The effect of
Botrytis cinerea
infection on ascorbate-glutathione
cycle in tomato leaves
Elz
;
bieta Kuz´niak *, Maria Skl
*
odowska
Department of Plant Physiology and Biochemistry,Uni6ersity of L*o´dz´,90-237L*o´dz´,Banacha12/16,Poland
Received 18 February 1999; received in revised form 21 June 1999; accepted 24 June 1999
Abstract
Changes in the total pool sizes of ascorbate and glutathione and their degree of oxidation as well as the activities of ascorbate peroxidase, dehydroascorbate reductase, glutathione reductase and glutathione transferase in tomato leaves afterBotrytis cinerea
infection have been studied. In infected leaves the concentration of reduced ascorbate was similar to that in control. A transient increase in dehydroascorbate content (55% above the control) and a decrease in the ascorbate redox status were observed only 3 days after inoculation. In the diseased leaves we found a significant progressive decrease, up to 50% 5 days after inoculation, in the reduced glutathione content while the oxidized glutathione concentration remained unaffected. Although a decline in reduced glutathione content was detected the ascorbate and glutathione redox ratios were maintained high up to the 5th day after inoculation.B.cinereainfection enhanced ascorbate peroxidase and glutathione reductase activities. Dehydroascorbate reductase activity was significantly decreased when compared with control, but its activity level in the inoculated leaves stayed rather constant. Total glutathione transferase activity remained unchanged. In the diseased tissues statistically significant (PB0.05) linear correlations between glutathione reductase and ascorbate peroxidase activities were found to occur 4 and 5 days after inoculation and ascorbate peroxidase activity was correlated with the total glutathione level and reduced glutathione content on the 4th day. The decreased reduced glutathione content found in the infected leaves could be a limiting factor for operation of the ascorbate-glutathione cycle related enzymes at the advanced stages of infection development. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Ascorbate-glutathione cycle;Lycopersicon esculentumMill.;Botrytis cinereainfection
www.elsevier.com/locate/plantsci
1. Introduction
There is considerable evidence that activated oxygen species (AOS) are involved in plant re-sponses to abiotic environmental stresses [1] as well as in several plant reactions to bacterial and fungal pathogen infection [2]. During infection AOS may be generated by both the invading pathogen and the host-plant. It has been reported
that AOS might be instrumental for necrotrophic plant pathogens such asBotrytis cinerea to kill the host tissue in initial stages of infection [3]. More-over, the ability of B. cinerea cultures grown on different substrates to generate H2O2 was
corre-lated with their ability to infect leaves [4]. Hydro-gen peroxide induced after pathoHydro-gen invasion has several effects in plants. It has been postulated to serve as a microbicidal agent at the sites of infec-tion and to be involved in the hypersensitive re-sponse associated plant cell death inhibiting the proliferation of the invading pathogen as well as in triggering later defence reactions: peroxidase catalyzed cell wall reinforcement, phytoalexin syn-thesis and defence-related gene activation [5]. However, when AOS are produced in excess, re-gardless of their exo- or endogenous origin, they
Abbre6iations:AOS, activated oxygen species; APX, ascorbate per-oxidase; As, reduced ascorbate; CDNB, 1-chloro-2,4-dinitrobenzene; DAs, dehydroascorbate; DHAR, dehydroascorbate reductase; DTNB, 5,5%-dithiobis-2-nitrobenzoic acid; GR, glutathione reductase; GSH, glutathione reduced; GSSG, glutathione oxidized; GST, glu-tathione-S-transferase.
* Corresponding author. Tel.: +48-42-6354414.
