsylvania, USA
Igor Jeroukhimov MD
Head of Trauma Unit Attending in General Surgery Division of Surgery, Hasaf Harofei Medical Center Affi liated with Tel Aviv University
Zerefi n, Israel
Maria Fernanda Jimenez MD
Assistant Professor of Surgery, Javeriana University, Bogota, Colombia
Robert Kelly MD
Division of General Surgery, Memorial Health Medical Center Savannah, Georgia, USA
David R. King MD Major USAF MC
Instructor of Surgery, Division of Trauma Emergency Surgery, Surgical Critical Care Massachusetts General Hospital
Contributors xi
Yoram Klein MD
Assistant Professor of Surgery Chief, Division of Trauma and Emergency Surgery, The Hebrew University School of Medicine - Kaplan Medical Center
Rehovot, Israel
Lydia Lam MD
Critical Care Fellow, Division of Trauma and Surgical Critical Care, Los Angeles County University of Southern California Hospital Los Angeles, California, USA
Teofi lo Lama MD
Trauma and Critical Care Services Saint Mary’s Medical Center West Palm Beach, Florida, USA
Peter A. Learn MD
Clinical Assistant Professor of Surgery University of Texas Health Science Center San Antonio, Texas
Wilford Hall, USAF Medical Center Lackland AFB, Texas, USA
Alan Lisbon MD
Associate Professor of Anaesthesia
Harvard Medical School, Acting Chair, Anesthesia Critical Care and Pain Medicine
Beth Israel Deaconess Medical Center Boston, Masschusetts, USA
Peter P. Lopez MD
Assistant of Professor of Surgery
Division of General and Laparoscopic Surgery University of Texas Health Science Center San Antonio, Texas, USA
Alexandra A. MacLean MD
Assistant Professor of Surgery, Weill Medical College Cornell University, Attending Surgeon
New York Hospital of Queens Flushing, New York, USA
Sapoora Manshaii MD Assistant Professor of Surgery Department of Surgery University of Minnesota Minneapolis, Minnesota, USA
Deborah L. Mueller MD FACS Associate Professor of Surgery
Division of Trauma and Emergency Surgery Department of Surgery, University of Texas Health Science Center
San Antonio, Texas, USA
Pararas Nikolaos MD PhD
Transplant Institute, Miller School of Medicine University of Miami
Miami, Florida
, USA
Doron Norman MD
Orthopedic Surgeon, Director, orthopedic surgery B Rambam Health Campus
Haifa, Israel
Terence O’Keeffe MB ChB BSC FRCS(ED) MSPH Division of Trauma, Critical Care and Emergency Surgery, Department of Surgery, University of Arizona Tucson, Arizona, USA
Adrian W. Ong MD
Assistant Professor of Surgery Drexel University College of Medicine Allegheny General Hospital
Department of Surgery Pittsburgh, Pennsylvania, USA
Michael D. Pasquale MD Associate Professor of Surgery Penn State College of Medicine
Chief, Division of Trauma/Surgical Critical Care Lehigh Valley Health Network
Allentown, Pennsylvania, USA
Edgar J. Pierre MD
Assistant Professor of Anesthesiology Surgery and Care Critical
Department of Anesthesiology, University of Miami Miami, Florida, USA
Brad H. Pollock MPH PhD Professor and Chairman
Department of Epidemiology and Biostatistics
School of Medicine, University of Texas Health Science Center at San Antonio
San Antonio, Texas, USA
Michelle A. Price MEd PhD
Assistant Professor of Surgery, University of Texas Health Science Center at San Antonio
San Antonio, Texas, USA
Basil A. Pruitt, Jr., MD Clinical Professor Department of Surgery
The University of Texas Health Science Center at San Antonio
San Antonio, Texas, USA
Juan Carlos Puyana MD Associate Professor
Surgery and Critical Care Medicine
University of Pittsburgh - Chief Medical Offi cer IMITS Center, UPMC
Pittsburgh, Pennsylvania, USA
Todd E. Rasmussen MD Lt Col USAF MC Chief Division of Surgery
Wilford Hall USAF Medical Center Lackland Air Force
Texas
Associate Professor of Surgery, The Uniformed Services University of the Health Sciences
Bethesda, Maryland, USA
Bipin K. Ravindran MD MPH
Fellow, Cardiology and Cardiac Electrophysiology Department of Medicine, Division of Cardiology University of Washington
John G. Schneider MD
Resident, Department of Surgery
University of Vermont College of Medicine Burlington, Vermont, USA
Carl I. Schulman MD MSPH FACS
DeWitt Daughtry Family Department of Surgery Division of Trauma and Critical Care
University of Miami Miller School of Medicine Miami, Florida, USA
Steven D. Schwaitzberg MD
Chief of Surgery, Cambridge Health Alliance Visiting Associate Professor Surgery
Harvard Medical School Cambridge, Masschusetts, USA
Wayne H. Schwesinger MD FACS Professor of Surgery
Division of General and Laparoendoscopic Surgery Department of Surgery
University of Texas Health Science Center San Antonio, Texas, USA
Maureen K. Sheehan MD FACS Assistant Professor
Division of Vascular Surgery
University of Texas Health Science Center San Antonio, Texas, USA
Joseph H. Shin MD FACS Chief of Plastic Surgery
Department of Surgery, Baystate Medical Center Tufts Medical School
Springfi eld, Masschusetts, USA
Joseph H. Shin MD FACS Chief of Plastic Surgery
Department of Surgery, Baystate Medical Center Tufts Medical School
Springfi eld, Masschusetts, USA
Paula K. Shireman MD FACS
Departments of Surgery and Medicine and
