Greenhouse-grown conditionally lethal tobacco plants obtained by
expression of plastidic glutamine synthetase antisense RNA may
contribute to biological safety
Andrea Migge
a, Thomas W. Becker
a,*
aLehrstuhl fu¨r Genetik,Fakulta¨t fu¨r Biologie,Uni6ersita¨t Bielefeld,Postfach10 01 31,D-33501Bielefeld,Germany
Received 3 June 1999; received in revised form 5 November 1999; accepted 8 November 1999
Abstract
A cDNA corresponding to plastidic glutamine synthetase (GS-2), an enzyme involved in photorespiration, was expressed in antisense orientation under the control of a leaf-specific soybean ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit gene promotor in transgenic tobacco to yield conditionally lethal plants. Three transgenic tobacco lines with decreased (at most 64%) foliar GS-2 activity were obtained. These plants grew normally when maintained in an atmosphere with a CO2 partial
pressure sufficiently high (300 Pa CO2) to suppress photorespiration. However, when photorespiration was initiated by the transfer
of the plants to air (35 Pa CO2), ammonium accumulated in the leaves. With time, the transgenic plants exhibited severe chlorotic
lesions and, eventually, the plants died. A stable atmosphere containing at least 300 Pa CO2 can be established easily in the
greenhouse but is unlikely to occur in a natural environment. Therefore, the transgenic tobacco plants with decreased leaf GS-2 activity may contribute to biological safety for production of desired proteins. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Glutamine synthetase; Photorespiration; Tobacco
www.elsevier.com/locate/plantsci
1. Introduction
Transgenic plants can be used to produce de-sired proteins. There is, however, an increasing social concern about the use of recombinant DNA technology. The production of desired proteins using transgenic plants may involve, for example, the risk that the genes encoding the desired proteins and/or the genes coding for the antibiotic-resistance, required as selection marker during the generation of the transgenic plants, could be trans-ferred from the transformed plant species to re-lated species growing in the same environment. Such concerns may lead to restrictions on the use of transgenic plants to produce desired proteins. It
is, therefore, desirable to develop methods that prevent or at least strongly restrict any involun-tary gene transfer from transformed plants used in biotechnology to other plants. We reasoned that transgenic plants that are viable and fertile in a specific greenhouse environment but not under natural conditions may contribute to biological safety if chosen to produce desired proteins.
Conditionally lethal plants can be obtained by deleting the activity of an enzyme involved in photorespiration [1]. In leaves, ribulose-1,5-bis-phosphate carboxylase/oxygenase catalyzes both the carboxylation and oxygenation of ribulose-1,5-bisphosphate. Carboxylation results in the forma-tion of two molecules of 3-phosphoglycerate (3-PGA) while oxygenation produces one molecule of 3-PGA and one of glycollate phosphate [2]. The 3-PGA is utilized within the photosynthetic car-bon reduction cycle to produce carbohydrates. Abbre6iations: Fd-GOGAT, ferredoxin-dependent glutamate
syn-thase; GS-2, plastidic glutamine synthetase;rbcS, ribulose-1,5-bispho-sphate carboxylase/oxygenase small subunit gene.
* Corresponding author. Tel.: +49-521-5602; fax:+49-521-5626.
Glycollate phosphate is metabolized via the photo-synthetic carbon oxidation cycle. The operation of this pathway results in the uptake of O2and in the
release of both CO2and ammonium. This process
is designated photorespiration [3]. The photores-piratory CO2 is in part reassimilated and in part
lost to the atmosphere [4] while the ammonium is reassimilated during the photorespiratory nitrogen cycle [5]. The major environmental cues affecting the rate of photorespiration are the ratio of CO2
to O2 and temperature [6].
Manipulation of the CO2 partial pressure of the
atmosphere allowed the selection of barley orAra -bidopsis thaliana mutants severely deficient in en-zymes of photorespiration from populations of seeds treated with mutagens [3]. These photores-piratory mutants grow normally if photorespira-tion is suppressed by increased ambient concentrations of CO2. When exposed to air,
how-ever, photosynthesis is rapidly impaired. With time, chlorotic lesions appear and, eventually, the mutants die [3].
