Changes in amino acid composition and nitrogen metabolizing
enzymes in ripening fruits of
Lycopersicon esculentum
Mill
Silvana B. Boggio
a, Javier F. Palatnik
a, Hans W. Heldt
b, Estela M. Valle
a,*
aDi6isio´n Biologı´a Molecular,Instituto de Biologı´a Molecular y Celular de Rosario(IBR-CONICET),Facultad de Ciencias Bioquı´micas y Farmace´uticas,UNR,Suipacha531,2000Rosario,Argentina
bAlbrecht–6on Haller–Institut fu¨r Pflanzenwissenschaften der Uni6ersita¨t Go¨ttingen,Abteilung fu¨r Biochemie der Pflanze,Untere Karspu¨le2,
37073Go¨ttingen,Germany
Received 25 March 2000; received in revised form 12 June 2000; accepted 12 July 2000
Abstract
The free amino acid content of tomato (Lycopersicon esculentumMill.) fruits from cultivars Platense, Vollendung and Cherry were determined during ripening. It was found that glutamate markedly increased in red fruits of the three cultivars under study. At this stage, the cv Cherry had the highest relative glutamate molar content (52%) of all the analyzed tomato fruit cultivars. Measurements of nitrogen-assimilating enzyme activities of these fruits showed a decrease in glutamine synthetase (GS, EC 6.3.1.2) during fruit ripening and a concomitant increase in NADH-glutamate dehydrogenase (GDH, EC 1.4.1.3) and aspartate aminotransferase (EC 2.6.1.1) activities. Western blot analysis of protein extracts revealed that while GS was principally present in green fruit extracts, GDH was almost exclusively observed in the extracts of red fruits. These results suggest a reciprocal pattern of induction between GS and GDH during tomato fruit ripening. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Amino acid; Glutamate dehydrogenase; Glutamine synthetase; Lycopersicon esculentum; Fruit ripening
www.elsevier.com/locate/plantsci
1. Introduction
The growth of tomato fruits goes through dif-ferent phases. The early phase of fruit develop-ment is characterized by high metabolic activity and a rapid cell division of the tissue, whereas at a later developmental phase the cells expand [1]. Fruit ripening begins when seeds are completely formed and the fruit reaches its final size [1,2]. This ripening process involves a series of coordi-nated events including changes at the physiological and biochemical levels [3]. During the initial phases of tomato ripening, chloroplasts differenti-ate into chromoplasts. This plastid transition is
accompanied by the expression of specific genes involved in chromoplast formation and the subse-quent synthesis of enzymes correlated with ripen-ing [4,5].
As sink organs, fruits are dependent on the translocation of sucrose, amino acids, and organic acids to the developing fruit cells. The rate of import of these photoassimilates from the leaves is governed by the metabolic activity of the fruit [6]. In the case of the tomato, green fruit cells contain most of the photosynthetically active chloroplasts that give the developing fruit its green appearance, and play a significant role in carbon dioxide scav-enging [7]. Nevertheless, the activity per mg of protein of ribulose-1,5-bisphosphate carboxylase in the tomato leaf was shown to be three times higher than in the green fruit pericarp [4]. In tomato fruits, carbohydrate metabolism has been principally studied so far [7 – 11], while there is less information regarding the metabolism of nitrogen
Abbre6iations: GABA, g-aminobutyric acid; GAD, glutamate de-carboxylase; GDH, glutamate dehydrogenase; GOGAT, glutamate synthase; GS, glutamine synthetase.
* Corresponding author Tel.: +54-341-4350661/4350596; fax: +
54-341-4390465.
E-mail address:[email protected] (E.M. Valle).
compounds such as amino acids. In developing fruits, about 70% of the total amino acid content found in the pericarp belongs to the glutamate family [12].g-Aminobutyric acid (GABA) was the
predominant N-form (almost 60% of the total
amino acid molar content) at the earlier growing stages of the tomato fruit and glutamine (ca. 30%) in mature green fruits [12].
There are few reports about the enzymes in-volved in amino acid biosynthesis in the fruit. Glutamine synthetase (GS), which catalyzes the synthesis of glutamine from glutamate, ATP and ammonium, was detected in green and red toma-toes [13] and in avocado fruit [14].