The Sam and Ann Barshop Institute for Longevity and Aging Studies at the University of Texas
Health Science Center
South Texas Veterans Health Care System San Antonio, Texas, USA
Kent R. Van Sickle MD
Division of General and Laparoendoscopic Surgery University of Texas Health Science Center
San Antonio, Texas, USA
Renato da Silva Freitas MD PhD Adjunct Professor of Plastic Surgery Federal University of Parana Curitiba, Brazil
Richard K. Simons MBChB FRCSC Associate Professor of Surgery University of British Columbia Vancouver, Canada
V. Seenu Reddy, MD MBA FACS Assistant Professor
Division of Thoracic Surgery Director Thoracic Aortic Surgery Department of Surgery
University of Texas Health Science Center San Antonio, Texas, USA
Peter M. Rhee MD MPH
Professor of Surgery, Chief, Section of Trauma Critical Care and Emergency Surgery
Arizona Health Sciences Center Department of Surgery
Tucson, Arizona, USA
Raul J. Rosenthal MD FACS The Bariatric Institute Cleveland Clinic Florida Weston, Florida, USA
Amanda Saab MD
Resident in Anesthesiology
Department of Anesthesiology, University of Miami Miami, Florida, USA
Shaul Sagiv MD
Assistant Professor of Orthopedic Surgery, Chief Division of Spine and Orthopedic Trauma The Hebrew University School of
Medicine – Kaplan Medical Center Rehovot, Israel
David Sahar MD
Division of Plastic and Reconstructive Surgery University of Texas Health Science Center San Antonio, Texas, USA
Anne Saladyga MD
William Beaumont Army Medical Center El Paso, Texas, USA
Edan Sarid MD Research Assistant,
The Yitzhak Robin Trauma Division Tel Aviv Sourasky Medical Center Department of Surgery B
Tel Aviv, Israel
Stephanie A. Savage MD Major USAF MC Surgical Critical Care/Chief of Trauma Wilford Hall Medical Center
Lackland AFB, Texas, USA
Mark D. Sawyer MD Consultant in Surgery
Division of Trauma, Critical Care, and General Surgery, The Mayo Clinic Rochester, Minnesota, USA
Patrick Schaner MD
Contributors xiii
Boulos Toursarkissian MD Associate Professor and Chief Division of Vascular Surgery
University of Texas Health Science Center San Antonio, Texas, USA
Olga N. Tucker MD FRCSI
The Academic Department of Surgery The Queen Elizabeth Hospital Birmingham, United Kingdom
Andreas G. Tzakis MD PhD FACS Professor of Surgery
Transplant Institute, Miller School of Medicine University of Miami
Miami, Florida, USA
George C. Velmahos MD PhD MSEd John F. Burke
Professor of Surgery
Harvard Medical School, Chief, Division of Trauma Emergency Surgery, and Surgical Critical Care Massachusetts General Hospital
Boston, Massachusetts, USA
Cynthia L. Villarreal MA Faculty Associate Department of Surgery
University of Texas Health Science Center at San Antonio
San Antonio, Texas, USA
Mohan N. Viswanathan MD Assistant Professor of Medicine
Division of Cardiology Section of Cardiac Electrophysiology
Department of Medicine
University of Washington School of Medicine Seattle, Washington, USA
J. Matthias Walz MD Assistant Professor
Departments of Anesthesiology and Surgery University of Massachusetts Medical School Worcester, Massachusetts, USA
Howard Wang MD
University of Texas Health Science Center San Antonio, Texas
Division of Plastic and Reconstructive Surgery San Antonio, Texas, USA
Steven E. Wolf MD
Betty and Bob Kelso Distinguished Professor in Burns and Trauma, Vice-Chairman for Research Department of Surgery, University of Texas Health Science Center
Chief and Task Area Manager, Clinical Trials United States Army
San Antonio, Texas, USA Dror Soffer MD
Director, The Yitzhak Rabin Trauma, Division Assistant Professor of Surgery, Tel Aviv Sourasky Medical Center, Department of Surgery B Tel Aviv, Israel
K. Vincent Speeg MD PhD
Professor of Medicine/Gastroenterology Transplant Center, University of Texas Health Science Center at San Antonio, Texas, USA
Kenneth D. Stahl MD FACS Assistant Professor of Surgery and
Director of Patient Safety, DeWitt Daughtry Family Department of Surgery, Division of Trauma and
Surgical Critical Care, Director of Patient Safety Research William Lehman Injury Research Center
The University of Miami Leonard M. Miller School of Medicine
Miami, Florida, USA
Scott R. Steele MD FACS
Colon and Rectal Surgery, Madigan Army Medical Center Department of Surgery
Assistant Professor of Surgery Uniformed Services University Fort Lewis, Washington, USA
Ronald Stewart MD
University of Texas Health Science Center San Antonio, Texas
Clinical Instructor, Trauma Surgery San Antonio, Texas
Institute of Surgical Research San Antonio, Texas, USA
Balachundhar Subramaniam MBBS MD
Assistant Professor of Anaesthesia, Harvard Medical School, Director of Cardiac Anesthesia Research Beth Israel Deaconess Medical Center
Boston, Masschusetts, USA
Erik J. Teicher MD
Division of Trauma/Surgical Critical Care Lehigh Valley Health Network
Allentown, Pennsylvania, USA
Pedro G.R. Teixeira MD Research Fellow
University of Southern California Division of Trauma Surgery and Surgical Critical Care
Los Angeles, California, USA
Burke Thompson MD MPH Associate Director of Trauma
Moses Cone Health System, Trauma Program Moses Cone Memorial Hospital
xiv
1
“The questions never change . . . just the answers!”
