Summary Shoot structure can be quantified as the ratio of maximum shoot silhouette area to maximum leaf silhouette (projected) area (Rmax ). I have used published studies on the effects of shade-shoot structure on photosynthetic production of evergreen conifer stands to test the hypothesis that the lower crown of stands of species with a high Rmax contributes signifi-cantly to photosynthetic production. Pruning studies were found inadequate to test this hypothesis rigorously. Eight stud-ies that used cuvettes to measure photosynthetic production in different crown layers are reviewed. Of six studies on species with Rmax values larger than 0.7, five found significant rates of photosynthesis in the lower half of the crowns. In contrast, pines, which have a low Rmax and a low leaf area index (≤ 3.5), had low rates of photosynthesis in the lowest crown zone. In field-grown shoots of all species examined, photosynthetic capacity was negatively related to Rmax .
Keywords: field photosynthesis, leaf silhouette area, photosyn-thetic production, shoot silhouette area.
Introduction
Leverenz and Hinckley (1990) and Leverenz (1992) found significant correlations between the architecture of shade shoots (quantified as the ratio of maximum shoot silhouette area to maximum leaf silhouette (projected) area, Rmax ) of different evergreen conifer species and maximum leaf area index, or maximum stand production. These correlations sup-port the hypothesis that, in closed evergreen conifer stands, the distribution and inclination of needles within shade-adapted shoots has a major effect on maximum photosynthetic produc-tion and thus maximum stand producproduc-tion. However, this posi-tive correlation does not hold on all sites, and species that are more productive on good sites can be less productive on poor sites (Aldhous and Low 1974). Thus shoot architecture can affect the ability of a species to respond to variations in nutrient availability or site water balance.
Leverenz and Hinckley (1990) hypothesized that species that produce shade shoots with a high Rmax will be able to develop a deeper canopy in terms of leaf area or leaf biomass as a result of a lower light compensation point than shade shoots with a low Rmax . It was also hypothesized that shade
shoots with a high Rmax will operate more efficiently at irradi-ances just above the light compensation point than shade shoots with a low Rmax . This would result in significant photo-synthetic production even at irradiances only slightly above the light compensation point. I have reviewed the literature on photosynthetic production at different canopy depths within stand-grown trees to determine whether species that have a high Rmax also have a high leaf area index and significant photosynthetic production in their lower crowns. Conversely, I have also examined whether species with a low Rmax have a low leaf area index and negligible photosynthetic production in their lower crowns.
Leverenz (1995) reported laboratory data indicating that shade-shoot structure itself is responsible for the correlations between Rmax and stand production through its effect on pho-tosynthetic efficiency. In the same study, no clear effects of variation in needle structure were observed. However, the illumination used was strongly collimated or almost totally diffuse, which is not the case in nature (Madgwick and Brum-field 1969, Grace 1971). Also, these laboratory measurements were made under steady-state conditions, and it is not known how natural fluctuations in irradiance with time may affect the results. For example, under natural illumination, a pine shoot may be no less photosynthetically efficient than a flat shoot because the changing position of the sun results in shadows that constantly move across individual cells of the needles, i.e., spatial and temporal variations in illumination direction may compensate for differences in shoot structure so that, if the pattern of shadows occurs with the right frequency, decreases in photosynthesis as a result of mutual shading may be small (Pearcy 1990). A second objective of this review, therefore, was to survey the literature to test the hypothesis that photo-synthetic performance in the field is related to shoot architec-ture.