NADH-gluta-mate synthase (GOGAT; EC 1.4.1.14) and
ferredoxin-GOGAT (EC 1.4.7.1), both enzymes catalyzing glutamate synthesis, were observed at low activities in the pericarp of green tomato fruits [15] and in intact fruit chloroplasts [16]. Glutamate dehydrogenase (GDH), which catalyzes the amina-tion of 2-oxoglutarate (synthetic reacamina-tion) and the deamination of glutamate (catabolic reaction), showed an increase in the enzyme protein content during ripening of avocado fruits [14]. Other amino acid metabolizing enzymes or transcripts for them expressed at different levels during tomato fruit ripening were reported: aspartate aminotrans-ferase, which was exclusively found in red fruits [17]; a putative glutamate decarboxylase (GAD; EC 4.1.1.15) [18] and arginine decarboxylase (EC 4.1.1.19), whose transcripts appeared to peak at the breaker stage [19], and a histidine decarboxy-lase (EC 4.1.1.22) mRNA, which accumulated dur-ing early fruit ripendur-ing and then declined [20].
The present study was carried out to contribute to the understanding of the metabolism of amino acids during the ripening of tomato fruits. For this purpose the molar contents of free amino acids were quantified in three different tomato cultivars (Platense, Cherry and Vollendung) at green, yellow and red ripening stages of the fruits. Concomi-tantly, the activities of nitrogmetabolizing en-zyme and proteins of GS, GOGAT and GDH were investigated.
2. Materials and methods
2.1. Plant material
Tomato plants cv Platense, Cherry and Vol-lendung were grown in a controlled environment
cabinet under a light intensity at the top of a fruit-containing plant of 700 mmol s−1 m−2. The
temperature ranged from 23°C during the light period (14 h) to 18°C in the dark and the relative humidity was 70%. Plants were grown in soil, continuously maintained under optimal irrigation and supplied daily with a standard nutrient medium [21]. Fully expanded 4th leaves of 30-day-old plants were used for GS activity and protein assays. Fruits were allowed to ripen naturally on the plant, and when mature, that is when fruit’s growing stops, they were harvested and grouped as ‘green, yellow and red’. Mature green fruits were classified as those still green on the outside, with no development of pink or red color inside and named ‘green’. The breaker stage (yellow) included fruits with the outside no more than 50% red, but with development of pink or red color in the interior. Fruits named ‘red’ were completely orange or red, but firm. Pericarp tissue of harvested fruits were obtained by removing the locule tissues and seeds and stored at −80°C until analysis.
2.2. Determination of amino acid content
Pericarp tissue was extracted with chloro-form:methanol [12,22]. The amino acid composi-tion in the methanolic phase was determined by derivatization with ninhydrin or o-phthaldialde-hyde and using an amino acid analyzer ALPHA PLUS (LKB 4151) or HPLC system Pharmacia-LKB [22].
2.3. Enzyme extraction and assays
Pericarp tissues were ground with a mortar and pestle at 4°C in extraction buffer (10 mM
ascor-bate, 2.5 mM DTT, 1 mM Na2EDTA, 10 mM
MgCl2, 10% glycerol, 50 mM HEPES – KOH, pH
8.0) plus 20% (w/w) insoluble
polyvinyl-pyrrolidone. The pericarp homogenate was filtered through Miracloth and centrifuged at 20 000×g
for 20 min. Enzyme activities were assayed follow-ing gel filtration through Sephadex G-25 equili-brated with the extraction buffer. For assaying transaminases, 40 mM pyridoxal-5%-phosphate was
and pestle at 4°C with the addition of extraction buffer (100 mM imidazole – HCl, 0.3% (v/v) 2-mercaptoethanol, 50% glycerol), and centrifuged 15 min at 21 000×g. Protein concentrations were determined in the supernatant as described [24]. The activity of GS was measured for the trans-ferase reaction by staining a non-denaturing poly-acrylamide gel (8%) [25] loaded with protein extracts (17 mg) in a Tris – glycine buffer system and run at 120 V for 2 h. After electrophoresis, the gel was incubated for 1 h at 37°C with GS
reaction mixture (80 mM NH2OH – HCl, 35 mM
Na – arsenate, 34 mM glutamine, 1 mM ADP, 100 mM imidazole – HCl, pH 7.1) and subse-quently the color was developed by the addition of FeCl3– HCl – thrichloroacetic acid [25]. GAD
was monitored by GABA formation from gluta-mate by derivatization with o-phthaldialdehyde and HPLC [22]. The assay medium was essen-tially as previously reported [26]. NAD-malate dehydrogenase (EC 1.1.1.37), NADP-malic en-zyme (EC 1.1.1.40), aspartate aminotransferase and alanine aminotransferase (EC 2.6.1.2) were measured by standard procedures [27]. One unit of enzyme activity (IU) is defined as the amount of enzyme catalyzing the transformation of 1 mmol of substrate per hour.