E-mail address:[email protected] (E. Kuz´niak)
E.Kuz´niak,M.Skl*odowska/Plant Science148 (1999) 69 – 76 70
exhibit deleterious impact on plant cells. To counteract the potential toxicity of AOS and, at the same time, to allow them to play their beneficial function in defence reactions, the an-tioxidative system must keep AOS under control. Catalases and peroxidases are the two major en-zymatic systems for H2O2 removal. In plant cells
H2O2 generated under stress conditions is
be-lieved to be mainly scavenged through reactions involving successive oxidations and reductions of ascorbate and glutathione known as the ascor-bate-glutathione (Halliwell-Asada) cycle [6], whereas catalases are mainly associated with H2O2 detoxification in peroxisomes [7]. The
ascorbate-glutathione cycle operates both in chloroplasts and in cytosol. The first step of the ascorbate-glutathione cycle is APX (EC 1.11.1.11) catalyzed reduction of H2O2 to water
with ascorbate as an electron donor. Monodehy-droascorbate, the primary product of As oxida-tion, disproportionates spontaneously to DAs that is reduced by DHAR (EC 1.8.5.1) using GSH as a reductant. GSSG is then regenerated by GR (EC 1.6.4.2) at the expense of NADPH. GSH can participate also in conjugation reac-tions catalyzed by GST (EC 2.5.1.18). GST is mainly involved in detoxification of xenobiotics [8] but it has been shown that pathogen attack induced an increase in GST mRNA in soybean cells [9].
In reference to their role in H2O2 scavenging,
different components of the ascorbate-glu-tathione cycle have been extensively studied in cells exposed to oxidative stress due to the impo-sition of environmental constraints [10]. How-ever, there are scant and conflicting data on the response of the ascorbate-glutathione cycle in-duced by infection. Inoculation of barley leaves with Blumeria graminis had no effect on the fo-liar ascorbate content or its redox state [11]. May et al. [12] showed that in Arabidopsis the depletion of glutathione levels by 70% did not alter its responses to the pathogens analyzed, whereas a strong GSH accumulation and alter-ation in GSH/GSSG redox balance have been observed in tomato cells challenged with
Cladosporium ful6um elicitor [13].
The current study examines changes in the to-tal pool sizes of ascorbate and glutathione and their degree of oxidation as well as changes in
the activities of the ascorbate-glutathione cycle associated antioxidant enzymes in B. cinerea in-fected tomato leaves.
2. Materials and methods
Tomato plants (Lycopersicon esculentum Mill.) cv ‘Perkoz’ were grown from seeds in soil, in a growth chamber under 16-h day and 8-h night cycles, with 350 mE m−2 s−1 light intensity, at
23°C. At the age of 5 weeks tomato plants were inoculated with Botrytis cinerea Pers. conidial suspension (1×106 conidia/ml). Each of the fully
expanded leaves was uniformly sprayed with conidial suspension. Control plants were sprayed with sterile distilled water. After inoculation plants were kept at 100% relative humidity. The second and the third leaves from the bottom were used for experiments 1, 2, 3, 4 and 5 days after B. cinerea inoculation. Leaves were har-vested in the middle of the 16-h light period.
2.1. Preparation of enzyme extracts
Leaves were homogenized (1:10 w/v) in an ice cold mortar using 50 mM potassium phosphate buffer pH 7.0 containing 1 M NaCl, 1% polyvinylpyrrolidone and 1 mM EDTA. For as-says of APX and DHAR extracts were prepared in the same medium also containing 1 mM sodium ascorbate and 2 mM 2-mercaptoethanol, respectively. After centrifugation (20 000×g, 15 min.) the supernatant was used for determination of APX, DHAR, GR and GST activities as well as glutathione content.
2.2. Enzyme assays
APX activity was assayed following the oxida-tion of As to DAs at 265 nm (o=13.7 mM−1
cm−1) by a modified method of Nakano and
Asada [14]. The assay mixture contained 50 mM potassium phosphate buffer pH 7.0, 0.25 mM sodium ascorbate, 25 mM H2O2 and enzyme
ex-tract (20 – 40 mg protein). Addition of H2O2
DHAR activity was determined by following the formation of As at 265 nm (o=14.6 mM−1cm−1)
according to Hossain and Asada [15] in 50 mM potassium phosphate buffer pH 6.4 containing 0.5 mM DAs and 2.5 mM GSH and enzyme extract (40mg protein). The non-enzymatic reduction of
DAs by GSH was subtracted. Enzyme activity was expressed in mmol As min−1 mg−1 protein.
GR activity was assayed spectrophotometrically by determination of GSSG-dependent oxidation of NADPH at 340 nm (o=6.22 mM−1 cm−1) as
described by Beutler [16]. The reaction solution contained 1 M Tris – HCl (pH 8.0) with 5 mM EDTA, 33 mM GSSG and 2 mM NADPH and enzyme extract (50mg protein). Enzyme activity
was expressed in units, each representing 1 nmol of oxidized NADPH per minute per mg protein.