Pruning experiments
Pruning or thinning experiments often result in the conclusion that the lower crown of conifer canopies does not contribute significantly to the production of the stand. For example, Waring (1991) concluded that leaf area indices in excess of 6 are inconsequential for photosynthetic production. However,
Shade-shoot structure, photosynthetic performance in the field, and
photosynthetic capacity of evergreen conifers
JERRY W. LEVERENZ
Department of Ecology and Environmental Sciences, Swedish University of Agricultural Sciences, Box 7072, S-750 07 Uppsala, Sweden Current address: The Arboretum, The Royal Veterinary and Agricultural University, Kirkegaardsvej 3A, DK2970 Hoersholm, Denmark
Received March 2, 1995
based on a review of pruning experiments within forest stands, Mar-Møller (1960) concluded the lower crown zones are im-portant for photosynthetic production. He found that in cases where pruning the lower branches from forest trees did not decrease production, other factors were confounding the re-sults. Thus in some studies, the foliage removed by pruning was largely or completely replaced by regrowth, and so no statistically significant effects of pruning were found. In other studies, there was no measurable effect of pruning on stem growth because pruning was done below the height on the stem where diameter growth was measured (Margolis et al. 1988).
Mar-Møller (1960) also reported a study (Hartig 1872) where severe drought in combination with pruning resulted in an increase in production. It is probable that there is an optimal leaf area index that is a function of site water balance (Gholz et al. 1976, Linder 1985). Pruning of stands with a supra-opti-mal leaf area index as a result of drought stress should lead to a less negative, or even a positive effect on growth. This would occur when leaf water potentials drop below a critical water potential for growth (Hsiao 1973) or photosynthesis (Aussenac and Granier 1978, Beadle et al. 1981). Therefore, to interpret accurately the results of pruning experiments, the effects of limiting water on tree growth must be clearly separated from the contribution of the lower canopy to photosynthetic produc-tion. There is good evidence that leaf area in evergreen conifers is controlled by Rmax through its affect on the light compensa-tion point (Leverenz and Hinckley 1990, Leverenz 1995). Thus, it may be hypothesized that there is selection pressure for a particular Rmax (low Rmax on drought-prone sites and high Rmax on well-watered sites) to obtain an optimal leaf area for maximizing tree growth and reproductive success.
Pruning of the lower crown zone may have little effect on growth in some instances simply because only a small fraction of the total leaf area or mass is removed. Oren et al. (1986) found that, in Picea abies (L.) Karst., the lower 40% of the canopy in terms of height contains only 5% of the foliage mass. Similarly, in Pseudotsugamenziesii (Mirb.) Franco, the lower 25 to 30% of the canopy (based on whorl number) may contain only 4 to 9% of the foliage mass (Jensen 1976, Brix 1981).
I conclude that most published pruning studies do not pro-vide a critical test of the hypothesis that the lowest one-third of the leaf mass or leaf area of a canopy contributes signifi-cantly to total canopy photosynthetic production.
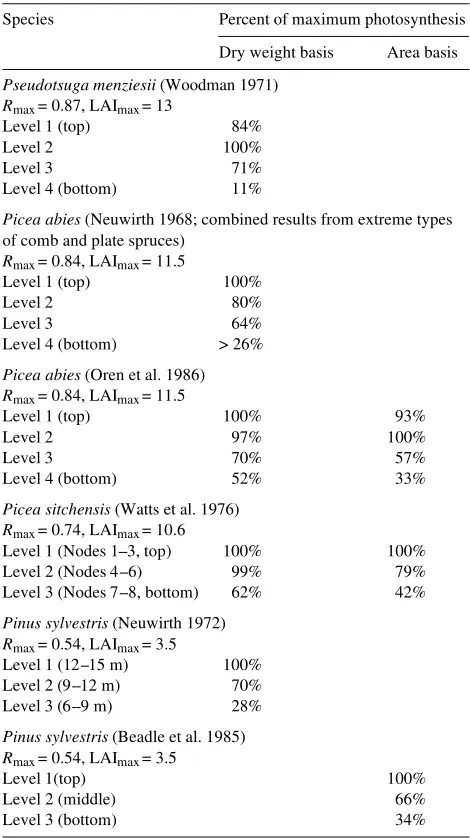
Photosynthetic production within different crown zones I found eight published studies on four species of evergreen conifers that reported on variation in photosynthetic produc-tion with crown depth. The species studied were: P. menziesii (Woodman 1971, Künstle and Mitscherlich 1975), P. abies (Neuwirth 1968, Oren et al. 1986, Häsler 1992), Picea sitchen-sis (Bong.) Carr. (Watts et al. 1976), and Pinus sylvestris L. (Neuwirth 1972, Künstle and Mitscherlich 1975, Beadle et al. 1985). Table 1 lists the results from this survey in order of decreasing Rmax and decreasing maximum leaf area index. Assuming the stands were not subject to severe stress, the LAI was substantially more than 6 for all species except
P. sylvestris. The data of Häsler (1992) and Künstle and Mit-scherlich (1975) are not listed in Table 1 because they sampled at only two heights in the tree crowns and used a limited number of cuvettes.