2.4. Protein extraction for SDS-PAGE and immunoblotting
Protein extracts were obtained by homogeniz-ing the leaf or pericarp with a mortar and pestle in 50 mM Tris – HCl (pH 7.5) and 1 mM EDTA, filtered through Miracloth, incubated with 2% SDS and 0.5 mM phenylmethylsulfonyl fluoride at 25°C and centrifuged at 20 000×g for 5 min.
The supernatant was precipitated with
thrichloroacetic acid (final concentration 20%) for 20 min in an ice bath, the pellet was washed twice with 80% acetone, resuspended in 2% SDS
and 0.5% b-mercaptoethanol and subjected to
SDS-PAGE. After electrophoresis proteins were either stained with a silver kit (BioRad) or
elec-troblotted to nitrocellulose membranes.
Im-munodetection was carried out according to the manufacturer’s procedure (ECL Amersham), us-ing antisera raised in rabbits against Zea mays
chloroplastic GS, Ferredoxin-GOGAT [28] or Vi
-tis 6inifera NADH-GDH [29].
3. Results
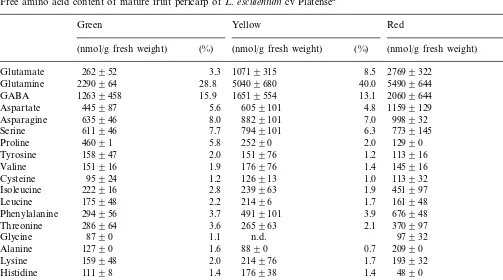
3.1. Relati6e free amino acid content of mature tomato fruit pericarp
Fruits at different ripening stages were
classified as green, yellow and red as described in Section 2. Table 1 shows the free amino acid contents of the pericarp of tomato fruit cv Platense during the transition from mature green to red. Glutamine was the most abundant free amino acid in all fruits, with the highest relative content (40%) in the yellow fruits. GABA, ubiq-uitous non-protein amino acid, represented the second most abundant free amino acid of green and yellow fruits (Table 1). During ripening, the relative molar content of some amino acids such as asparagine, serine, proline, tyrosine and valine declined, while aspartate and glutamate increased (Table 1). From all the amino acids tested, gluta-mate content showed the largest change during ripening. In fact, the glutamate content of red fruits was almost 10-fold higher than in green fruits (Table 1). In order to check if this rise was dependent on the ripening process itself, other tomato cultivars, Cherry and Vollendung, were grown under exactly the same conditions (illumi-nation, irrigation and nutrition) (see Section 2). The free amino acid contents of these fruit peri-carps were determined and the results are shown in Table 2. In all cultivars under study, a marked increase in glutamate content during the course of fruit ripening was observed (Tables 1 and 2). The cv Cherry showed the highest glutamate con-tent (52%) of all ripe fruits tested (Table 2). GABA was found to be especially abundant in the pericarp of green fruits and its relative molar content changed differently during ripening, de-pending on the cultivar assayed (Tables 1 and 2). The total molar content of free amino acids per gram of fresh fruit varied considerably among the cultivars tested and ripe fruits of cv Vollendung showed the highest molar content of total free amino acids (Table 2).
3.2. Pattern of enzyme expression in ripening fruits
synthesizing the most abundant free amino acids (Table 1) was investigated. Pericarp tissue was chosen for this study based on previously reported results [1,13,30,31]. Analyses of enzyme activities were performed in extracts of mature green and red fruit pericarps and the results are shown in Table 3. NADH-GDH activity was 2-fold higher in red fruits than in the green ones.
Ferredoxin-and NADH-GOGAT activities and enzyme
proteins, mainly detected in the leaves, showed a weak signal in green fruits only (data not shown). Considerable GAD activity was found in green fruits, whereas it could not be detected in ripe red fruits. In yellow fruits, GAD activity was less than 20% of that in green fruits (4.2 IU mg protein−1).