—Owen Wangensteen
Preface
JOHN HUNTER, FATHER OF EVIDENCE-BASED SURGERY
The first surgeon to apply evidence to the field of surgery was probably John Hunter (1728–1793, Fig. P.1). His approach to medicine is exemplified by his management of gunshot wounds.
Conventional practice dictated that army surgeons open up a gunshot wound—a technique known as “dilatation”— prize out the musket ball or shot with their fingers or forceps prior to cleaning away any debris and dressing the wound. The principle of dilatation stemmed from the belief that gunpowder was poisonous, dating back to its first use in European warfare in the thirteenth century. This doctrine almost certainly increased death and suffering. The acts of incising flesh within a wound were exceedingly painful before the advent of anaesthetic agents and often lead to tremendous loss of blood. In addition, dilatation frequently introduced fatal infection as military surgeons often treated their casualties on muddy, manure-ridden battlefields.
During the conquest of Belle-Ile in 1761, during Britain’s Seven Year War with France, Hunter observed the outcomes of five French soldiers but had been shot in the exchange of gunfire who had managed to hide out in an empty farmhouse. “The first four men had nothing done to their
wounds; indeed very little was done to the men themselves; for they lay in an uninhabited house for more than four days with hardly any subsistence,” Hunter noted. “The wounds were never dilated, nor were they dressed all this time. . . . All of them healed as well, and as soon as the like accidents do in others who have all the care that possibly can be given of them.” Therefore, neglected through acci-dent rather than design, their injuries had healed better than those of their British counterparts who had been subjected to the surgeon’s knife. Hunter believed that wounded soldiers had a better chance of survival by letting nature take its course. While his colleagues dismissed his examples as mere curiosities, Hunter adapted his methods to suit his observations in the first systematic application of scientific evidence to practice.
Hunter’s aim was that young surgeons attending his lectures would always “ask the reasons of things”. He wanted them to take nothing for granted, to subject every common super-stition and unproven therapy to scrutiny. Essentially, he aimed to equip them to elevate surgery to the rank of a science. (Adapted from Knife Man, by W. Moore, 2005)
EVIDENCE-BASED SURGERY
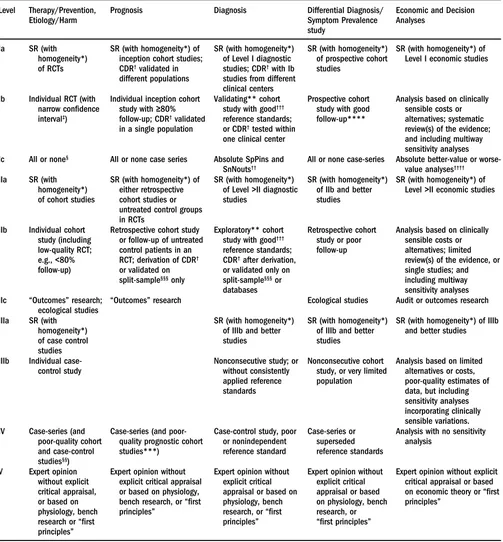
Using evidence-based studies, this textbook focuses on the critical management questions of the day. The book uses publications from the past decade and predominantly cites those published manuscripts that provide Level I and II evidence using the Oxford scale (with permission from the Centre for Evidence-Based Medicine) (see Table P.1).
