Zinc is an essential micronutrient. Genes responsible for zinc uptake have now been identified from yeast and plants. These genes belong to an extended family of cation transporters called the ZIP gene family. Zinc efflux genes that belong to another transporter family, the CDF family, have also been identified in yeast and Arabidopsis. It is clear that studies in yeast can greatly aid our understanding of zinc metabolism in plants.
Addresses
*Department of Biological Sciences, 6044 Gilman, Dartmouth College, Hanover, New Hampshire 03755, USA;
e-mail: [email protected]
†Nutritional Sciences Program, Department of Biochemistry, 217 Gwynn Hall, University of Missouri, Columbia, Missouri 65211, USA; e-mail: [email protected]
Current Opinion in Plant Biology1999, 2:244–249 http://biomednet.com/elecref/1369526600200244 © Elsevier Science Ltd ISSN 1369-5266
Abbreviations
CDF cation diffusion facilitator ZIP ZRT, IRT-like protein
Introduction
Zinc plays an amazing number of critical roles in all organ-isms — it is an essential component of more than 300 enzymes including RNA polymerase, alkaline phos-phatase, alcohol dehydrogenase, Cu/Zn superoxide dismutase, and carbonic anhydrase. Moreover, greater than 3% of the protein sequences inferred from the two eukary-otic genomes completely sequenced to date, that is the genomes of S. cerevisiae and C. elegans, contain sequence motifs characteristic of zinc binding structural domains [1]. These include several motifs found in DNA binding pro-teins, including the C2H2 zinc finger domain (see Note added in proof), the C6 zinc cluster domain, and the C4
hormone receptor domain. Because zinc is a hydrophilic, highly charged species, it cannot cross biological mem-branes by passive diffusion, but rather it must be transported. It is taken up from the soil solution as a diva-lent cation [2]. Once taken up, zinc is neither oxidized nor reduced; thus, the role of zinc in cells is based on its behav-ior as a divalent cation that has a strong tendency to form tetrahedral complexes (for a review see [3]).
Zinc deficiency is probably the most widespread micronu-trient deficiency limiting crop production and quality in cereals [4•]. A global sampling of 190 soils from 25 countries
found that 49% were low in zinc. Unlike other micronutri-ent deficiencies, zinc deficiency is ubiquitous: it occurs in cold and warm climates, in drained and flooded soils and in acid and alkaline soils. This review describes how recent studies of zinc uptake and its regulation in yeast have now led to new insight into how plants obtain zinc from soils.
Zinc uptake in yeast
Kinetic studies of zinc uptake by yeast cells grown with dif-ferent amounts of zinc in the medium suggested the presence of at least two uptake systems [5•]. One system has
a high affinity for zinc with an estimated apparent Kmof
10 nM Zn2+and is only active in zinc-limited cells [6]. The
second system has a lower affinity for zinc (apparent Kmof
100 nM Zn2+) and is detectable in zinc replete cells [7].
The ZRT1gene encodes the transporter protein of the high affinity system [6]. The level of ZRT1 mRNA correlates with activity of the high affinity system; overexpressing ZRT1increases high affinity uptake whereas disrupting the ZRT1gene eliminates high affinity activity and results in poor growth of the mutant on zinc-limiting media. In simi-lar studies, it was determined that the ZRT2gene encodes the transporter of the low affinity uptake system [7]. More recently, the ZRT1 protein has been found to be glycosy-lated and localized to the plasma membrane of the cell [8••]. Additional, as yet uncharacterized zinc uptake
sys-tems are also present in S. cerevisiaeas demonstrated by the observation that the zrt1 zrt2mutant is viable [7].
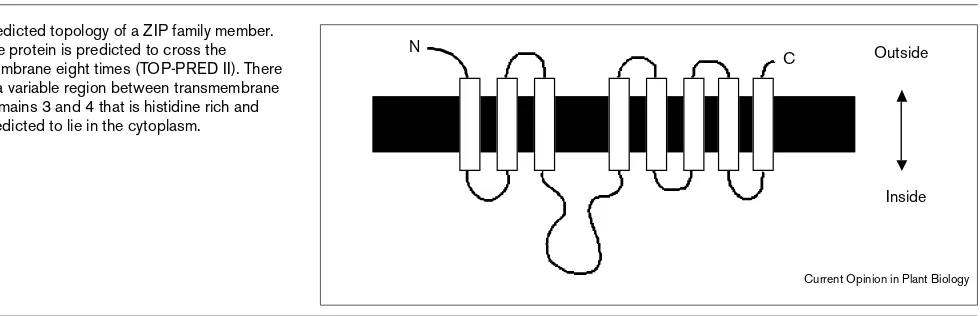
The ZRT1 and ZRT2 proteins share 44% sequence identi-ty and 67% similariidenti-ty. They each contain eight potential transmembrane domains and have a similar predicted membrane topology in which the amino- and carboxy-ter-minal ends of the protein are located on the outside surface of the plasma membrane (Figure 1). These proteins were originally identified because of their similarity to the IRT1 transporter fromArabidopsis [9]. The IRT1gene was cloned by functional expression in a yeast mutant (fet3 fet4) defec-tive for iron uptake. Our current hypothesis is that IRT1 is an Fe2+transporter that takes up iron from the soil, a
pro-posal that is consistent with the observation that yeast expressing IRT1 possess a novel Fe2+ uptake activity.