Based on the data reported by Woodman (1971) for P. menziesii (Rmax = 0.87), photosynthetic production was only negligible in the lowest crown zone (Level 4). This zone contained 10% of the living branches of the tree, but less than 10% of the total foliage mass, and represented the zone where shoots die for lack of light. The bulk of the crown (90%) had rates of photosynthesis that were 70% of the zone with the highest rates.
Similar results were obtained for P. abies (Rmax = 0.84) based on the data in Figures 2 and 4 of the papers by Neuwirth (1968) and Oren et al. (1986), respectively. In agreement with Mar-Møller (1960), Neuwirth (1968) concluded that care Table 1. Relative photosynthetic production within different crown zones of stand-grown trees.
Species Percent of maximum photosynthesis
Dry weight basis Area basis
Pseudotsuga menziesii (Woodman 1971)
Rmax = 0.87, LAImax = 13
Level 1 (top) 84%
Level 2 100%
Level 3 71%
Level 4 (bottom) 11%
Picea abies (Neuwirth 1968; combined results from extreme types of comb and plate spruces)
Rmax = 0.84, LAImax = 11.5
Level 1 (top) 100%
Level 2 80%
Level 3 64%
Level 4 (bottom) > 26%
Picea abies (Oren et al. 1986)
Rmax = 0.84, LAImax = 11.5
Level 1 (top) 100% 93%
Level 2 97% 100%
Level 3 70% 57%
Level 4 (bottom) 52% 33%
Picea sitchensis (Watts et al. 1976)
Rmax = 0.74, LAImax = 10.6
Level 1 (Nodes 1--3, top) 100% 100%
Level 2 (Nodes 4--6) 99% 79%
Level 3 (Nodes 7--8, bottom) 62% 42%
Pinus sylvestris (Neuwirth 1972)
Rmax = 0.54, LAImax = 3.5
Level 1 (12--15 m) 100% Level 2 (9--12 m) 70% Level 3 (6--9 m) 28%
Pinus sylvestris (Beadle et al. 1985)
Rmax = 0.54, LAImax = 3.5
Level 1(top) 100%
Level 2 (middle) 66%
should be taken not to underestimate the contribution of the lower crown zone to the total photosynthetic production of the entire crown. The study by Oren et al. (1986) showed that the upper shade crown made a large contribution to photosynthetic production per unit leaf area, especially when expressed per unit of invested leaf mass (values obtained by dividing the mean rates of photosynthesis by their mean specific leaf areas). However, even in the lowest canopy zone, photosynthetic production (dry weight basis) was 52% of that in the most productive zone. Similarly, Häsler (1992) found that photosyn-thetic production (needle area basis) in the lower crown of a P. abies tree was 56% of that in the upper crown. However, the data reported by Häsler (1992) do not provide a rigorous test of the hypothesis because it is unclear if the sampled trees were growing in a closed stand.
A summary of data published by Watts et al. (1976) for P. sitchensis is shown in Table 1. Rates of photosynthesis on an area basis were calculated from their Figure 7, and data for the same stand (Figure 7 in Norman and Jarvis 1974) were then used to convert from an area to a mass basis. The relatively high Rmax of 0.74 of P. sitchensis was associated with an LAI substantially above 6 and with significant rates of photosyn-thesis (dry weight basis) in the lowest third of the crown (whorl basis).