Preface xv
Table P.1 Oxford Centre for Evidence-Based Medicine Levels of Evidence (May 2001) Level Therapy/Prevention,
SR (with homogeneity*) of inception cohort studies; CDR† validated in
different populations
SR (with homogeneity*) of Level I diagnostic studies; CDR† with Ib
SR (with homogeneity*) of Level I economic studies
Ib Individual RCT (with
narrow confidence interval‡)
Individual inception cohort study with ≥80% follow-up; CDR† validated
in a single population
Analysis based on clinically sensible costs or alternatives; systematic review(s) of the evidence; and including multiway sensitivity analyses
Ic All or none§ All or none case series Absolute SpPins and
SnNouts††
All or none case-series Absolute better-value or worse-value analyses††††
IIa SR (with
homogeneity*) of cohort studies
SR (with homogeneity*) of either retrospective cohort studies or untreated control groups in RCTs
SR (with homogeneity*) of Level >II diagnostic studies
SR (with homogeneity*) of IIb and better studies
SR (with homogeneity*) of Level >II economic studies or follow-up of untreated control patients in an RCT; derivation of CDR†
or validated on split-sample§§§ only
Exploratory** cohort study with good†††
reference standards; CDR† after derivation,
or validated only on split-sample§§§ or
databases
Retrospective cohort study or poor follow-up
Analysis based on clinically sensible costs or alternatives; limited review(s) of the evidence, or single studies; and including multiway sensitivity analyses
IIc “Outcomes” research;
ecological studies
“Outcomes” research Ecological studies Audit or outcomes research
IIIa SR (with
homogeneity*) of case control studies
SR (with homogeneity*) of IIIb and better studies
SR (with homogeneity*) of IIIb and better studies
SR (with homogeneity*) of IIIb and better studies study, or very limited population
Analysis based on limited alternatives or costs,
Analysis with no sensitivity analysis or based on physiology, bench research, or “first principles”
Expert opinion without explicit critical appraisal or based on physiology, bench
Expert opinion without explicit critical appraisal or based on economic theory or “first principles”
Users can add a minus sign, −, to denote the level that fails to provide a conclusive answer because of either a single result with a wide confidence interval (such that, e.g., an. ARR in an RCT is not statistically significant but whose confidence intervals fail to exclude clinically important benefit or harm); or a systematic review with troublesome (and statistically significant) heterogeneity. Such evidence is inconclusive, and therefore can only generate Grade D recommendations.
Abbreviations: CDR, clinical decision rule; RCT, randomized controlled trial; SR, systematic review.
*By homogeneity we mean a systematic review that is free of worrisome variations (heterogeneity) in the directions and degrees of results between individual studies. Not all systematic reviews with statistically significant heterogeneity need be worrisome, and not all worrisome heterogeneity need be statistically significant. As noted, studies displaying worrisome heterogeneity should be tagged with a – at the end of their designated level.
†CDRs are algorithms or scoring systems that lead to a prognostic estimation or a diagnostic category.
‡See foregoing note for advice on how to understand, rate, and use trials or other studies with wide confidence intervals.
§Met when all patients died before the Rx became available, but some now survive on it; or when some patients died before the Rx became available, but none now
die on it.
Table P.2 Grades of recommendation A Consistent Level I studies
B Consistent Level II or II studies or extrapolations from Level I studies C Level IV studies or extrapolations from Level II or III studies
D Level V evidence or troublingly inconsistent or inconclusive studies of any level
Extrapolations are where data are used in a situation that has potentially clinically important differences than the original study situation.
Table P.1 (Continued )
§§By poor-quality cohortstudy we mean one that failed to clearly define comparison groups and/or failed to measure exposures and outcomes in the same (preferably
blinded), objective way in both exposed and nonexposed individuals and/or failed to identify or appropriately control known confounders and/or failed to carry out a sufficiently long and complete follow-up of patients. By poor-quality case-control study we mean one that failed to clearly define comparison groups and/or failed to measure exposures and outcomes in the same (preferably blinded), objective way in both cases and controls and/or failed to identify or appropriately control known confounders.
§§§Split-sample validation is achieved by collecting all the information in a single tranche, then artificially dividing this into “derivation” and “validation” samples. ††An absolute SpPin is a diagnostic finding whose specificity is so high that a positive result rules in the diagnosis. An absolute SnNout is a diagnostic finding whose
sensitivity is so high that a negative result rules out the diagnosis.
‡‡Good, better, bad, and worse refer to the comparisons between treatments in terms of their clinical risks and benefits.
†††Good reference standards are independent of the test and applied blindly or objectively to applied to all patients. Poor reference standards are haphazardly applied,
but still independent of the test. Use of a nonindependent reference standard (where the test is included in the reference, or where the testing affects the reference) implies a Level IV study.
††††Better-value treatments are clearly as good but cheaper, or better at the same or reduced cost. Worse-value treatments are as good and more expensive, or worse
and the equally or more expensive.
**Validating studies test the quality of a specific diagnostic test, based on prior evidence. An exploratory study collects information and trawls the data (e.g., using a regression analysis) to find which factors are significant.
***By poor-quality prognostic cohort study, we mean one in which sampling was biased in favor of patients who already had the target outcome, the measurement of outcomes was accomplished in < 80% of study patients, outcomes were determined in an unblinded, nonobjective way, or there was no correction for confounding factors.