NADP-malic enzyme activity was only found in
Table 1
Free amino acid content of mature fruit pericarp ofL.esculentumcv Platensea
Green Yellow Red
(nmol/g fresh weight)
(nmol/g fresh weight) (%) (%) (nmol/g fresh weight) (%)
Glutamine 2290964 28.8
15.9
12639458 16519554 13.1 20609644 12.8
GABA
445987 5.6 6059101
Aspartate 4.8 11599129 7.2
6.2 998932
7.0 8829101
Asparagine 635946 8.0
611946 4.8
Isoleucine 222916 2.8 239963
21496 1.7 161948
Leucine 175948 2.2 1.0
4919101 3.9 676948
Phenylalanine 294956 3.7 4.2
2.3 370997
2.1 265963
Threonine 286964 3.6
1.1
1261192139 100 1606792334 100
S(total) 794291220 100
aResults are means 9S.D. (nmol/g fresh weight) of four replicates with different fruits of approximately the same fresh weight and chlorophyll or lycopene content for each maturation stage. n.d.: not detectable; %: of total nmol amino acid/g pericarp fresh weight.
Table 2
Relative free amino acid molar content of mature fruit pericarps of two tomato cultivars during ripeninga
Cherry (nmol/g fresh weight) Vollendung (nmol/g fresh weight)
Red
Glutamine 26209708 (35) 308091040 (45) 9979263 (16)
28009662 (30) 3220948 (20)
Table 3
Specific enzyme activities of mature green and red tomato fruit pericarp cv Platensea
Enzyme activities (IU (mg protein)−1)
Green fruit Red fruit
3.190.3 1.690.3
NADH-GDH
GAD 25.892.4 n.d.
6.590.5 n.d. Alanine amino
tranferase
Aspartate amino n.d. 12.392.1 tranferase
0.290.0
NAD-malate 2.290.2
dehydrogenase
n.d. NADP-malic enzyme 26.593.0
aThe specific activity of enzymes were determined in freshly prepared pericarp extracts. Each value represents the mean
9S.E. of three experiments. n.d.: not detectable.
other hand, NAD-malate dehydrogenase activity was 10-fold higher in red fruits than in green fruits (Table 3), probably reflecting the increase in respi-ration known to occur in a climacteric fruit like tomato [33]. Thus, the increase in the relative content of glutamate in ripe fruits can be corre-lated with an increase in the activities of GDH,
aspartate aminotransferase and NAD-malate
dehydrogenase.
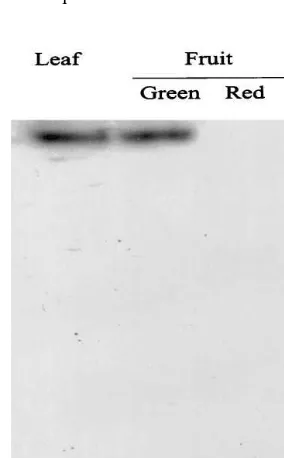
Measurements of GS activity turned out to be problematic, as there were activity losses observed during the assays with the fruit pericarp extracts. Therefore, GS activity was assayed after separa-tion of an extract sample by non-denaturing poly-acrylamide gel electrophoresis followed by activity staining (Fig. 1). A clear band of GS activity was observed exclusively in the lanes where leaf and green fruit extracts were loaded. No signal was detected when ripe fruit extracts were applied, even after overloading the gel with a five fold amount of extract (not shown). These results indi-cate that GS was predominantly active in only one oligomeric state.