Total GST activity was measured using CDNB as modified by Carmagnol et al. [17]. The product of CDNB conjugation with GSH absorbs at 340 nm (o=9.6 mM−1 cm−1). The reaction mixture
con-tained 100 mM sodium phosphate (pH 6.25), 0.75 mM CDNB and 30 mM GSH and enzyme extract (50 mg protein). Enzyme activity was expressed
in units, each representing the formation of 1 nmol of S-conjugate per minute per mg protein.
2.3. Determination of ascorbate
Tomato leaves were homogenized in a cold mor-tar placed on ice using 6% trichloroacetic acid. A modification of the colorimetric bipirydyl method of Okamura [18], as described by Kno¨rzer et al. [19], was used.
2.4. Determination of glutathione
For the determination of glutathione the same extracts as for enzyme assays were used. The levels of non-protein sulfhydryl groups (−SH) were de-termined colorimetrically using DTNB as described by Brehe and Burch [20]. For specific assay of GSSG the GSH can be masked by derivatisation with 2-vinylpyridine in the presence of trietanoloamine for 60 min at 20°C (250 ml of the extract, 5 ml
2-vinylpyridine and 15 ml triethanoloamine). To
evaluate whether there are statistically significant correlations between GSH content and GR and GST activities, GSH concentration calculated in nmol mg−1 protein (data not shown) was used.
Protein was determined by the Bradford method [21], with standard curves prepared using bovine serum albumin.
All assays were performed spectrophotometri-cally (UNICAM UV 300 UV-visible spectrometer) at 25°C.
2.5. Statistical analysis
The results presented are the means (n=6 – 12) of three independent experiments. Sample variability is given as the standard error of the mean. The significance of differences between mean values was determined by a non-parametric Mann – Whitney Rank Sum Test. Differences at PB0.05 were con-sidered significant. Linear correlation coefficients were evaluated using the STATISTICA (Edition 1998) software. The level of significance was set at
PB0.05.
3. Results
In tomato plants light to dark brown localized lesions, the first visible symptoms of B. cinerea
infection, appeared on the second and the third leaves used for experiments starting from the 3rd day after inoculation. No new lesions were observed until the 5th day. The youngest leaves remained symptomless up to the end of the experimental period. At the 5th day the fungus started to sporu-late and a subset of lesions on the lowermost leaves developed into spreading lesions and thereafter a typical grey mould became evident.
3.1. Ascorbate and glutathione contents
The As content was not influenced byB.cinerea
E.Kuz´niak,M.Skl*odowska/Plant Science148 (1999) 69 – 76 72
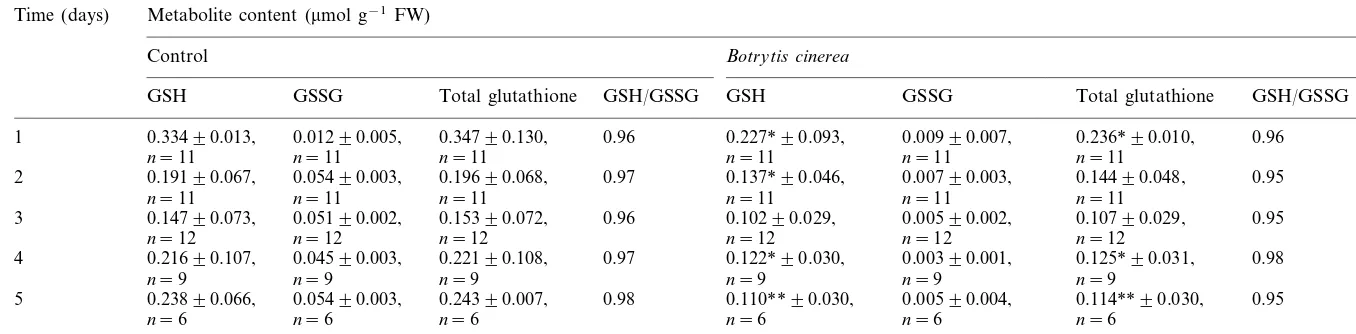
In B. cinerea infected leaves a significant de-crease in GSH concentration when compared to control was found (Table 2). It ranged from 32% (PB0.05) to 54% (PB0.01) 1 and 5 days after inoculation, respectively. Similarly to GSH, a pro-gressive decrease in total glutathione concentra-tion was observed during infecconcentra-tion development. On the 1st day after inoculation the total foliar glutathione content of 0.236mmol/g FW was 32%
(PB0.05) lower than in control and on the 5th day it declined to 0.11mmol/g FW, i.e. 53% (PB
0.01) below the control value. A less pronounced decrease in GSH as well as in total glutathione concentrations was also observed in the non-inoc-ulated leaves up to the 3rd day (Table 2). The GSSG concentration in infected leaves was com-parable to that in healthy plants except a transient decrease to 62% of control observed on the 4th day. The redox state of glutathione (GSH/GSSG) stayed roughly constant in infected leaves and varied from 0.98 to 0.95.
