Characteristics of B2O3 and Fe added into BaFe12O19 permanent magnets prepared at
different milling time and sintering temperature
Perdamean Sebayang, Ayu Yuswita Sari, Delovita Ginting, Yola Allan, Nasruddin M. N., and Kerista Sebayang
Citation: AIP Conference Proceedings 1711, 020004 (2016); doi: 10.1063/1.4941613
View online: https://doi.org/10.1063/1.4941613
View Table of Contents: http://aip.scitation.org/toc/apc/1711/1
Published by the American Institute of Physics
Articles you may be interested in
Synthesis and characterization of barium hexaferrite with manganese (Mn) doping material as anti-radar AIP Conference Proceedings 1801, 040007 (2017); 10.1063/1.4973096
Synthesis and magnetic study of magnetically hard-soft SrFe12-yAlyO19 - x Wt.% Ni0.5Zn0.5Fe2O4 nanocomposites
AIP Advances 7, 055602 (2017); 10.1063/1.4978398
Preparation of barium hexaferrite powders using oxidized steel scales waste AIP Conference Proceedings 1711, 020002 (2016); 10.1063/1.4941611
Physical characteristics and magnetic properties of BaFe12O19/SrTiO3 based composites derived from mechanical alloying
AIP Conference Proceedings 1725, 020098 (2016); 10.1063/1.4945552
Site occupancy and magnetic properties of Al-substituted M-type strontium hexaferrite Journal of Applied Physics 117, 243904 (2015); 10.1063/1.4922867
Characteristics of B
2
O
3
and Fe Added Into BaFe
12
O
19
Permanent Magnets Prepared At Different Milling Time
and Sintering Temperature
Perdamean Sebayang
1)
, Ayu Yuswita Sari
1)
, Delovita Ginting
2)
, Yola
Allan
2)
,
Nasruddin M. N.
2)
, Kerista Sebayang
2)
1
Research Center for Physics LIPI, Kawasan PUSPIPTEK, Serpong – Indonesia, 15314 2
Postgraduate Program, Faculty of Mathematic and Natural Science, University of Sumatera Utara-Indonesia, 20155
Corresponding author: [email protected]
Abstract. The objective of present work is to investigate the characteristic of BaFe12O19, B2O3-BaFe12O19 and
Fe-BaFe12O19 magnets fabricated at different milling time and sintering temperature. The characteristic of perrmanen magnet
BaFe12O19 with different content of B2O3 and Fe which was fabricated at different milling time and sintering temperature
were investigated. The powder mixtures were prepared by dry and wet milling at various milling time. The powder were mixtured and prepared by dry and wet milling at various milling time. The mixture powder was then compacted by anisotropic with compressive pressure of 50 N/cm2. The green bodies were sinter at 1050, 1100, 1150 and 1200o C and hold for 1 h, separately. The density, magnetic flux density and B-H curve were measured by Archimedes principle, Gauss meter and Permagraph, respectively. The microstructure and phase composition characterization were performed by SEM and XRD. The results of this study are presented in this paper. It shows that addition of Fe (in wet milling) and B2O3 (in dry milling) respectively give a potential benefit to reduce the sintering temperature and improve the magnetic
flux density of barium hexaferrite.
INTRODUCTION
Generally, the hard ferrite magnets are expressed as M.O6Fe2O3 (M = Ba, Sr, Pb or mixture of them) [1].The hard
ferrite magnets M series are experessed as M.O6Fe2O3 (M = Ba, Sr, Pb or mixture of them) [1]. Especially barium
and strontium hexaferrites (Ba.Fe12O19 and Sr.Fe12O19) have high saturation magnetizations (Ms) of 72 emu g-1 and
at least 74 emu g-1 , respectively [Durmus, Z., Durmus, A., and Kavas, H., 2015, J Mater Sci 50, 1201-1213 (2015)]. The Barium hexaferrite is widely used due to its thermal, electrical and chemical high stability and high remanence and coercivity [2]. In general, they can be classified into two categories: isotropic and anisotropic ferrites. In the case of isotropic magnets, the material shows random orientation of the c-axis of the grains and has equal magnetic properties in all directions. The remanence Br and coercive force Hc of ferrite magnets are around 2 kG and 1.5 to 20 kOe, respectively, depending on the processing of the ferrite material. For anisotropic hard ferrite magnets, enhancement of remanent magnetization of the material is obtained by orientating of the c-axis of the plate-like hexagonal ferrite crystals to the direction of an external magnetic field that was applied during the shaping process of the material. The remanent magnetization of anisotropic ferrite is nearly twice the value of the isotropic ones [3].
additives for obtaining desired structural and magnetic properties in the ferrite magnets [3]. Ceramic-metal nanocomposites have also received increasing attention because of their unique mechanical, electrical and magnetic properties. Previous works [4, 5] have successfully prepared the magnetic ceramic-metal nanocomposites, such as BaFe12O19/Fe magnetic composite by using high energy ball milling. This material has shown interesting magnetic
properties, with potential for some industrial applications.