3.3. Western blot analysis of GDH and GS proteins in mature fruits
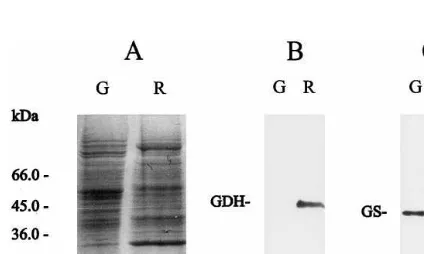
To determine the contents of the enzymes in-volved in the glutamate and glutamine metabolism of the tomato fruits during the different ripening stages, SDS-PAGE and Western blot analyses of GDH and GS in the pericarp extracts from cv Platense were carried out (Fig. 2). The total protein profiles in SDS-PAGE differed consider-ably between green and red fruits (Fig. 2A), which is in agreement with earlier results obtained with lines of cv Cherry [4]. The immunoblotting experi-ment revealed that GDH and GS had opposite expression patterns. The level of the GDH protein increased significantly during the ripening process (Fig. 2B), even to a greater extent than the in-crease in NADH-GDH activity shown in Table 3. Similar results were also reported for avocado fruits [14]. Conversely, GS protein was found to be present mainly in mature green fruits (Fig. 2C), concomitantly with the detection of GS activity (Fig. 1). It may be noted that rabbit anti-serum raised against purified maize GS2 also recognizes GS1 in Western blot experiments, although the efficiency for detecting GS2 is higher than for GS1 [28]. Since a criterion used to distinguish between
Fig. 1. GS activity staining of pericarp extracts of mature fruit of L. esculentum cv Platense. The activity of GS was measured for transferase reaction by staining a non-denatur-ing gel [25]. Protein extracts (17mg) were loaded onto a native polyacrylamide gel (8%). After electrophoresis, the gel was incubated for 1 h at 37°C with GS reaction mixture and the color developed by the addition of FeCl3– HCl – TCA as described in Section 2.
each isoenzyme is the distinct molecular mass of their subunits [34], experiments were performed for identifying the GS isoenzymes present in tomato green fruit. Western blot analysis of protein extracts from tomato 4th leaf and pericarp of mature fruit were performed in a 12% polyacry-lamide denaturing gel and the results are shown in Fig. 3. Two bands were clearly present in both green tissues, most likely representing GS2 (44 – 45 kDa) and GS1 (38 – 40 kDa) subunits. The relative intensity of each signal was similar in the leaf extracts, while in mature green fruits the faster-mi-grating band was predominant (Fig. 3).
4. Discussion
Fruit development and growth are dependent on the translocation of photoassimilates to the fruit cells [3]. At present, the physiological factors controlling the metabolic activities that influence fruit growth are not well understood. Much effort has been devoted to the study of sink strength during later phases of fruit development in respect to sucrose metabolism and accumulation [8 – 11],
whereas little is known about amino acid
metabolism during fruit ripening. As during ripen-ing the fruits intensify their taste and flavor, changes in the composition of different com-pounds are to be expected. Free amino acids are essential nonvolatile compounds involved in the overall taste of many foods, particularly glutamate and aspartic acid contribute to the taste of toma-toes [35].
Glutamine and glutamate are the main amino
acids translocated by the phloem system of L.
esculentum, and glutamine and GABA are the predominant N-forms in developing fruits [12]. In the present study, the pattern of devolepmental changes in the free amino acid content of ripening fruits of three tomato cultivars (Platense, Cherry and Vollendung), grown under identical condi-tions, were determined. Care was taken to ensure that the plants were all exposed to the same water-ing and nitrogen nutrition regime. The relative molar content of the more abundant amino acids varied considerable during ripening. Nevertheless, a remarkable increase in the glutamate content of ripening fruits was apparent in the three cultivars under study (Tables 1 and 2). Cherry variety had the highest glutamate content (52% of all amino acids), which coincided with a decrease in the glutamine plus GABA content. An increase in the glutamate content during tomato fruit ripening was also observed in all other cultivars assayed so far, independent of the growing conditions (un-published observations). Nagata and Saijo [31] showed that fruits of a tomato mutant defective in ripening (rin for ripening inhibitor) had less than half of the glutamate content as compared to the corresponding ripe wild type fruits. Taken to-gether, these results indicate that the accumulation of glutamate in tomato fruits is a consequence of the ripening process.
To elucidate the capacity of the fruit to metabo-lize amino acids, the activities of enzymes involved in the metabolism of glutamine and glutamate
Fig. 2. Polypeptide patterns of GS and GDH inL.esculentum
cv Platense fruits. Pericarp protein extracts (20 mg) were fractionated in SDS-PAGE (12.5%), blotted on nylon mem-brane and immunoassayed using antisera against Zea mays
GS2 and Vitis6inifera GDH and ECL as detection system.