In inoculated leaves the levels of antioxidants in their physiologically active form, i.e. GSH and As, were correlated with each other on the 2nd and 4th days, as shown by r=0.691 (PB0.05) and
r=0.889 (PB0.05), respectively. Similarly, the to-tal glutathione concentration correlated with toto-tal ascorbate content: r=0.669 (PB0.05) and r=
0.858 (PB0.05) for the 2nd and 4th days, respectively.
3.2. Enzyme acti6ities
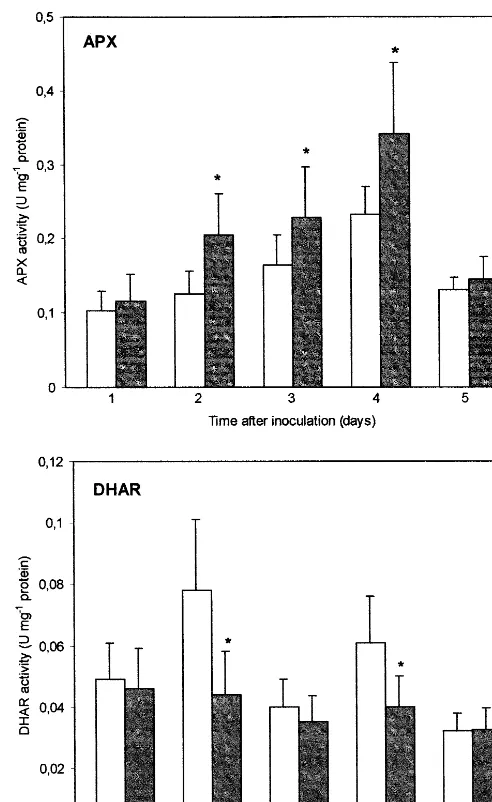
In B. cinerea infected leaves enhanced APX activity was found (Fig. 1). Compared with con-trol leaves significant APX activity increases of 64, 39 and 47% (PB0.05) were observed 2, 3 and 4 days after inoculation, respectively. In contrast to APX, infection caused DHAR activity to decrease (Fig. 1). The strongest DHAR activity reduction found 2 and 4 days after inoculation was 44% (PB0.05) and 35% (PB0.05), respectively, com-pared to control leaves. However, DHAR activity level changed substantially in non-inoculated con-trol leaves whereas in the inoculated ones it re-mained roughly stable during the time course of the experiment. In the infected tissues GR activity was enhanced (Fig. 2). Statistically significant (PB0.05) GR activity increase, compared to con-trol, was detected from the 3rd to the 5th days of experiment, when infected plants showed visible disease symptoms. During the course of experi-ment total GST activity was unchanged in infected leaves when compared to control (Fig. 2). GR and GST activities were positively correlated during the first 3 days of the experiment, both in control and infected leaves. The statistically significant (PB0.05) correlation coefficients for the 1st, 2nd and 3rd days were as follows: r=0.685,r= 0.817 and r=0.826 in control leaves and r=0.856, r=
0.902 and r=0.625 in the infected leaves.
Table 1
Ascorbate content in control and infected tomato leavesa
Metabolite content (mmol g−1FW)
Time (days)
aValues are the means of three different experiments9S.E.
*PB0.05, **PB0.01,
E
.
Kuz
´niak
,
M
.