For powder preparation, commercial milling techniques are used in magnetic materials technology to reduce the particle size from multidomain to single domain direction. The effect of milling and annealing on intrinsic coercivity of BaFe12O19 powder has been studied for dry-milled and wet-milled powder. In both cases, it was observed that
prolonged milling decreases both coercivity and magnetic saturation [6].
In this work, the synthesis and characterization of structure and magnetic properties of sintered BaFe12O19 with
various B2O3 and Fe additives were carried out. The goals of this work is to study the effect of milling time under
dry and wet milling condition, sintering temperature and the addition of B2O3 and Fe on physical and magnetic
properties of BaFe12O19 permanent magnets. The particle size analyzer (PSA), X-ray diffraction (XRD), scanning
electron microscope (SEM), Gauss meter and permagraph have been used to investigate and analyze the
Energy Milling (HEM) at various milling time under wet and dry milling condition, separately. The milling process was carried out by using stainless steel ball and jar mill with ball and powder ratio of 10:1 at 120 rpm.
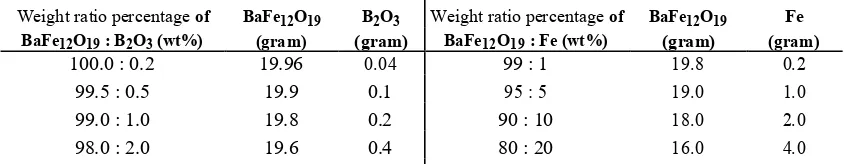
TABLE 1. Weight ratio percentage of BaFe12O19, B2O3and Fe.
Weight ratio percentage of BaFe12O19 B2O3 Weight ratio percentage of BaFe12O19 Fe
BaFe12O19 : B2O3 (wt%) (gram) (gram) BaFe12O19 : Fe (wt%) (gram) (gram)
In the wet milling method, the process was carried out for 0, 10, 20, and 40 hours and for the dry milling method, the process was performed for 12, 24, 48 and 60 hours. The particle size and true density of original powder and milled powder were measured by particle size analyzer (PSA) and picnometer, respectively. From the both process, the optimum milling time were obtained. In the second step of this study, we also considered the addition of varying amount of B2O3 (0.2, 0.5, 1, 2 wt%) and Fe (1, 5, 10, 20) in order to improve the physical and magnetic properties
of barium hexaferrite. The milling time was fixed according to the optimum aforementioned condition: 48 h for dry milling time of B2O3 addition and 20 h for wet milling time of Fe addition. The powder were then dried in an oven
at 1000C for 1h, mixed with 3 wt% Celuna (WE-158) and compacted by using magnetic field press (anisotropic process) at 50 N/cm2 press. The molding dies are made from steainless steel with 12 mm in diameter. 2 gram of powder was used for each sample.
RESULTS AND DISCUSSION
The effects of Milling Time and Sintering Temperature
True density and average particle size of barriumhexaferrite powder before and after milling under wet and dry milling condition with varying milling time of 10, 20, 40 and 12, 24, 48, 60, respectively are shown in FIGURE 1.
(a). (b).
FIGURE 1.True density and average particle size of BaFe12O19 prepared at varying milling time under (a) wet milling and (b)dry milling conditions.
According to the results shown in Figure 1, the true density and particle size of BaFe12O19 are around 4.37g/cm3
and 21.4µm, respectively. As the milling time increase, the true density increases and the average particle size tends to decrease. The optimum true density and average particle size of barium hexaferrite were achieved at 20 h for wet milling (4.70 g/cm3 and 7.56 µm) and 48 h for dry milling (4.76 g/cm3 and 3.97 µm). For dry milling, at milling time more than 48 hours (Fig. 1b ), the powder seems to become agglomeration. The results of this study suggest that in both conditions, the true density and particle size of BaFe12O19 powder show an opposite behavior. In addition, the results show that as the milling time increases, the the average particle size of powder has a tendency to decrease.
Table 2 shows the bulk density and magnetic flux density of original BaFe12O19, wet and dry milled powder
sintered at 1100oC, 1150oC and 1200oC for one-hour holding time.