Conditionally lethal photorespiratory mutants of barley or A. thaliana [3] may potentially con-tribute to biological safety when chosen to pro-duce desired proteins. However, barley can not be transformed easily so far while A. thaliana is difficult to handle in a biotechnological produc-tion process due to the small size of the plants and the short life cycle of this species. One possibility to overcome these difficulties may be to pheno-copy photorespiratory mutants of barley or A. thaliana in transgenic tobacco. We attempted to obtain transgenic tobacco lines with decreased ac-tivity of plastidic glutamine synthetase (GS-2). The GS-2 was chosen as the target of our approach because the reaction catalyzed by GS-2 represents the first step of the refixation of photorespiratory ammonium [7].
In the case of barley, the probability to obtain mutants severely deficient in GS-2 activity from mutagenized seeds was appreciably high because only a single-copy GS-2 gene as the target of mutagenesis is present in the barley genome [8]. Using the same approach, the isolation of tobacco mutants with decreased GS-2 activity may, how-ever, be considerably more difficult because two distinct GS-2 genes were detected in the am-phidiploid genome of tobacco [9]. We have, there-fore, chosen the antisense strategy [10] to obtain transgenic tobacco plants with decreased GS-2
activity. The antisense strategy allows the inhibi-tion of the expression of a multigene family for which no mutants are available and difficult to isolate [11].
2. Material and methods
2.1. Plasmid construction
The GS-2 cDNA insert of pcGS2-17 [9] contain-ing the codcontain-ing region and the 5%- and 3%-flanking regions was isolated as an EcoRI –EcoRI frag-ment (1.6 kb) from pBluescript and ligated in reverse orientation into the EcoRI-restricted bi-nary vector pAQ4.1 [12] between the soybean ribulose-1,5-bisphosphate carboxylase/oxygenase small subunit gene (rbcS) promotor (1.1 kb) [13] and the polyadenylation signal of the octopine synthase gene of Agrobacterium tumefaciens (2.3 kb) [14]. The resulting plasmid (13.6 kb) was des-ignated pGS20. This plasmid also carries the neomycin phosphotransferase II (nptII) and nptIII genes to provide a kanamycin-selectable marker in plants and in bacteria, respectively. After verifica-tion of the orientaverifica-tion of the inserted GS-2 gene by restriction analysis, pGS20 was mobilized from E. coli into the disarmed A. tumefaciens strain LB4404 by triparental mating [15].
2.2. Transformation of tobacco
Tobacco (Nicotiana tabacum L. var Petite Ha-vanna SR1; INRA, Versailles, France) was trans-formed via A. tumefaciens-mediated gene transfer using a leaf disc inoculation technique [16]. Trans-genic plants were regenerated and grown in a CO2-enriched atmosphere (300 Pa CO2) obtained
as previously described [17] by injection of CO2
into an air stream. Rooted transformants were first maintained for 6 weeks on Murashige Skoog medium with kanamycin (100 mg ml−1) and
cefo-taxim (500 mg ml−1) and then grown to maturity
in garden soil under white light from fluorescent lamps providing a photosynthetically active irradi-ance of 120 mmol photons m−2 s−1 and a 16 h
2.3. Assay of GS acti6ity
Total soluble tobacco leaf protein was extracted at 0°C using a potassium phosphate buffer (200 mM, pH 7.5) supplemented with 5 mM EDTA, 12.5 mM 2-mercaptoethanol and 2 mM phenyl-methyl sulfonyl fluoride (tissue to volume ratio is 0.5). In the semi-biosynthetic GS assay [19], the physiological substrate ammonium is replaced by hydroxylamine. The GS catalyses the formation of
g-glutamylhydroxamate (GHA) from
hydroxy-lamine and glutamate. The GHA was determined colorimetrically at 540 nm after complexation with acidified ferric chloride. Our reaction mixture con-tained 100 ml imidazole buffer (450 mM, pH 7.2),
100 ml MgCl2 (450 mM), 100 ml NH2OH·HCl (60
mM), 100 ml ATP (80 mM), 100 ml L-glutamate
(870 mM) and 300 ml H2O. The reaction was
started by adding 50 ml protein extract and
termi-nated after incubation for 15 min at 30°C by adding 850 ml of a solution containing 0.37 M
FeCl3, 0.2 M trichloroacetic acid and 0.67 N HCl.