In contrast to the above five studies on species with shade shoots with a high Rmax , Künstle and Mitscherlich (1975) concluded that, in P. menziesii (Rmax = 0.87), the lower half of the crown did not contribute significantly to photosynthetic production. The reason for this discrepancy is unclear.
A comparison of the data for P. sitchensis, P. abies and P. menziesii with the data for P. sylvestris (Rmax = 0.54) re-ported by Neuwirth (1972) and Beadle et al. (1985) indicated that the lower crown zone of P. sylvestris was less productive than the lower crowns of the other three species despite the much lower LAI of the pine species. Both studies report that photosynthetic production of the lowest crown zone was about 30% of that of the highest crown zone (Neuwirth 1972, Beadle et al. 1985). Thus, the P. sylvestris canopies had both lower LAI and lower rates of photosynthesis in their lower crown compared with the upper crown. Unfortunately, it is not possi-ble to relate these photosynthetic data for P. sylvestris to the actual leaf area or leaf mass within the different canopy zones; however, the maximum leaf area index for P. sylvestris is so low (about 3, and at most 4) that the LAI of the total crown would be equivalent to, or less than, the upper half of the crowns of the other species. Nevertheless, there was still a sharp decrease in photosynthetic performance with decreasing crown depth in the P. sylvestris stands.
I conclude that five of these six studies support the hypothe-sis (Leverenz and Hinckley 1990) that species that produce shade shoots with a high Rmax will have substantial rates of photosynthesis in the bulk of their lower crowns despite having an LAI of more than 6. Only the very lowest foliage (10% or less of the total leaf mass) appears not to contribute signifi-cantly to total photosynthetic production.
Photosynthetic responses to light by individual shoots To test the hypothesis that shoot architecture itself underlies the observed differences in photosynthetic production. It is necessary to test the effects under natural illumination. I com-pared the photosynthetic performance of different species based on the following criteria: the initial slope of the irradi-ance response curve of photosynthesis (the maximum quantum yield based on incident light), rate of dark respiration, light compensation point, rate of bending of the light response curve (apparent convexity), and maximum rate of photosynthesis (Leverenz 1995).
Under nonstress conditions, the initial slope of the irradi-ance response curve of photosynthesis may be modeled as (Leverenz 1995):
φi ≤αφaRmax , (1)
where φi is the maximum initial slope (equivalent to apparent quantum yield) based on incident irradiance, φa is the maxi-mum initial slope based on absorbed irradiance or illuminance, and α is leaf absorptance under the conditions of illumination. Interceptance of irradiance will be reduced for all directions of illumination other than the one that maximizes Rmax . This will decrease the initial slope based on incident light. In the field,
φi will not be larger than φaRmax if the total irradiance at the shoot is accurately estimated.
Troeng and Linder (1982) estimated that the mean maxi-mum φi for P. sylvestris shoots in the field was 0.27 ± 0.002, which when divided by 0.5 to correct for Rmax gives an esti-mated φa of 0.054. The expected quantum yield at 20 °C is 0.061 (Leverenz and Öquist 1987), which is about 12% higher than predicted by Equation 1. On a projected needle area basis, a φi of 0.052 is obtained from Figure 4 of the paper published by Benecke (1980), which when divided by 0.5 to correct for Rmax gives a φa of 0.104 compared with an expected yield at 17 °C of 0.063 (Leverenz and Öquist 1987). In this case, the apparent quantum yield is 1.65 times higher than predicted by Equation 1. Dick et al. (1991) also reported high φi values for Pinus contorta Dougl. shoots.
φi, weight ≤αφaRmax /S, (2)
where S is the specific leaf area in units of m2 g−1, the φ i, weight values determined from field measurements were not signifi-cantly different from those predicted from laboratory measure-ments.