Skl
*
odowska
/
Plant
Science
148
(1999)
69
–
76
73
Glutathione content in control and infected tomato leaves
Time (days) Metabolite content (mmol g−1FW)
Botrytis cinerea
Control
GSSG Total glutathione GSH/GSSG GSH GSSG Total glutathione GSH/GSSG GSH
0.96 0.96 0.227*90.093, 0.00990.007,
0.01290.005, 0.34790.130, 0.236*90.010, 1 0.33490.013,
n=11 n=11
n=11
n=11 n=11 n=11
0.137*90.046, 0.19690.068, 0.97
0.19190.067, 0.14490.048, 0.95
2 0.05490.003, 0.00790.003,
n=11
n=11 n=11 n=11 n=11 n=11
0.10790.029, 0.96 0.10290.029, 0.00590.002,
0.15390.072, 0.95
0.05190.002, 3 0.14790.073,
n=12
n=12
n=12 n=12
n=12 n=12
0.97 0.122*90.030,
0.22190.108, 0.00390.001,
0.04590.003, 0.125*90.031,
0.21690.107, 0.98
4
n=9
n=9
n=9
n=9 n=9 n=9
0.24390.007, 0.98 0.110**90.030,
0.05490.003, 0.00590.004, 0.23890.066,
5 0.114**90.030, 0.95
n=6
n=6 n=6
n=6 n=6 n=6
aValues are the means of three different experiments9S.E.
*PB0.05,
E.Kuz´niak,M.Skl*odowska/Plant Science148 (1999) 69 – 76 74
Fig. 1. The effect ofB. cinereaattack on APX and DHAR activities in tomato leaves. White bars, non-inoculated con-trols; black bars, inoculated leaves. Bars represent S.E. of means. *Indicates values that differ significantly from the control atPB0.05.
4. Discussion
We observed that young tomato leaves were less sensitive toB.cinereainfection than older ones. The spreading lesions with a typical grey mould form of disease were visible only on the oldest leaves taken for experiments starting from the 5th day after inoculation. Taking into account that senescence is one of the most important factors for intact organ susceptibility toB.cinerea[22], we suggest that the changes in ascorbate-glutathione cycle observed in tomato plants infected with this necrotrophic fungal pathogen could be at least partly related to senes-cence, which favours disease development, and not to the hypersensitive reaction.
Fig. 2. The effect of B. cinerea attack on GR and GST activities in tomato leaves. White bars, non-inoculated con-trols; black bars, inoculated leaves. Bars represent S.E. of means. *Indicates values that differ significantly from the control atPB0.05.
Only in the infected leaves did we find some correlations between antioxidants and the enzymes of the ascorbate-glutathione cycle to occur on the 4th day after inoculation. GR activity was found to be positively correlated with DHAR activity (r=
0.774,PB0.05). Similarly, positive correlations of GR activity with GSH concentration (r=0.698,
B. cinereainfection has been shown to influence differentially the two antioxidant compounds and enzymes involved in their regeneration. The activity of APX, which is proposed to be predominantly responsible for H2O2decomposition in plants, was
significantly higher in infected leaves. The induction of APX suggests that it was mobilized as a detox-ification mechanism for AOS generated during tomato-B. cinereainteraction [3]. El-Zahaby et al. [23] showed a substantial APX activity increase in susceptible barley cultivars and a less pronounced increase in the resistant cultivar after powdery mildew infection, whereas Vanacker et al. [11], using the same pathosystem, showed that the foliar APX activity decreased in the resistant isoline whereas it was unchanged in the susceptible one.
Unlike some other studies [23,24], we found no apparent changes in As content in infected tomato leaves, except the significant decrease observed 3 days after inoculation, accompanied by the appear-ance of visible disease symptoms. The decline in As level was associated with a transient DAs accumu-lation and with a decrease in the ascorbate redox status. We did not observe a prolonged maintenance of higher DAs content in infected leaves although the DHAR activity remained markedly lower than in control from the 1st to 4th day after inoculation. DAs may not be found to accumulate after infection because (1) the activity of monodehydroascorbate reductase compensates for the decrease in DHAR activity or (2) DAs formed decomposes to products such as tartrate and oxalate [25]. Moreover, it has been recently shown that at least four different proteins can catalyse in vitro the glutathione-depen-dent DAs reduction [26].
In B. cinerea infected leaves we observed a massive decrease in the glutathione pool at the expense of GSH, while the amount of GSSG remained unaffected. Moreover, the GSH/GSSG ratio remained high during the plant-pathogen interaction. In our study GR activity seems to be sufficient to maintain the high redox state of glu-tathione in infected leaves, thus preventing the accumulation of GSSG. Go¨nner and Schlo¨sser [27] reported a marked decline in glutathione content in barley leaves infected with highly virulent Drech
-sleraspecies in comparison to that in leaves inocu-lated with weakly virulent ones.