(A) SDS-PAGE followed by Coomassie Brilliant Blue stain-ing. (B) and (C) Western blot analysis with anti-GDH and anti-GS2, respectively. G, means mature green fruit; R, means red fruit.
Fig. 3. Western blot analysis of GS in the leaf and fruit ofL.
esculentumcv Platense. Protein extracts (25mg) were fraction-ated in SDS-PAGE (12% acrylamide), blotted on nylon mem-brane and immunoassayed using antiserum againstZea mays
were measured in the pericarp protein extract of green and red fruits. Results showed an important change in the expression pattern of the nitrogen metabolizing enzymes during tomato fruit ripen-ing. Two groups of enzymes could be defined (Figs. 1 and 2 and Table 3), one group is ex-pressed mainly in green fruits, like GS, GAD, alanine aminotransferase and NADP-malic en-zyme, and another group in red fruits, represented by NADH-GDH, aspartate aminotransferase and NAD-malate dehydrogenase. GS was found in green fruits only and the GS protein was detected by Western blot analysis. Electrophoresis on a native gel yielded a single band with GS trans-ferase activity. Higher plant GS has been reported to be active as an octamer [36] and a tetramer [25]. Our result indicates that in the leaves and green fruits of tomato, cultivated under our grow-ing conditions, only one oligomeric stage of GS seemed to be active. Two types of GS isoenzymes exist in most plants examined, cytosolic (GS1) and plastidic (GS2), based on their subcelullar localization. In earlier analysis of tomato leaves, a single polypeptide was detected for each GS1 and GS2 isoenzyme [37]. In tomato cotyledons GS2 was found to be represented by two different polypeptides of 44 and 45 kDa, while GS1 oc-curred as a single polypeptide of 40 kDa [34]. In the case of fruits, the prevalence of the 40 kDa polypeptide suggests that the cytosolic GS was the main isoenzyme present.
Alanine aminotransferase and NADP-malic en-zyme are two other enen-zymes exclusively present in the pericarp of mature green fruits. The activity of the latter was reported to be highest in the locular gel of mature-green tomato fruits [32]. The large decline in the activity of alanine aminotransferase observed in ripe fruits correlates with the low alanine content of these fruits (Tables 1 and 3). In tomato fruits, a mRNA encoding a putative GAD was found at particularly high levels in the early breaker stage of normal tomato ripening and continued to accumulate after the onset of ripening in rin mutant fruit [18]. In this study, GAD was detected mainly in mature green fruits and its activity declined to less than 20% in yellow fruits, and was undetectable in red fruits. It is likely that more than one GAD isoform occurs in tomato.
GDH protein and activity were highly induced in ripe fruits. A similar increase in GDH protein
content was also noticed in avocado fruits at the end of the ripening process [14]. The role of GDH in nitrogen assimilation is a matter of debate. The decline in GS and the induction of GDH in ripe fruits, suggest a reciprocal regulation of both en-zymes during tomato fruit ripening. Similar obser-vations were reported in avocado fruits [14] and maize roots [38].
The increase in the relative content of gluta-mate in ripe fruits related to green fruits is corre-lated with an increase in the levels of GDH, aspartate aminotransferase and NAD-malate de-hydrogenase, and also with a decline of GS in ripe fruits. In this way the utilization of glutamate might be diminished. Recently an increase in NADP-isocitrate dehydrogenase was found in the late stage of tomato ripening [39], suggesting that 2-oxoglutarate is generated in ripe fruits.
Considerable variation in the expression pattern of amino acid metabolizing enzymes occurs dur-ing the ripendur-ing process of tomato fruit. However the contribution of each enzyme to the amino acid pool remains to be assigned. Current work is in progress to clarify this matter.
Acknowledgements
The authors are indebted to Otto Wedemeyer for taking care of tomato plants. We wish to
thank Tatsuo Sugiyama, Nagoya University,
Japan and K.A. Roubelakis-Angelakis, University of Crete, Greece, for the generous gift of the antisera against the Zea mays chloroplastic GS,
Fd-GOGAT and Vitis 6inifera NADH-GDH. We
also thank Anna Brandeck for her continuous help with Argentina and Germany communica-tions. EMV is member of CONICET (Argentina) and JFP is a fellow of the same institution. This work was supported by a grant from the Volk-swagen Foundation (Germany).