The rates of dark respiration estimated by fitting the convex-ity equation were similar for the two species (0.12 mg g−1 h−1) (cf. Hodges and Scott 1968, Leverenz 1995). The light com-pensation point (Γi) was twofold higher for P. sylvestris (1.2 Klux) than for P. menziesii (0.6 Klux). A more extensive sampling of shade shoots by Künstle and Mitscherlich (1975) showed that, on average, Γi of P. menziesii was 0.66 times that of P. sylvestris. Based on laboratory measurements (Leverenz 1995), I predicted that Γi of P. menziesii would be 0.53 times that of P. sylvestris. Because the rates of dark respiration were similar in the two species (Künstle and Mitscherlich 1976), the differences in Γi are largely attributable to differences in shoot structure (Leverenz 1995).
The apparent convexity (rate of bending, θa) for the P. sylvestris (0.60) shoot was 0.61 times that of the P. menziesii (0.98) shoot (Figure 1). A ratio of 0.23 is predicted from the laboratory data of Leverenz (1995). The smaller difference in
θa in the field may reflect the more diffuse light environment compared with that of the laboratory. However, low values of
θa (from 0.0 to 0.36) have also been reported for P. contorta shoots (Rmax = 0.5) under natural illumination in the field (Dick et al. 1991). The estimated light-saturated rate of photosynthe-sis (Asat , dry weight basis) was 35% higher in the P. sylvestris shoot (10.7 mg g−1 h−1) than in the P. menziesii shoot (8.0 mg g−1 h−1), which is in agreement with the laboratory results of Leverenz (1995).
Hodges and Scott (1968) measured photosynthetic light response curves of shoots of several conifer trees when illumi-nances were low and water stress minimal. Illuminance was measured inside the chambers with selenium photocells. The results of their measurements are reproduced in Figure 2. The
light compensation point was about twofold higher, and the initial slope was about twofold lower for P. sylvestris than for Abiesgrandis Lindl. (Figure 2). Abiesprocera Rehd. had the next highest Γi and next lowest φi followed by P. menziesii and P. sitchensis. These rankings are in close agreement with the laboratory results of Leverenz (1995) for unilaterally illumi-nated shoots. Within a species, the differences in φi values between the field and laboratory measurements are attributable to tree-to-tree variations in Rmax (Leverenz and Hinckley 1990, Leverenz 1995).
Long-term photosynthetic production under the shade of a forest stand has also been ranked with respect to species (Table 1 in Hodges and Scott 1968) with A. grandis shoots having the highest average rates during daylight hours, fol-lowed by Tsuga heterophylla (Raf.) Sarg., P. sitchensis, P. menziesii, A. procera and lastly P. sylvestris. These results support the contention that data from steady-state, unilateral illumination in the laboratory can be used to infer relative photosynthetic efficiency and performance under natural illu-mination when stress is minimized. The results on individual shoots under natural illumination in the field are in agreement with the hypotheses that shade-shoot structure itself strongly affects photosynthetic production, the development of leaf area, and production in dense conifer stands on fertile sites. However, these results differ from the results reported by Carter and Smith (1985) who found no strong effect of shoot structure on photosynthesis at low irradiances, and the results of Benecke (1980) and Dick et al. (1991) who reported high quantum yields for pine shoots.
Maximum photosynthetic capacity
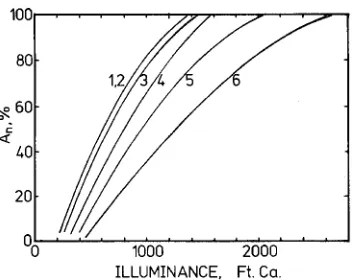
Among evergreen conifer species, there is a slight negative correlation between Asat and Rmax for shade shoots (Leverenz Figure 1. Net photosynthesis (An) in relation to illuminance for a
Pseudotsuga menziesii shoot (s) and a Pinus sylvestris shoot (d). Illuminance was measured with a spherical sensor. Data points were taken from Figure 5 in Künstle and Mitscherlich (1970). The solid lines represent the best fit of the convexity equation to the data by nonlinear least squares.