We found that in the uninoculated control leaves some of the studied parameters underwent similar changes as those described for the diseased tissues,
e.g. APX activity increased and GSH concentration decreased. In control plants the GSH content, calculated in nmol/mg protein, gradually declined up to the end of the experimental period (data not shown). We suggest that the changes in ascorbate-glutathione cycle in control leaves could be at least partly the effect of the concomitant senescence process. Similarly, in senescing pea leaves GSH content decreased 16-fold during 11 days [28]. However, the possibility that these changes have been induced as a consequence of keeping plants at 100% relative humidity for initiating B. cinerea
infection can not be excluded.
According to the obtained data the strongly decreased GSH level seems to be the limiting factor for operation of the ascorbate-glutathione cycle during the advanced stage of infection development, before the appearance of spreading lesions. This is supported by a significant positive correlation be-tween GR and APX activities found to occur 4 and 5 days after inoculation (r=0.797, PB0.05 and
r=0.894,PB0.05, respectively). Moreover, on the 4th day APX activity in the diseased tissues was correlated with the total glutathione level (r=
0.680,PB0.05) and GSH content (r=0.690,PB
0.05).
In conclusion, the decrease in GSH content and the concomitant stability in As concentration dur-ing tomato-B. cinerea interaction seem to indicate that GSH may play an important role in biochem-ical reactions against disease development. The shortage of GSH supply influenced the activity of ascorbate-glutathione related enzymes at the ad-vanced stage of infection development. However, while APX, GR and GST exist in several isoen-zymes at different subcellular sites they could be selectively induced after pathogen attack. Further studies are necessary for a better understanding of the role of ascorbate-glutathione cycle in plant responses to fungal pathogen infection. To deter-mine the contribution of the distinct isoforms in plant defence the chloroplastic ascorbate-glu-tathione cycle is currently being studied.
Acknowledgements
E.Kuz´niak,M.Skl*odowska/Plant Science148 (1999) 69 – 76 76
Department of Plant Protection, Skierniewice, Poland) for providing B. cinerea strain.
References
[1] K. Kirtikara, D. Talbot, Alteration in protein accumula-tion, gene expression and ascorbate-glutathione pathway in tomato (Lycopersicon esculentum) under paraquat and ozone stress, J. Plant Physiol. 148 (1996) 752 – 760. [2] C.J. Baker, E.W. Orlandi, Active oxygen in plant
patho-genesis, Annu. Rev. Phytopathol. 33 (1995) 299 – 321. [3] A. v Tiedemann, Evidence for a primary role of active
oxygen species in induction of host cell death during infection of bean leaves with Botrytis cinerea, Physiol. Mol. Plant Pathol. 50 (1997) 151 – 166.
[4] W. Edlich, G. Lorenz, H. Lyr, E. Nega, E.-H. Pommer, New aspects on the infection mechanism of Botrytis cinerea Pers, Neth. J. Plant Pathol. 95 (1989) 53 – 62. [5] M.C. Mehdy, Y.K. Sharma, K. Sathasivan, N.W. Bays,
The role of activated oxygen species in plant disease resistance, Plant Physiol. 98 (1996) 365 – 374.
[6] G. Noctor, C.H. Foyer, Ascorbate and glutathione: keeping active oxygen under control, Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 (1998) 249 – 279.
[7] H. Willekens, D. Inze´, M. Van Montagu, W. Van Camp, Catalases in plants, Mol. Breed. 1 (1995) 207 – 228. [8] J.O.D. Coleman, M.M.A. Blake-Kalff, T.G.E. Davies,
Detoxification of xenobiotics by plants: chemical modifi-cation and vacuolar compartmentation, Trends Plant Sci. 4 (1997) 144 – 151.
[9] A. Levine, R. Tenhaken, R. Dixon, C. Lamb, H2O2from
the oxidative burst orchestrates the plant hypersensitive disease resistance response, Cell 79 (1994) 583 – 593. [10] C.H. Foyer, H. Lopez-Delgado, J.F. Dat, I.M. Scott,
Hydrogen peroxide- and glutathione-associated mecha-nisms of acclimatory stress tolerance and signalling, Physiol. Plant. 100 (1997) 241 – 254.