2.4. Ammonium analysis
Leaf samples were homogenized in 50% (v/v) ethanol (pH 3). The extract was acidified with HCl prior to the removal of the liquid phase under vacuum at 40°C. The residue was resuspended in water. Ammonium was isolated from this solution by isothermic distillation and then determined col-orimetrically at 436 nm using the Nessler reagent [20]. Our reaction mixture contained 200ml NaOH
(2 N), 200 ml K-Na-tartrate (10% [w/v]) and 9 ml
H2O. After adding 300 ml of the sample and
incubation for 10 min at 25°C, 400 ml Nessler
reagent were added. The reaction mixture was incubated for 30 min at 25°C. The Nessler reagent was prepared by mixing solution A (5 g KJ/5 ml H2O) with solution B (2.3 g HgCl2/36 ml H2O)
followed by stirring for 5 min prior to the addition of solution C (4 g NaOH/20 ml H2O).
3. Results and discussion
In tobacco, the genes corresponding to GS-2 are predominantly expressed in the leaves (i.e. at the site of photorespiration) [9]. We have, therefore, attempted to decrease the activity of GS-2 specifi-cally in the leaves of transgenic tobacco by
em-ploying a strong and leaf-specific promotor to drive a chimeric GS-2 antisense gene. In transgenic tobacco, the soybean rbcS promotor [13] is strongly preferentially active in the leaves [12]. Therefore, a binary vector containing a full-length tobacco GS-2 cDNA [9] in reverse orientation downstream of the soybean rbcS promotor [13] was constructed and introduced into tobacco by A. tumefaciens-mediated gene transfer. Transgenic tobacco plants were regenerated in an atmosphere containing 300 Pa CO2 to ensure the survival of
potentially obtained transgenic lines with de-creased foliar GS-2 activity. Previously, we have shown that a CO2 partial pressure of 300 Pa is
sufficient to suppress or at least strongly impair photorespiration [17,21]. Three transgenic tobacco lines (designated GSc, GSd and GSe) were ob-tained that express a GS-2 gene in reverse orienta-tion under the control of the soybean rbcS promotor.
To verify the degree of antisense inhibition, the activity of GS-2 was measured in leaf extracts of transformants grown under non-photorespiratory conditions in a high CO2 atmosphere (300 Pa
CO2). In plant leaves, the GS may be represented
by two groups of proteins, one being located in the chloroplasts (GS-2), the other (GS-1) being restricted to the cytosol of the phloem companion cells [22]. In tobacco, however, the abundance of GS-1 is at the limit of detection by immunoblot analysis [9]. Therefore, the (total) GS activity mea-sured in tobacco leaf extracts predominantly reflects the activity of GS-2. As shown in Fig. 1, the foliar GS-2 activity of the individual transgenic tobacco lines was decreased to 45% (GSc), 36% (GSd) and 56% (GSe) of wild-type levels. In each transgenic line, the decrease in GS-2 activity was paralleled by a decreased accumulation of GS-2 specific transcripts (data not shown). Taken to-gether, these results show that a substantial degree of antisense inhibition with respect to GS-2 gene expression was achieved in transgenic tobacco.
In tobacco, the leaves are the major site of primary ammonium assimilation [23]. Despite the rather strong inhibition of foliar GS-2 activity, the transgenic tobacco plants were phenotypically in-distinguishable from wild-type plants when grown in a high CO2 atmosphere (300 Pa CO2). Plant
and transgenic plants were observed under high CO2 conditions (data not shown). Thus, under
non-photorespiratory conditions, transgenic to-bacco plants tolerate more than 60% reduction in the activity of leaf GS-2 without any growth
retar-dation. This result indicates that the residual foliar GS-2 activity has sufficient capacity for ammo-nium assimilation via the GS-2/Fd-GOGAT cycle to provide adequate input of nitrogen for normal growth of the transgenic plants, if photorespira-tory ammonium production is suppressed. A pre-vious study with barley mutants has shown that these mutants even tolerate a decrease of 90% in leaf GS-2 activity without any growth retardation provided the plants are maintained in a high CO2
atmosphere [24].
The rate at which CO2 and ammonium are
released during photorespiration at ambient CO2
partial pressure (35 Pa CO2) in the leaves of
C3-plants has been estimated to be as high as
30 – 40% of the rate of net CO2 uptake [4,25].