Figure 2. Net photosynthesis (An) in relation to illuminance for shoots of six conifer species (redrawn from Figure 1 of Hodges and Scott 1968). The curve to the far left (1,2) is approximated from the overlap-ping curves for Tsuga heterophylla (Rmax = 0.85) and Abies grandis (Rmax = 0.99). The other curves are for (3) Picea sitchensis (Rmax = 0.74), (4) Pseudotsuga menziesii (Rmax = 0.87), (5) Abies procera
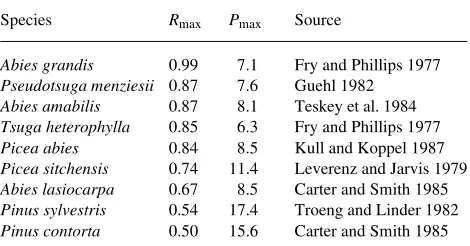
1995), indicating that there is a negative correlation between photosynthetic capacity and stand production. However, the variations in Asat among species were small compared to the variation within a species. A survey of the literature of the maximum measured rates of photosynthesis (Amax ) of different species with known Rmax also reveals that the ability to main-tain high rates of photosynthesis under favorable conditions is negatively correlated with the ability to produce shade shoots with a high Rmax (Table 2). The correlation becomes even more negative when the rates of photosynthesis are expressed per unit of shoot projected area rather than leaf projected area, because the species with the highest rates of photosynthesis have the lowest Rmax .
Combining the data summarized by Leverenz (1992, Fig-ure 2) and the data in Table 2 yields a significant negative correlation between volume production (Gv,r , expressed as a % of that of P. abies) and Amax (Gv,r = 156.2 − 5.41Amax , R2 = 0.77, P = 0.009). These data support the hypothesis that, in dense stands on fertile sites, efficiency of photosynthesis at low light (which is strongly affected by shoot structure) is more impor-tant for stand production than maximum rate of photosynthesis (which is strongly affected by biochemical differences per unit leaf area), i.e., architectural variation is more important than variation in leaf biochemistry in determining the variation in growth and productivity among species (Küppers 1994, Lev-erenz 1995).
Acknowledgments
The Swedish Forest and Agricultural Research Council provided fi-nancial support.
References
Aldhous, J.R. and A.J. Low. 1974. The potential of western hemlock, western red cedar, grand fir and noble fir in Britain. Her Majesty’s Stationary Office, London, 105 p.
Aussenac, G. and A. Granier. 1978. Quelques résultats de cinétique journaliére du potentiel de séve chez les arbres forestiers. Ann. Sci. For. 35:19--32.
Beadle, C.L., R.E. Neilson, P.G. Jarvis and H. Talbot. 1981. Photosyn-thesis as related to xylem water potential and carbon dioxide con-centration in Sitka spruce. Physiol. Plant. 52:391--400.
Beadle, C.L., R.E. Neilson, H. Talvot and P.G. Jarvis. 1985. Stomatal conductance and photosynthesis in a mature Scots pine forest. 1. Diurnal, seasonal and spatial variation in shoots. J. Appl. Ecol. 22:557--571.
Benecke, U. 1980. Photosynthesis and transpiration of Pinus radiata
D. Don under natural conditions in a forest stand. Oecologia 44:192--198.
Brix, H. 1981. Effects of thinning and nitrogen fertilization on branch and foliage production in Douglas-fir. Can. J. For. Res. 11:502--511. Carter, G.A. and W.K. Smith. 1985. Influence of shoot structure on light interception and photosynthesis in conifers. Plant Physiol. 79:1038--1043.
Dick, J.M., P.G. Jarvis and R.R.B. Leakey. 1991. Influence of male and female cones on needle CO2 exchange rates of field-grown
Pinus contorta Dougl. trees. Funct. Ecol. 5:422--432.