****Good follow-up in a differential diagnosis study is > 80%, with adequate time for alternative diagnoses to emerge (e.g., 1–6 months acute, 1–5 years chronic).
Source: Produced by Bob Phillips, Chris Ball, Dave Sackett, Doug Badenoch, Sharon Straus, Brian Haynes, Martin Dawes, since November 1998.
xvii
1
Foreword
This textbook focuses on important surgical management issues where one or more problems are addressed using scientific evidence from the published literature. This fore-word describes the criteria used for weighing the evidence provided by published research studies. Why evidence-based medicine? The primary use of evidence-evidence-based medicine (EBM) is to help make informed decisions by combining individual clinical expertise with the best available external clinical evidence. This approach optimizes decision making for the care of individual patients (1).
Surgical management issues presented herein are ori-ented toward interventions. Although gathering evidence from intervention studies is the most common use of EBM, the objectives of patient-oriented research studies can alter-natively include determining the etiology of a health prob-lem, determining the accuracy and utility of new tests, and identifying prognostic markers. In this book, EBM is used to assess the safety and efficacy of new treatments and reha-bilitative or preventive interventions. The evidence from multiple studies is often combined to make clinical infer-ences and select the most appropriate treatment plan for individual patients. The goal of this chapter is to describe the ways evidence is evaluated and integrated.
ASSESSING THE VALIDITY OF INTERVENTION STUDIES
Four attributes define the strength of evidence provided by a published intervention study. The first is the level of the evidence—dictated by the type of study design that was used. The second is the quality of evidence—directly related to lack of bias. The third is statistical precision—the degree to which true effects can be distinguished from spurious effects due to random chance. The fourth is the choice of a study endpoint to measure an effect—an end-point’s appropriateness to truly represent a clinically mean-ingful effect—and the magnitude of the observed effect. For practical reasons, the selection of study subjects is almost always a compromise. The degree to which a chosen study population represents an intended target population must also be considered; selection bias can compromise a study’s weight of evidence.
Study Design
Several different types of studies are used in clinical research. Case reports and case series can document the effects of an intervention or clinical course. However, these are subject to selection bias, often use subjective outcome assessment,
and are imprecise due to the small samples. Case reports and case series have no control groups for comparisons. Case-control studies include subjects who have developed the outcome of interest (cases) and a group of unaffected subjects (controls). Case-control studies can be performed in a more timely manner and are often much less expensive than other study designs. However, a temporal relationship between cause and effect can only be inferred, and not directly measured, because of the retrospective in nature of case-control studies. Also, case-control studies are subject to biased recall of antecedent exposures. Selection bias is an important concern, especially the selection of controls. Case-control studies are most often used for very rare out-comes or when there is a long induction period between an exposure and the outcome.
Prospective cohort studies recruit subjects who are free of the outcome of interest. Subjects are then dynami-cally followed over time for the occurrence of the outcome. Recruitment may be selective and based on accruing an equal number of subjects into preselected exposures cate-gories; matching on other factors is possible to reduce con-founding and improve the precision of comparisons across exposure groups. Alternatively, recruitment to cohort stud-ies need not be based on predetermined categorstud-ies of expo-sure; these are typically studies with several exposures of interest. An alternative design is the historical cohort study. These studies use preexisting information, often in a com-prehensive database, to historically classify exposure status. The database is then gleaned for information about subse-quent outcome events. Except for randomized controlled trials, prospective cohort studies are more expensive than other designs. An exposure of interest, such as a new sur-gical procedure versus a conventional procedure, may be linked to unknown or unmeasurable potential confounders. Because cohort studies are not randomized, the distribu-tion of these unknown or unmeasurable confounders may not be balanced between the treatment groups, thus lead-ing to confoundlead-ing. Prospective studies are more time con-suming than case-control studies. A major advantage of cohort designs are that they provide a clear picture of the temporal relationship between a cause and an effect. Match-ing can efficiently reduce confoundMatch-ing. A cohort study is generally simpler and less expensive to conduct than a randomized controlled trial.
There are a number of internal validity considerations. Measurement bias is inaccuracy related to the method of measuring values for a study. Examples include miscali-brated blood pressure readings, inaccurate height measure-ments, flawed laboratory methods that give erroneous values, or less than optimal coding that fails to accurately reflect clinically meaningful categories. Observer bias is inac-curacy related to measuring a study outcome where the observer knows the intervention group assignment. Observer bias is more likely to occur when the chosen outcome measure is subjective. Examples of softer, more subjective measures include the occurrence of symptoms or toxicities, patient self-report measures, and interpretations of physi-cal examination findings. If observers know which treat-ment a patient is receiving, their outcome assesstreat-ments may be biased. Blinded designs are sometimes used to reduce observer bias. Double blinding is a technique in which neither the observer nor the patient knows the treatment assignment. However, blinding may be impractical for many surgical interventions (such as total limb versus partial limb amputation) or for regimens with very idiosyncratic symptom or toxicity profiles. Confounding bias is the mix-ing up of effects so that the primary effect under study cannot be separated from the influence of extraneous fac-tors. For example, failing to account for preoperative dis-ease severity in a randomized trial evaluating two surgical approaches might lead to confounding if the severity dis-tribution differed between groups.