References
[1] G. Gillaspy, H. Ben-David, W. Gruissem, Fruits: a developmental perspective, Plant Cell 5 (1993) 1439 – 1451.
[2] C.J. Brady, Fruit ripening, Annu. Rev. Plant Physiol. 38 (1987) 155 – 178.
[4] B. Piechulla, R.E. Glick, H. Bahl, A. Melisand, W. Gruissem, Changes in photosynthetic capacity and pho-tosynthetic protein pattern during tomato fruit ripening, Plant Physiol. 84 (1987) 911 – 917.
[5] M.R. Marano, N. Carrillo, Chromoplast formation dur-ing tomato fruit ripendur-ing. No evidence for plastid DNA methylation, Plant Mol. Biol. 16 (1991) 11 – 19. [6] L. Ho, Metabolism and compartmentation of imported
sugars in sink organs in relation to sink strength, Annu. Rev. Plant Physiol. Plant Mol. Biol. 39 (1988) 355 – 378. [7] R.M. Smillie, S.E. Hetherington, W.J. Davies, Photo-synthetic activity of the calyx, green shoulder, pericarp, and locular parenchyma of tomato fruit, J. Exp. Bot. 50 (1999) 707 – 718.
[8] S. Chengappa, M. Guilleroux, W. Phillips, R. Shields, Transgenic tomato plants with decreased sucrose syn-thase are unaltered in starch and sugar accumulation in the fruit, Plant Mol. Biol. 40 (1999) 213 – 221.
[9] M.A. D’Aoust, S. Yelle, B. Nguyen-Quoc, Antisense inhibition of tomato fruit sucrose synthase decreases fruit setting and the sucrose unloading capacity of young fruit, Plant Cell 11 (1999) 2407 – 2418.
[10] A.A. Schaffer, M. Petreikov, Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation, Plant Physiol. 113 (1997) 739 – 746. [11] E.M. Klann, B. Hall, A.B. Bennett, Antisense acid
inver-tase (TIV1) gene alters soluble sugar composition and size in transgenic tomato fruit, Plant Physiol. 112 (1996) 1321 – 1330.
[12] E.M. Valle, S.B. Boggio, H.W. Heldt, Free amino acids content of phloem sap and fruits inLycopersicon escu
-lentum, Plant Cell Physiol. 39 (1998) 458 – 461.
[13] F. Gallardo, S. Ga´lvez, M.A. Quesada, F.M. Ca´novas, I. Nun˜ez de Castro, Glutamine synthetase activity dur-ing the ripendur-ing of tomato, Plant Physiol. Biochem. 26 (1988) 747 – 752.
[14] K.A. Loulakakis, A.K. Roubelakis-Angelakis, A.K. Kanellis, Regulation of glutamate dehydrogenase and glutamine synthetase in avocado fruit during develop-ment and ripening, Plant Physiol. 106 (1994) 217 – 222. [15] F. Gallardo, F.R. Canto´n, A. Garcia-Gutierrez, F.M.
Ca´novas, Changes in photorespiratory enzymes and glu-tamate synthases in ripening tomatoes, Plant Physiol. Biochem. 31 (1993) 189 – 196.
[16] M. Bu¨ker, D. Schu¨nemann, S. Borchert, Enzymic prop-erties and capacities of developing tomato (Lycopersicon esculentum L.) fruit plastids, J. Exp. Bot. 49 (1998) 681 – 691.
[17] E.M. Valle, J.F. Palatnik, S.B. Boggio, Amino acids assimilation in tomato, in: P. Mathis (Ed.), Photosynthe-sis: from Light to Biosphere, Kluwer, Dordrecht, 1995, pp. 411 – 414.
[18] P.P. Gallego, L. Whotton, S. Picton, D. Grierson, J.E. Gray, A role for glutamate decarboxylase during tomato ripening: the characterisation of a cDNA encoding a putative glutamate decarboxylase with a calmodulin-binding site, Plant Mol. Biol. 27 (1995) 1143 – 1151. [19] R. Rastogi, J. Dulson, S.J. Rothstein, Cloning of tomato
(Lycopersicon esculentum Mill.) arginine decarboxylase gene and its expression during fruit ripening, Plant Phys-iol 103 (1993) 829 – 834.