Fry, D.J. and I.D.J. Phillips. 1977. Photosynthesis of conifers in relation to annual growth cycles and dry matter production. II. Seasonal photosynthetic capacity and mesophyll ultrastructure in
Abies grandis, Picea sitchensis, Tsuga heterophylla, and Larix leptolepis growing in S.W. England. Physiol. Plant. 40:300--306. Gholz, H.L., F.K. Fitz and R.H. Waring. 1976. Leaf area differences
associated with old-growth forest communities in the western Ore-gon Cascades. Can. J. For. Res. 6:49--57.
Grace, J. 1971. The directional distribution of light in natural and controlled environment conditions. J. Appl. Ecol. 8:155--164. Guehl, J.-M. 1982. Potentiel de photosynthèse hivernale du douglas
(Pseudotsuga mensiesii Mirb.) en relation avec le règime ther-mique. Ann. Sci. For. 39:239--258.
Hartig, R. 1872. Einfluss verschiden starker Aufästung und Ent-nadelung auf der Zuwachs der Weymouthskiefer und gemeinen kiefer. Zeit. für Forst- u. Jagdwesen. 4:240--254.
Häsler, R. 1992. Long-term gas exchange measurements in a mature
Picea abies of a subalpine forest. In Production and Management of Mountain Forests. Eds. Y. Yang and J. Zhang. Science Press, Beijing, pp 162--169.
Hodges, J.D. and D.R.M. Scott. 1968. Photosynthesis in seedlings of six conifer species under natural environmental conditions. Ecol-ogy 49:973--981.
Hsiao, T.C. 1973. Plant responses to water stress. Annu. Rev. Plant Physiol. 24:519--570.
Jensen, E.C. 1976. The crown structure of a single codominant Douglas-fir. M.Sc. Thesis. University of Washington, Seattle, WA. Künstle, E. and G. Mitscherlich. 1970. Assimilationsund Transpira-tionsmessungen in einem Stagenholz. Allg. Forst-u. J.-Ztg. 141:89--94.
Künstle, E. and G. Mitscherlich. 1975. Photosynthese, Transpiration und Atmung in einem Mischbestand im Schwarzwald. I. Teil: Pho-tosynthese. Allg. Forst-u. J.-Ztg. 146:45--63.
Künstle, E. and G. Mitscherlich. 1976. Photosynthese, Transpiration und Atmung in einem Mischbestand im Schwarzwald. III. Teil: Atmung. Allg. Forst-u. J.-Ztg. 147:169--177.
Küppers, M. 1994. Canopy gaps: competitive light interception and economic space filling----a matter of whole-plant allocation. In
Exploitation of Environmental Heterogeneity by Plants. Eds. M.M. Caldwell and R.W. Pearcy. Academic Press Inc., San Diego, pp 111--144.
Table 2. Maximum measured photosynthetic rates (Pmax , µmol m−2 s−1) of shoots of field-grown evergreen conifers. Rates are based on projected needle area. There was a significant negative correlation between the maximum rates of photosynthesis listed in this table and the estimates of Rmax reported by Leverenz and Hinckley (1990): Pmax = −20.75Rmax + 25.85, R2 = 0.79, P = 0.001.
Species Rmax Pmax Source
Abies grandis 0.99 7.1 Fry and Phillips 1977
Pseudotsuga menziesii 0.87 7.6 Guehl 1982
Abies amabilis 0.87 8.1 Teskey et al. 1984
Tsugaheterophylla 0.85 6.3 Fry and Phillips 1977
Picea abies 0.84 8.5 Kull and Koppel 1987
Picea sitchensis 0.74 11.4 Leverenz and Jarvis 1979
Abies lasiocarpa 0.67 8.5 Carter and Smith 1985
Pinus sylvestris 0.54 17.4 Troeng and Linder 1982
Kull, O. and A. Koppel. 1987. Net photosynthetic response to light intensity of shoots from different crown positions and age in Picea abies (L.) Karst. Scand. J. For. Res. 2:157--166.