[11] H. Vanacker, T.L.W. Carver, C.H. Foyer, Pathogen-in-duced changes in the antioxidant status of the apoplast in barley leaves, Plant Physiol. 117 (1998) 1103 – 1114. [12] M.J. May, J.E. Parker, M.J. Daniels, C.J. Leaver, C.S.
Cobbett, An Arabidopsismutant depleted in glutathione shows unaltered responses to fungal and bacterial patho-gens, Mol. Plant Microbe Interact. 9 (1996) 349 – 356. [13] M.J. May, K.E. Hammond-Kosack, J.D.G. Jones,
In-volvement of reactive oxygen species, glutathione metabolism, and lipid peroxidation in the Cf -gene-de-pendent defence response of tomato cotyledons induced by race-specific elicitors of Cladosporium ful6um, Plant
Physiol. 110 (1996) 1367 – 1379.
[14] Y. Nakano, K. Asada, Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts, Plant Cell. Physiol. 22 (1981) 867 – 880.
[15] M.A. Hossain, K. Asada, Purification of dehydroascor-bate reductase from spinach and its characterization as a thiol enzyme, Plant Cell. Physiol. 25 (1984) 85 – 92. [16] E. Beutler, Glutathione reductase, in: Red Cells
Metabolism, second ed., Grune & Stratton, New York, 1975, pp. 69 – 70.
[17] F. Carmagnol, P.M. Sinet, J. Rapin, H. Jerome, Glu-tathione S-transferase of human red blood cells assay, value in normal subjects and in two pathological circum-stances — hyperbilirubinemia and impaired renal func-tion, Clin. Chim. Acta 117 (1981) 209 – 217.
[18] M. Okamura, An improved method for determination of
L-ascorbic acid and L-dehydroascorbic acid in blood plasma, Clin. Chim. Acta 103 (1980) 259 – 268.
[19] O.C. Kno¨rzer, J. Durner, P. Bo¨ger, Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress, Physiol. Plant. 97 (1996) 388 – 396.
[20] J.E. Brehe, H.B. Burch, Enzymatic assay for glutathione, Anal. Biochem. 74 (1976) 315 – 319.
[21] M.M. Bradford, A rapid and sensitive method for the quantification of microgram quantities of protein utiliz-ing the principle of protein-dye bindutiliz-ing, Anal. Biochem. 72 (1976) 248 – 254.
[22] Y. Elad, K. Evensen, Physiological aspects of resistance toBotrytis cinerea, Phytopathology 85 (1995) 637 – 643. [23] H.M. El-Zahaby, G. Gullner, Z. Kira´ly, Effects of
pow-dery mildew infection of barley on the ascorbate-glu-tathione cycle and other antioxidants in different host-pathogen interactions, Phytopathology 85 (1995) 1225 – 1230.
[24] J. Fodor, G. Gullner, A.L. A´ da´m, B. Barna, T. Ko¨mives, Z. Kira´ly, Local and systemic responses of antioxidants to tobacco mosaic virus infection and to salicylic acid in tobacco, Plant Physiol. 114 (1997) 1443 – 1451.
[25] C.H. Foyer, Ascorbic acid, in: R.G. Alscher, J.L. Hess (Eds.), Antioxidants in Higher Plants, CRC Press, Boca Raton, FL, 1993, pp. 31 – 58.
[26] M.C. De Tullio, L. De Gara, C. Paciolla, O. Arrigoni, Dehydroascorbate-reducing proteins in maize are in-duced by the ascorbate biosynthesis inhibitor lycorine, Plant Physiol. Biochem. 36 (1998) 433 – 440.
[27] M.V. Go¨nner, E. Schlo¨sser, Oxidative stress in interac-tions betweenA6ena sati6aL. andDrechsleraspp,
Phys-iol. Mol. Plant Pathol. 42 (1993) 221 – 234.
[28] A. Jime´nez, J.A. Herna´ndez, G. Pastori, L.A. del Rı´o, F. Sevilla, Role of the ascorbate-glutathione cycle of mito-chondria and peroxisomes in the senescence of pea leaves, Plant Physiol. 118 (1998) 1327 – 1335.