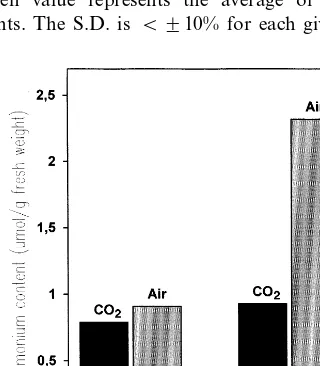
Therefore, a strong accumulation of ammonium in the leaves was expected upon transfer to air of the transgenic tobacco plants with decreased foliar GS-2 activity. However, upon illumination in air (35 Pa CO2), the high CO2-grown (300 Pa CO2)
transgenic tobacco GSd plants accumulated only about 2.7-fold more ammonium in the leaves than wild-type plants (Fig. 2). Similar results were ob-tained in identical experiments with GSc or GSe plants (data not shown). Our data are consistent with previous observations with barley mutants with reduced GS-2 activity, which suggested that a threshold of about 40% GS-2 activity exists below which ammonium accumulation takes place [7]. There is, therefore, a clear similarity between the C3 plants barley and tobacco.
Photosynthesis is inhibited rapidly following ex-posure of barley mutants severely deficient in GS-2 to air [26] and, in turn, the generation of photores-piratory ammonium may be impaired under these circumstances [5]. The (putative) inhibition of pho-torespiratory ammonium release may, at least in part, explain the relatively small accumulation of ammonium in the leaves following illumination in air of high CO2-grown transgenic tobacco with
reduced foliar GS-2 activity. In addition, some of the photorespiratory ammonium may have been lost from the plants, since even wild-type plants have an ammonium compensation point and lose some ammonium to the atmosphere [27]. Finally, the residual leaf GS-2 activity may have still been sufficient to re-assimilate a considerable amount of the photorespiratory ammonium generated upon illumination in air.
Fig. 1. The activity of GS (mmol mg−1 chlorophyll h−1) in
the leaves of the wild-type (SR1) or of tobacco lines trans-formed with pGS20. The plants were grown for 4 weeks in a high CO2 atmosphere (300 Pa CO2) in a 16-h photoperiod
under white light (120mmol photons m−2s−1). Leaf samples
were collected 1 h after the beginning of the light period. Each given value represents the average of five identical experiments. The S.D. is B 910% for each given value.
Fig. 2. The concentration (mmol g−1fresh weight) of
ammo-nium in leaves of tobacco wild-type (SR1) or GSd plants that had been grown in a 16-h photoperiod under white light (120 mmol photons m−2s−1) in a high CO
2atmosphere (300 Pa
CO2) for 28 days and were then illuminated (420 mmol
photons m−2 s−1) in air (35 Pa CO
2) for 4 h. Each given
Fig. 3. Comparison of representative tobacco wild-type (SR1) or GSd plants that had been grown in a 16 h photoperiod under white light (120mmol photons m−2s−1) in a high CO2
atmosphere (300 Pa CO2) for 28 days and were then exposed to air (35 Pa CO2) for 14 days. The arrowhead indicates the flowers prematurely developed by air-exposed GSd plants.
tion took place outside the high CO2 greenhouse
atmosphere. Transgenic pollen, once escaped from the greenhouse, could lead to pollination of re-lated plant species in the surrounding area. The frequency of such a pollination event is probably very low. The antisense marker may be recessive
and, therefore, the resulting transgenic
het-erozygotes could survive. This possibility is not eliminated by the technology based on condition-ally lethal transgenic plants with reduced foliar GS-2 activity. Nevertheless, these plants may con-tribute to biological safety if employed to produce desired proteins in greenhouse conditions.
Acknowledgements
The authors are grateful to Inge Broer (Univer-sita¨t Rostock, Germany) for kindly providing the binary vector pAQ4.1. T.W.B. wishes to express his gratitude to Prof. Alfred Pu¨hler (Universita¨t Bielefeld, Germany) for the opportunity to work at the Lehrstuhl fu¨r Genetik. The present work was supported by the Deutsche Forschungsge-meinschaft (Be 1108/5-1; Be 1108/5-3).