TABLE 2.Bulk density and magnetic flux density of BaFe12O19magnets with and without milling.
Sintering Original powder Wet milling (20 h) Dry milling (48 h)
condition Bulk density Magnetic flux Bulk density Magnetic flux Bulk density Magnetic flux
(g/cm3) density (g/cm3) density (g/cm3) density
(gauss) (gauss) (gauss)
1100oC, 1 h 4.86 760 4.61 620 4.63 798.5
1150oC, 1 h 4.88 744 4.71 594 4.56 617
1200 oC, 1 h 4.98 786 4.66 609 4.55 744
The optimum bulk density of compact-original powder, wet milled and dry milled powder is 4.98, 4.71 and 4.63 g/cm3, respectively. The magnetic flux densities at the aforesaid density are 786, 594 and 798.5 Gauss, respectively.
The results as presented in Table 2 indicate that there isn’t strong correlation between bulk density and magnetic
flux density. Specimen that has optimum bulk density does not always have optimum magnetic flux density. This could be related to the presence of pores which reduces the magnetic flux density of barium hexaferrite.
(a). (b). (c). FIGURE 2.SEM image of sintered a) BaFe12O19powder, b) BaFe12O19powder after wet milling for 20 h and c)
BaFe12O19powder after dry milling for 48 h.
As can be seen in Fig. 2, BaFe12O19 powder without milling process has grain size about 2-6µm. For 48 h dry
milling sample, the specimen has a bigger grain size among before milling and sintered wet milled powder. This suggests that the dry milling process seems to enhance the grain growth of barium hexaferrite.
The Effects of Fe and B
2O
3Additions
The average particle size of BaFe12O19-5wt% Fe after wet milling process for 20 hours and BaFe12O19-0.5wt%
B2O3 after dry milling process for 48h is 3.73 µm and 1.10 µm , respectively.
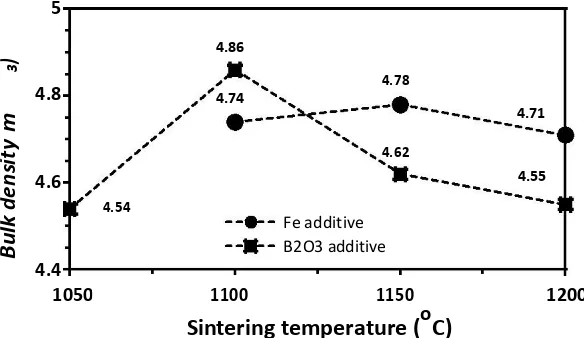
Figure 3 shows the correlation between sintering temperature and bulk density of BaFe12O19 with B2O3 and Fe
addition. The sintering temperature was determined from the optimum bulk density of specimen. Therefore, it can be seen that the optimum sintering temperature for barium hexaferrite with 0.5 wt% B2O3 and 5 wt% Fe addition is
FIGURE 3.Correlation of sintering temperature versus bulk density of BaFe12O19sample with B2O3and Fe addition.
The bulk densities of specimens were 4.86 g/cm3 for 0.5 wt% B2O3 addition and 4.78g/cm3 for 5 wt% Fe additions. In general, the bulk density of material shows a linear correlation with sintering temperature. However, it tend to decrease when deformation of material take places (> sintering temperature). The bulk density of material decreases due to the grain of particles growth and enlargement of pores in grain boundaries. Meanwhile, the magnetic flux density of material is affected by oriented particle in c-axis [3].
The bulk density and magnetic flux density of BaFe12O19 sample as a function of Fe content: 0, 1, 5, 10 and
(a). (b).
FIGURE 4.Bulk density and magnetic flux density of BaFe12O19sample with the addition of (a) Fe (wt%)-sintered at1150oC for 1 h, and b) B2O3 (wt%)-sintered at 1100oC for 1 h.
The results show that the optimum bulk density and magnetic flux density are obtained by 5 wt% Fe addition (4.78 g/cm3 and 883 Gauss) and 0.5wt% B2O3 addition (4.86 g/cm3 and 832.9 Gauss). Comparing the magnetic flux
density of sintered original powder, it suggests that the addition of B2O3 and Fe can improve the magnetic flux
density and decrease the sintering temperature of barium hexaferrite. The SEM images of BaFe12O19 with 0.5 wt%
B2O3 and 5 wt% Fe additions are shown in the Fig. 5a and 5b, respectively.
(a). (b).