[20] S. Picton, J.E. Gray, S. Payton, S.L. Barton, A. Lowe, D. Grierson, A histidine decarboxylase-like mRNA is involved in tomato fruit ripening, Plant Mol. Biol. 23 (1993) 627 – 631.
[21] J.B. Jones, Hydroponics: its history and use in plant nutrition studies, J. Plant Nutr. 5 (1982) 1005 – 1030. [22] B. Riens, G. Lohaus, D. Heineke, H.W. Heldt, Amino
acid and sucrose content determined in the cytosolic, chloroplastic and vacuolar compartments and in the phloem sap of spinach leaves, Plant Physiol. 97 (1991) 227 – 233.
[23] K.A. Loulakakis, K.A. Roubelakis-Angelakis, Intracel-lular localization and properties of NADH-glutamate dehydrogenase from Vitis 6inifera L.: purification and
characterization of the major leaf isoenzyme, J. Exp. Bot. 41 (1990) 1223 – 1230.
[24] G.L. Peterson, A simplification of the protein assay method of Lowry et al. which is more generally applica-ble, Anal. Biochem. 83 (1977) 346 – 356.
[25] G. Ma¨ck, Glutamine synthetase isoenzymes, oligomers and subunits from hairy roots of Beta 6ulgaris L. var.
lutea, Planta 205 (1998) 113 – 120.
[26] A.D. Carroll, G.G. Fox, S. Laurie, R. Phillips, R.G. Ratcliffe, G.R. Stewart, Ammonium assimilation and the role of aminobutyric acid in pH homeostasis in carrot cell suspensions, Plant Physiol. 106 (1994) 513 – 520.
[27] M.V. Bergmeyer, Methods of Enzymatic Analysis, VCH, Weinheim, Germany, 1983.
[28] H. Sakakibara, S. Kawabata, H. Takahashi, T. Hase, T. Sugiyama, Molecular cloning of the family of glutamine synthetase genes from maize: expression of genes for glutamine synthetase and ferredoxin-dependent gluta-mate synthase in photosynthetic and non-photosynthetic tissues, Plant Cell Physiol. 33 (1992) 49 – 58.
[29] K.A. Loulakakis, K.A. Roubelakis-Angelakis, Im-munocharacterization of NADH-glutamate dehydroge-nase from Vitis 6inifera L., Plant Physiol. 94 (1990) 109 – 113.
[30] D. Laval-Martin, J. Farineau, J. Diamond, Light versus dark carbon metabolism and photosynthetic activity in cherry tomato fruits. I. Occurrence of photosynthesis. Study of intermediates, Plant Physiol. 60 (1977) 872 – 876.
[31] M. Nagata, R. Saito, Changes in free amino acid con-tents of tomato fruits during ripening, especially changes in glutamine, Nippon Shokuhin Kogyo Gakkaishi 39 (1992) 64 – 67.
[32] M. Knee, F.L. Finger, NADP+-malic enzyme and
or-ganic acid levels in developing tomato fruits, J. Am. Soc. Hort. Sci. 117 (1992) 799 – 801.
[33] J. Andrews, The climacteric respiration rise in attached and detached tomato fruits, Postharv. Biol. Technol. 6 (1995) 287 – 292.
[35] S. Fuke, S. Konosu, Taste-active components in some foods: a review of Japanese research, Physiol. Behav. 49 (1991) 863 – 868.
[36] G.R. Stewart, A.F. Mann, P.A. Fentem, Enzymes of glutamate formation: glutamate dehydrogenase, glu-tamine synthetase and glutamate synthase, in: B.J. Miflin (Ed.), Book Title?, Academic Press, London, 1980, pp. 271 – 327.
[37] J. Pe´rez-Rodrı´guez, V. Valpuesta, Expression of glu-tamine synthetase genes during natural senescence
of tomato leaves, Physiol. Plant. 97 (1996) 576 – 582.
[38] A. Oaks, I. Stulen, K. Jones, M.J. Winspear, S. Mishra, I.L. Boesel, Enzymes of nitrogen assimilation in maize roots, Planta 148 (1980) 483 – 484.
[39] F. Gallardo, S. Ga´lvez, P. Gadal, F.M. Ca´novas, Changes in NADP+-linked isocitrate dehydrogenase
during tomato fruit ripening. Characterization of the predominant cytosolic enzyme from green and ripe peri-carp, Planta 196 (1995) 148 – 154.