Statistical Precision
Statistical precision for a study results in the ability to dis-tinguish real effects from those due to random chance, that is, chance associations. For example, with just 10 subjects (5 in each group) in an RCT comparing a new postsurgical antibiotic regimen to a conventional regimen for sepsis prophylaxis is likely to result in an extreme finding that can be attributed to random chance, not the true biological drug effect. Chance errors are less likely to occur with larger sample sizes. Trials are always planned to limit the likelihood of chance errors; acceptable levels of error (for Type I and Type II statistical errors) are selected, and the target minimum detectable effect size is chosen. Formal sample size/power calculations are performed during the study’s design to ensure adequate statistical precision.
External Validity
External validity is a function of whether a study’s results can be generalized. The question is, “Does the study pop-ulation possess unique characteristics that might modify the effect of an intervention in a way that would render it ineffective in some other group?” Subjects accrued to a trial may not be representative of the population to which the intervention is intended to be applied. There is a tendency for published surgical and nonsurgical intervention studies to enroll subjects at larger academic institutions. The char-acteristics for these referred patients may not be represen-tative of patients seen at smaller nonacademic centers. Even within a center, subjects that volunteer to participate in a study may not be representative of the institution’s entire clinical population.
Selection bias can occur with the self-selection of indi-viduals who volunteer to participate in a research study. of RCTs is their lower likelihood of confounding bias.
Whereas controlling for known confounders can be per-formed using techniques such as restriction, stratified block design, or statistical adjustment, randomization tends to balance the distribution of unknown or unmeasurable con-founders between treatment groups. RCTs can also be more easily blinded. The disadvantages of RCTs are recruit-ment barriers (particularly for subjects who prefer not to be experimented on) and, because of their prospective nature, higher costs than nonprospective designs such as case-control studies. Even with those limitations, RCTs rep-resent the gold standard; they provide the strongest weight of evidence.
Other study designs are used less frequently in med-ical research. Cross-sectional studies collect both exposure and outcome information simultaneously and may be more applicable for prevalent rather than acute conditions. Cross-sectional studies do not address cause and effect temporal relationships. Cross-over designs are studies in which all subjects serve as their own controls. Half the study popu-lation receives the primary treatment first and then crosses over to receive the second treatment. The other half receives the treatments in reverse order. An assumption of cross-over studies is that the residual effects of a treatment dis-appear by the time the groups are crossed over. This is clearly not applicable for many surgical interventions where a subject’s condition is permanently altered by the therapy (e.g., limb amputation). Pharmaceutical trials where the washout period for the new drug is too long or of unknown duration cannot be evaluated with cross-over designs.
Bias
The strength of scientific evidence provided by an individ-ual study is dependent on a number of key factors. All of these factors must be properly considered before attempt-ing to make clinical inferences from a published study. Ideally, results are published for studies that are both inter-nally and exterinter-nally valid. Compromised validity lowers a study’s weight of evidence.
The design of all patient-oriented research studies is strongly associated with the degree to which bias can poten-tially impact the study results and conclusions. The internal validity for a particular study is affected by observer bias, measurement bias, confounding, and statistical precision. These potential problems manifest themselves in different ways for different study designs.
Foreword xix
SYSTEMATIC REVIEWS
Systematic reviews are a staple of EBM (2). They provide the best means for combining evidence from multiple stud-ies. They follow a defined protocol to identify, summarize, and combine information. Systematic reviews may restrict the inclusion of studies to specific study designs, such as RCTs, or they may include a broader set of designs. Sys-tematic reviews can be very labor intensive and costly. They may attempt to use information from unpublished studies. There are significant challenges in combining evidence from studies that use different designs, or different endpoints, or that vary by other methodological characteristics.
A protocol for a systematic review uses a strict set of guidelines for selecting and amalgamating information from the literature. The Cochrane Collaboration (see www. cochrane.org) guidelines for developing a systematic review protocol requires a background section explaining the con-text and rationale for the review; a statement of the objec-tives; a clear definition of the inclusion and exclusion criteria for studies (including study designs, study popula-tions, types of intervenpopula-tions, and outcome measures); the search strategy for identification of studies; and the meth-odological approach to the review process, including the selection of trials, assignment of methodological quality, data handling procedures; and data synthesis. Data syn-thesis includes statistical considerations such as choice of summary effect measures, assessment of heterogeneity of effect across studies, subgroup analyses, use of random or fixed effects statistical models, and assessment of publica-tion bias.