Leverenz, J.W. 1992. Shade shoot structure and productivity of ever-green conifer stands. Tests of three alternative hypotheses. Scand. J. For. Res. 7:345--353.
Leverenz, J.W. 1995. Shade shoot structure of conifers and the photo-synthetic response to light at two CO2 partial pressures. Funct. Ecol. 9:413--421.
Leverenz, J.W. and T.M. Hinckley. 1990. Shoot structure, leaf area index and productivity of evergreen conifer stands. Tree Physiol. 6:135--149.
Leverenz, J.W. and P.G. Jarvis. 1979. Photosynthesis in Sitka spruce. VIII. The effects of light flux density and direction on the rate of net photosynthesis and the stomatal conductance of needles. J. Appl. Ecol. 16:919--932.
Leverenz, J.W. and G. Öquist. 1987. Quantum yields of photosynthe-sis at temperatures between −2 °C and 35 °C in a cold-tolerant C3 plant (Pinus sylvestris) during the course of one year. Plant Cell Environ. 10:287--295.
Linder, S. 1985. Potential and actual production in Australian forest stands. In Research for Forest Management. Eds. J.J. Landsberg and W. Parsons. CSIRO, Melbourne, Australia, pp 11--34. Madgwick, H.A.I. and G.L. Brumfield. 1969. The use of
hemispheri-cal photographs to assess light climate in the forest. J. Appl. Ecol. 57:537--542.
Margolis, H.A., R.R. Gagnon, D. Pothier and M. Pineau. 1988. The adjustment of growth, sapwood area, heartwood area, and sapwood saturated permeability of balsam fir after different intensities of pruning. Can. J. For. Res. 18:723--727.
Mar-Møller, C. 1960. The influence of pruning on the growth of conifers. Forestry 33:37--53.
Neuwirth, G. 1968. Photosynthese und Transpiration von Kamm----und Plattenfichten (Picea abies L.). Arch. Forstwes. 17:613--620. Neuwirth, G. 1972. Bestandesstruktur, Stoffproduktion und
Stoffbi-lanz eines 35jähringen Kiefernbestandes. Arch. Naturschutz u. Landschaftsforsch. 12:101--120.
Norman, J.M. and P.G. Jarvis. 1974. Photosynthesis in Sitka spruce (Picea sitchensis (Bong.) Carr.). III. Measurements of canopy struc-ture and interception of radiation. J. Appl. Ecol. 11:375--398. Oren, R., E.-D. Schulze, R. Matyssek and R. Zimmermann. 1986.
Estimating photosynthetic rate and annual carbon gain in conifers from specific leaf weight and leaf biomass. Oecologia 70:187--193. Pearcy, R.W. 1990. Sunflecks and photosynthesis in plant canopies.
Annu. Rev. Plant Physiol. Plant Mol. Biol. 41:421--453.
Teskey, R.O., C.C. Grier and T.M. Hinckley. 1984. Change in photo-synthesis and water relations with age and season in Abies amabilis. Can. J. For. Res. 14:77--84.
Troeng, E. and S. Linder. 1982. Gas exchange in a 20-year-old stand of Scots pine. II. Variation in net photosynthesis and transpiration within and between trees. Physiol. Plant. 54:15--23.
Waring, R.H. 1991. Responses of evergreen trees to multiple stresses.
In Response of Plants to Multiple Stresses. Eds. H.A. Mooney, W.E. Winner and E.J. Pell. Academic Press Inc., San Diego, pp 371--390. Watts, W.R., R.E. Neilson and P.G. Jarvis. 1976. Photosynthesis in
Sitka spruce (Picea sitchensis (Bong.) Carr.). VII. Measurements of stomatal conductance and 14CO2 uptake in a forest canopy. J. Appl. Ecol. 13:623--638.