FIGURE 5.SEM image of a). BaFe12O19-5wt%Fe sintered at 1150oC and b). BaFe12O19-0.5wt%B2O3 sintered at 1100oC
A different in grain size: large and small grain can be distinguished clearly in the sintered BaFe12O19 with 5wt%
Fe addition. Meanwhile in the sintered BaFe12O19-0.5wt%B2O3, the material shows more homogeneous
microstructure.
X-ray diffraction patterns of original BaFe12O19, BaFe12O19-5wt%Fe and BaFe12O19-0.5wt%B2O3 are shown in
Fig. 6. According to the results as shown above, sintered original powder is composed of single phase of BaFe12O19.
On the other hand, two phases as BaFe12O19 and hematite (Fe2O3) phases were detected in the barium hexaferrite
with 0.5 wt% B2O3 and with 5 wt% Fe addition. This indicates that the addition of 0.5 wt% B2O3 and 5wt% Fe
leads to the formation of hematite.
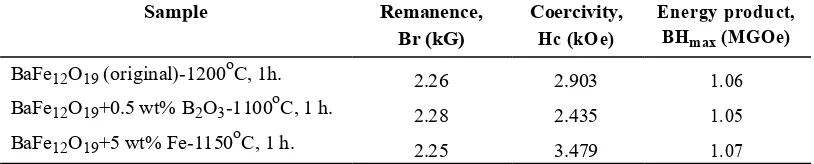
Table 3 presents the remanence Br, coercivityHcj and energy product BHmax of BaFe12O19, BaFe12O19-0.5 wt%
B2O3 and BaFe12O19-5 wt% Fe sintered at 1100C, and 1150oC for 1 h, respectively.
TABLE 3.Magnetic properties of BaFe12O19with B2O3and Fe addition
Sample Remanence, Coercivity, Energy product,
Br (kG) Hc (kOe) BHmax (MGOe)
BaFe12O19 (original)-1200oC, 1h. 2.26 2.903 1.06
BaFe12O19+0.5 wt% B2O3-1100oC, 1 h. 2.28 2.435 1.05
BaFe12O19+5 wt% Fe-1150oC, 1 h. 2.25 3.479 1.07
FIGURE 6. XRD patterns of (a) original BaFe12O19powder sintered at 1200oC, (b) 5 wt% B2O3added-BaFe12O19(dry milled for48 h and sintered at 1100oC for 1 h), c) 5 wt% Fe added-BaFe12O19 (wet milled for 20 h and sintered at 1150oC for 1h.
The results indicate that the remanence Br, coercivityHcj and energy product BHmax of barium hexaferrite with 5
wt% Fe addition sintered at 1150oC for 1 h are 2.25 kG, 3.479 kOe and 1.07 MGOe, respectively. On the other hand, the aforementioned properties of barium hexaferrite with 0.5 wt% B2O3 addition sintered at 1100oC for 1 h
are 2.28 kG, 2.435 kOe and 1.05 MGOe, respectively. For BaFe12O19 samples without milling sintered at 1200oC
for 1 h, the magnetic properties are as follows: magnetic remanence, Br = 2.26 kG, coercivity, Hcj = 2.903 kOe and
energy product, BHmax = 1.06 MGOe.
Summary
In this study, the effects of milling time and sintering temperature on the physical and magnetic prop erties of barium hexaferrite have been performed. In addition, in order to improve the properties of barium hexaferrite, the addition of Fe (wet milling) and B2O3 (dry milling) has also been carried out. The most important finding of this
study is that the addition of Fe and B2O3 with wet and dry milling process, respectively give a potential benefit to
reduce the sintering temperature and improve the magnetic flux density of barium hexaferrite.
ACKNOWLEDGMENTS
This work was supported by Research Center for Physics, Indonesian Institute of Sciences under contract DIPA 2014 (No. 0131/IPT.1.03/A/2014).
REFERENCES
1. Mahgoob A. and Hudeish A.Y 2013, Middle-East Journal of Scientific Reseach15, 834-839 (2013).
2. F. Tudorache, P.D. Popa, F. Brinza and S. Tascu, The International Congress on Advances in Applied Physics and Material Science, Antalya 2011: ActaPhysicaPolonica A 121, 95-97 (2012).
3. O. T. Özkan, H. Erkalfa, A. Uildirim, Journal of the European Ceramic Society14, 351-358 (1994). 4. P.G. Bercoff, H.R. Bertorello, Journal of Magnetism and Magnetic Materials187, 169-176 (1998). 5. P.G. Bercoff, H.R. Bertorello, Journal of Magnetism and Magnetic Materials205, 261-269 (1999). 6. S.J. Campbell, E. Wu, W.A. Kaczmarek and K.D. Jayasuriya, Hyperfine Interaction92, 933-941 (1994).