Meta-Analysis
Systematic reviews often (but not always) include a meta-analysis. The goals of meta-analysis are to provide a precise estimate of the effect and determine if the effect is robust across a range of populations (3). Often a component of systematic reviews, meta-analyses tally the results of each study identified by the reviewer and then calculate the average of those results, if appropriate. Data are first extracted from each individual study and then used to calculate a point estimate of effect along with a measure of uncertainly, for example, the 95 percent confidence inter-val. This is repeated for each of the studies included in the meta-analysis. Then a decision is made about whether the results can be pooled to calculate an average result across all the studies. The decision to combine or not combine studies is made by an assessment of the heterogeneity of effect across studies. Observed statistical heterogeneity suggests that the true underlying treatment effects in the trials are not identical; that is, the observed treatment effects have a greater difference than one should expect due to random error alone. Importantly, uncovering heterogeneity may be the primary goal of a meta-analysis. Analysis of heterogeneity may elucidate previously unrecognized dif-ferences between studies. Only in the absence of significant heterogeneity can study results be combined and a sum-mary measure of effect calculated. Calculation of sumsum-mary measures relies on a mathematical process that gives more weight to the results from studies that provide more infor-mation (usually those with larger study populations) or with higher quality. Often, data for all included studies are Both researchers and participants may bring a multitude
of characteristics to a clinical study, some inherent and some acquired. These can include factors such as gender, race/ethnicity, hair, eye and skin color, personality, mental capability, physical status, and psychological attitudes such as motivation or willingness to participate. Differences in the distribution of these factors between a source popula-tion and a protocol-enrolled study populapopula-tion may intro-duce selection bias. For example, some investigators may preferentially select more athletic-looking subjects for an elective orthopedic surgery clinical trial. Multicenter trials may improve the generalizability of a study, but such stud-ies may still suffer from selection bias.
WEIGHT OF EVIDENCE
Study design, lack of bias, statistical precision, and external validity are elements that affect a study’s weight of evi-dence. Each of these factors must be considered when evaluating a published study. For practical reasons, the investigator who is designing a new study is always con-fronted with trade-offs between these factors and cost. For example, having highly restrictive eligibility criteria reduces confounding but lowers the generalizability of a study. The choice of a more objective endpoint for an antibiotic trial (e.g., death versus confirmed sepsis) decreases observer bias at the cost of decreased statistical precision—fewer deaths compared to the number of incident sepsis cases. Investiga-tors are faced with many challenges when designing inter-vention studies. Because resources are almost always limited, design compromises are made that ultimately impact the overall weight of evidence provided by a study.
LITERATURE REVIEWS
Reviews of published studies can take multiple forms. Reviews can be done of single studies. Single studies may be used as the basis for making treatment decisions. There may be a very large RCT that appropriately evaluated a single clinical endpoint with high validity. This may be sufficient for medical decision making. Alternatively, narrative reviews or systematic reviews evaluate multiple publications.
NARRATIVE REVIEWS
Narrative reviews often address a broad set of clinical ques-tions and are thus less focused on a specific question. They appear more often in the literature and are more qualita-tive and less quantitaqualita-tive. In contrast, systematic reviews are usually focused on a specific clinical issue, incorporate objective criteria for selection of published studies, include an evaluation of quality and worthiness, and often use a quantitative summary to synthesize combined results.
with weaker study designs, such as cohort studies (Level 2), followed by case-control studies (Level 3), case series (Level 4), and at the lowest level, expert opinion (Level 5). Grades of recommendations are based on consistency of higher level studies: An A grade shows consistency across Level 1 studies; a B grade shows consistency across Level 2 or 3 studies or extrapolations from Level 1 studies; a C grade shows consistency across Level 4 studies or extrap-olations from Level 2 or 3 studies; a D grade shows Level 5 evidence or inconsistency across studies of any level.
SUMMARY
EBM is not limited to the evaluation of RCTs and meta-analysis. A broader range of external evidence can be brought to bear on addressing clinical questions (1). Prac-tice guidelines developed using EBM can have a positive impact on patient outcomes. EBM guidelines have reduced mortality from myocardial infarctions and also improved care for persons with diabetes and other common medical problems. EBM supplements physicians’ judgments that might otherwise be based solely on anecdotal clinical expe-rience. Surgical practice can benefit from EBM and should be incorporated into the standard of care.
REFERENCES
1. Sackett DL, Rosenberg WMC, Gray JAM, et al. Evidence based medicine: What it is and what it isn’t. Br Med J 1996; 312: 71–2.
2. Egger M, Smith GD, Altman DG, eds. Systematic Reviews in Health Care. Meta-Analysis in Context, 2nd edition. London: BMJ Publishing Group, 2001.
3. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to Meta-Analysis. New York: Wiley, 2009.
plotted on a graph know as a forest plot, which includes a graphical representation of the magnitude of effect for each study and its degree of uncertainly (plotted as confi-dence intervals). Meta-analysis can evaluate the impact of potential confounders on the treatment effect.