References
[1] C.R. Somerville, W.L. Ogren, The inhibition of photo-synthesis in Arabidopsis mutants lacking glutamate syn-thase activity, Nature 286 (1980) 257 – 259.
[2] F. Va´cha, The role of oxygen in photosynthesis, Photo-synthetica 31 (1995) 321 – 334.
[3] R.C. Leegood, P.J. Lea, M.D. Adcock, R.E. Ha¨usler, The regulation of photorespiration, J. Exp. Bot. 46 (1995) 1397 – 1414.
[4] A. Gerbaud, M. Andre´, An evaluation of the recycling in measurements of photorespiration, Plant Physiol. 83 (1987) 933 – 937.
[5] R.E. Ha¨usler, R.D. Blackwell, P.J. Lea, R.C. Leegood, Control of photosynthesis in barley leaves with reduced activities of glutamine synthetase or glutamate synthase, Planta 194 (1994) 406 – 417.
[6] Z. Chen, R.J. Spreitzer, How various factors influence the CO2/O2 specificity of ribulose-1,5-bisphosphate car-boxylase/oxygenase, Photosynth. Res. 31 (1992) 157 – 164.
[7] R.D. Blackwell, A.J.S. Murray, P.J. Lea, Inhibition of photosynthesis in barley with decreased levels of glu-tamine synthetase activity, J. Exp. Bot. 38 (1987) 1799 – 1809.
[8] J. Freeman, A.J. Marquez, R.M. Wallsgrove, R. Saare-lainen, B.G. Forde, Molecular analysis of barley mutants deficient in chloroplast glutamine synthetase, Plant Mol. Biol. 14 (1990) 297 – 311.
The transfer of high CO2-grown transgenic
to-bacco GSd plants with reduced leaf GS-2 activity into air resulted in the appearance of severe chlorotic lesions (Fig. 3) and, eventually, the trans-genic plants died. Prior to that, premature flower-ing was observed (Fig. 3). This response may have been triggered directly or indirectly by the in-creased foliar ammonium concentration [28]. The flowers were, however, lost before seeds were pro-duced. Seeds derived from high CO2-grown
trans-genic tobacco with reduced leaf GS-2 activity did not yield viable seedlings when germinated in air (data not shown).
We have obtained transgenic tobacco plants with reduced foliar GS-2 activity and shown that these plants are conditionally lethal with respect to the ambient CO2 partial pressure. Stable
atmo-spheric CO2 concentrations that are high enough
to allow these plants to grow normally are unlikely to occur in a natural environment. Such condi-tions can, however, be established and maintained in the greenhouse [29]. The conditionally lethal transgenic tobacco plants with reduced leaf GS-2 activity are unable to survive any prolonged expo-sure to air, e.g. in case that these plants are involuntarily removed from the high CO2
green-house atmosphere.
germina-[9] T.W. Becker, M. Caboche, E. Carrayol, B. Hirel, Nucle-otide sequence of a tobacco cDNA encoding plastidic glutamine synthetase and light inducibility, organ specifi-city and diurnal rhythmispecifi-city in the expression of the corresponding genes of tobacco and tomato, Plant Mol. Biol. 19 (1992) 367 – 379.
[10] M. Cannon, J. Platz, M. O’Leary, C. Sookdeo, K. Cannon, Organ-specific modulation of gene expression in transgenic plants using antisense RNA, Plant Mol. Biol. 15 (1990) 39 – 47.
[11] S. Rodermel, M. Abbott, L. Bogorad, Nuclear-organelle interactions: nuclear antisense gene inhibits ribulosebis-phosphate carboxylase enzyme levels in transformed to-bacco, Cell 55 (1988) 673 – 681.
[12] H.J. Quandt, I. Broer, A. Pu¨hler, Tissue-specific activity and light-dependent regulation of a soybean rbcS pro-motor in transgenic tobacco plants monitored with the firefly luciferase gene, Plant Sci. 82 (1992) 59 – 69. [13] D. Facciotti, J. O’Neal, S. Lee, C. Shoemaker,
Light-in-ducible expression of a chimaeric gene in soybean trans-formed with Agrobacterium, Biotechnology 3 (1985) 241 – 246.
[14] J. Gielen, M. de Beuckeleer, J. Seurinck, H. Debroeck, H. de Greeve, M. van Montagu, J. Schell, The complete nucleotide sequence of the TL-DNA of the Agrobac
-terium tumefaciens plasmid pTiAch5, EMBO J. 3 (1984)
835 – 846.