Publication Bias
All studies are subject to Type I errors where evidence is found to reject a null hypothesis when there is no true effect, or Type II errors where evidence is found to not reject the null hypothesis when a true effect exists. Studies with statistically significant results (“positive” studies) are more likely to be accepted for publication than those with-out statistically significant results (“negative” studies). Even adequately powered studies with very low Type II error rates are less likely to be accepted for publication than are smaller positive studies.
LEVELS OF EVIDENCE AND GRADES
OF RECOMMENDATIONS
All reviews evaluate historical information and are there-fore subject to systematic bias and random error. For dif-ferent study objectives (e.g., determining the impact of a therapeutic or preventive intervention), the Oxford Centre for Evidence-Based Medicine Levels of Evidence displays the level of evidence based on a review of the literature, study design, and quality. The highest level of evidence for a therapeutic intervention is provided by systematic reviews of large RCTs that show homogeneity of effect across trials (Level 1a); the next highest is for an individual RCT with a narrow confidence interval (Level 1b); this is followed by an all-or-none effect related to the introduction of a treatment (Level 1c). The level of evidence decreases
Brad H. Pollock MPH PhD
xxi
1
Introduction
Sir William Osler said, “To study the phenomena of disease without books is to sail an uncharted sea, while to study books without patients is not to go to sea at all” (1). Today we can expand on that aphorism by saying, “to practice surgery without scientific understanding is like sailing without a rudder.” Surgeons must use their understand-ing of disease pathogenesis and knowledge of treatment effectiveness to define the science of surgery, which enables them to achieve early diagnosis and apply appropriate treatment with resultant maximum salvage and optimum outcomes.
Surgical practice has always been based on the scien-tific understanding of the day. That understanding has slowly evolved to a knowledge base that is continually expanding and being refined by sophisticated high-technology laboratory studies and the randomized con-trolled clinical trials of today. Until the 19th century, scientific understanding was commonly based on personal observa-tion and opinion or, at best, anatomic dissecobserva-tions and com-parisons. The impact of recommendations based on scientific thought generated in that fashion and the credence accorded such were related directly to the prominence and reputa-tion of the individual making the recommendareputa-tion. His-torically, surgical authority, as the determinant of surgical treatment, has migrated from individual to individual and from country to country, for example, from Hippocratic Greece to Galenic Rome.
The retardation of surgical progress and impairment of patient care due to ascientific surgical dogma are well illustrated by the tortured history of wound care. Hippo-crates (460–377 B.C.) recommended making pus form in the wound as soon as possible for the counterintuitive pur-pose of reducing inflammation (2). When Rome gained medical ascendancy, Galen (130–200 A.D.), by being a proponent of suppuration as a beneficial, even essential component of wound healing, furthered the concept of “laudable” pus (2,3). In the 13th century, Theodoric pub-lished Chirurgia , in which he advanced the then-heretical opinion that the formation of pus was not necessary for wound healing. That opinion was largely ignored, even though the concept of pus-free healing was supported by Henri de Mondeville of France in his 14th-century textbook Chirurgie (2). Guy de Chauliac further extended the author-ity of French surgeons by publication of his seven-part work, La Grande Chirurgie , in 1363. Unfortunately, de Chauliac fully supported the importance of laudable pus and has thus been credited by some with having arrested progress in wound care for more than five centuries (4).
The limitations of authoritarian opinion and dogma in the absence of scientific understanding are further illus-trated by the “gunpowder as poison” controversy. In 1460, Heinrich von Pfolspeundt prepared his Buch Der Bündth-Ertznei , in which he mentioned “powder-burns” caused by gunshots (5). The concept of poisoned gunshot wounds was extended by Brunschwig, who in his 1497 book Dis Ist Das Buch Der Chirurgia Hantwirckung Der Wundartzny recommended using boiling oil or cautery to make wounds suppurate. In the next century, during the siege of Turin, Ambroise Paré noted the absence of severe inflammation in casualties treated without the customary boiling oil. Despite that observation, Paré persisted in a search for a “perfect” salve to stimulate suppuration in the belief that suppuration was required for optimum healing (6). In 16th-century England, Clowes advocated avoiding cautery, but in the next century, Richard Wiseman, whom some consider to have been the father of English surgery, reintroduced cautery and recommended incorporating raw onions in the dressings to counteract the effects of the gunpowder (2,7). In the last decade of the 18th century (1794), John Hunter, on the basis of his experience in the treatment of war wounds, proposed in his posthumously published book A Treatise on the Blood, Inflammation, and Gun-shot Wounds that a gunshot wound should be treated like other wounds. Hunter’s stature at the time was so great that early American surgeons such as Jones, Morgan, and Ship-pen commonly traveled to England to work with him and complete their training. Consequently, his opinion was considered to have resolved the controversy. Unfortunately, Hunter also recommended that gunshot wounds should not be opened or made larger (6). That recommendation, which made the subsequent appearance of laudable pus virtually certain, did little to improve the control of infec-tion in war wounds. Infecinfec-tions in patients with war wounds remained common and were associated with prohibitively high mortality rates, for example, 97% in patients with pyemia in the U.S. Civil War (8).