[15] E. van Haute, H. Joos, M. Maes, G. Warren, M. van Montagu, J. Schell, Intergenic transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for reversed genetics of Ti plasmids of Agrobacterium tumefaciens, EMBO J. 2 (1983) 411 – 418.
[16] R. Horsch, J.E. Fry, N. Hoffmann, D. Eichholtz, S. Rogers, R.T. Fraley, A general method for transferring genes into plants, Science 227 (1985) 1229 – 1231. [17] K. Beckmann, C. Dzuibany, K. Biehler, H. Fock, A.
Migge, T.W. Becker, Photosynthesis and fluorescence quenching, and the mRNA levels of plastidic glutamine synthetase or mitochondrial serine hydroxymethyltrans-ferase (SHMT) in the leaves of the wild-type and of the SHMT-deficient stm mutant of Arabidopsis thaliana in relation to the rate of photorespiration, Planta 202 (1997) 379 – 386.
[18] A. Migge, G. Meya, E. Carrayol, B. Hirel, T.W. Becker, Regulation of the subunit composition of tomato plas-tidic glutamine synthetase by light and the nitrogen source, Planta 200 (1996) 213 – 220.
[19] R.M. Wallsgrove, J.C. Turner, N.P. Hall, A.C. Kendall, S.W. Bright, Barley mutants lacking chloroplast glu-tamine synthetase. Biochemical and genetic analysis, Plant Physiol. 83 (1987) 155 – 158.
[20] S.H. Yuen, A.G. Polland, Determination of nitrogen in agricultural materials by the Nessler reagent, J. Sci. Food Agric. 3 (1952) 441 – 447.
[21] C. Dzuibany, S. Haupt, H. Fock, K. Biehler, A. Migge, T.W. Becker, Regulation of nitrate reductase transcript levels by glutamine accumulating in the leaves of a ferredoxin-dependent glutamate synthase-deficient gluS
mutant of Arabidopsis thaliana, and by glutamine pro-vided via the roots, Planta 206 (1998) 515 – 522. [22] B. Hirel, G.H. Miao, D.P.S. Verma, Metabolic and
developmental control of glutamine synthetase genes in legume and non-legume plants, in: D.P.S. Verma (Ed.), Control of Plant Gene Expression, CRC Press, Boca Raton, FL, 1993, pp. 443 – 458.
[23] A. Oaks, A re-evaluation of nitrogen assimilation in roots, Bioscience 42 (1992) 103 – 110.
[24] K.W. Joy, R.D. Blackwell, P.J. Lea, Assimilation of nitrogen in mutants lacking enzymes of the glutamate cycle, J. Exp. Bot. 43 (1992) 139 – 145.
[25] K. Biehler, H.P. Fock, Evidence for the contribution of the Mehler-peroxidase reaction in dissipation of excess electrons in drought-stressed wheat, Plant Physiol. 112 (1996) 265 – 272.
[26] R.D. Blackwell, A.J.S. Murray, P.J. Lea, K.W. Joy, Photorespiratory amino donors, sucrose synthesis and the induction of CO2 fixation in barley deficient in
glutamine synthetase and glutamate synthase, J. Exp. Bot. 39 (1988) 845 – 858.
[27] J.K. Schjoerring, A. Kyllingsbaek, J.V. Mortensen, S. Byskov-Nielsen, Field investigations of ammonia ex-change between barley plants and atmosphere. I. Con-centration profiles and flux densities of ammonium, Plant Cell Environ. 16 (1993) 161 – 167.
[28] R. Vincent, V. Fraisier, S. Chaillou, A. Limami, E. Deleens, B. Phillipson, C. Douat, J.-B. Boutin, B. Hirel, Overexpression of a soybean gene encoding cytosolic glutamine synthetase in shoots of transgenic Lotus cor
-niculatusL. plants triggers changes in ammonium assimi-lation and plant development, Planta 201 (1997) 424 – 433.
[29] T. Stuhlfauth, K. Klug, H. Fock, The production of secondary metabolites by Digitalis lanata during CO2
enrichment and water stress, Phytochemistry 26 (1987) 2735 – 2739.