Moreover, in Arabidopsis, IRT1mRNA is expressed in roots and is induced by iron-limiting growth conditions. Although IRT1 was originally identified as an iron trans-porter, we now know from studies in yeast that IRT1 is able to transport both Mn and Zn in addition to Fe [10]. In addi-tion to sharing sequence similarity and numbers of potential transmembrane domains, ZRT1, ZRT2, and IRT1 each have a potential metal-binding domain between transmembrane domains three and four that is predicted to be cytoplasmic. For example, in ZRT1, this sequence is HDHTHDE and in IRT1, this motif is HGHGHGH. Although the function of this motif is currently unknown, its conserved location in these three proteins and its poten-tial for metal binding suggests that it plays an important role in metal ion uptake or its regulation.
Through DNA sequence database comparisons and addi-tional expression cloning studies, it is now clear that these
Zeroing in on zinc uptake in yeast and plants
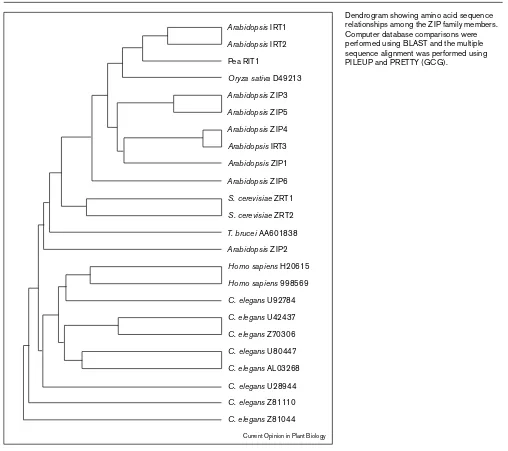
three metal ion transporters are members of a family of proteins found in a diverse array of eukaryotic organisms [11]. This family is referred to collectively as the ZIP fam-ily for ‘ZRT, IRT-like protein.’ At this time, 24 ZIP members have been identified and these genes fall into two discrete subfamilies based on amino acid similarities (Figure 2). Subfamily I includes 11 genes in plants (9 from Arabidopsis, one from pea and one from rice), the two yeast genes (i.e. ZRT1and ZRT2), and a gene from the protozoan T. brucei. Subfamily II includes 8 genes in the nematode C. elegansand two in humans. There are also two more dis-tantly related family members, one in humans and one in mouse that are not shown on the dendrogram in Figure 2 [11]. All but two of these proteins contain the putative metal-binding domain described above.
Zinc uptake in plants
Using a method similar to the one used to isolate IRT1, the ZIP1, ZIP2, and ZIP3genes of Arabidopsis were isolated by functional expression cloning in a zrt1 zrt2mutant yeast strain; expression of these genes in yeast restored zinc-lim-ited growth to this mutant [12••]. Biochemical analysis of
metal uptake has demonstrated that these genes encode zinc transporters. Yeast expressing ZIP1, ZIP2, and ZIP3 each have different time-, temperature-, and concentra-tion-dependent zinc uptake activities with apparent Km values of 10–100 nM Zn2+. These values are similar to the
levels of free Zn2+ available in the rhizosphere [13].
Moreover, no Fe2+or Fe3+uptake activity has been
detect-ed with any of these proteins in uptake experiments using
55Fe. We propose that each of these three genes plays a
role in zinc transport in plants and that they represent the first zinc transporter genes to be cloned from any plant species. A fourth Arabidopsis ZIP homolog, ZIP4, was iden-tified in the DNA sequence databases, but its expression did not confer zinc uptake activity in yeast [12••]. This
may not be surprising as ZIP4 is predicted to have a chloro-plast targeting sequence and, therefore, may not localize properly in yeast cells.
Some plants are better able to grow on zinc deficient soils and hence have been termed zinc efficient. The physiological
mechanisms responsible for differential tolerance to zinc deficiency among genotypes are not present. Rengel and Hawkesford [14] have previously reported the presence of a 34 kDa polypeptide that is strongly induced in a zinc effi-cient variety of wheat grown under zinc deficiency. The 34 kDa polypeptide localized to the plasma membrane of roots, leading the authors to speculate that it might be a structural or regulatory component of the plasma membrane zinc transporter. The ZIP proteins we have identified to date are predicted to range in size from 36 to 39 kDa, sug-gesting that the wheat protein may be a related transporter.
A second gene family that could potentially play a role in zinc transport in plants is the Nramp family. This family of transport proteins has been identified in plants [15,16] as well as other eukaryotes [17]. A recent study has demon-strated that one member, DCT1/Nramp2, is capable of transporting a variety of metal ions including zinc [18]. Although DCT1/Nramp2 is clearly important for iron uptake in mammals [19,20], evidence for a role of DCT1 in zinc transport in any organism is still lacking.
Do phytosiderophores play a role in
zinc uptake?
Grasses release metal chelators, called phytosiderophores, both under iron and zinc deficiency [21,22]. Although the role of phytosiderophores in iron nutrition is well accepted [23••], whether phytosiderophores play an important role
in zinc nutrition remains an unanswered question. In a recent study comparing wheat genotypes that differed in their tolerance to zinc deficiency, the tolerant genotype released more phytosiderophores, and showed both greater uptake of zinc by roots and greater transport of zinc to the shoots than did the genotype sensitive to zinc deficiency [24]. Although chelate splitting is not a prerequisite for the uptake of zinc from zinc-phytosiderophores by maize, free zinc is taken up more readily than chelated zinc [25].
What’s special about zinc hyperaccumulators?
Plant species which are endemic to metalliferous soils are metal tolerant, either by excluding metals or by virtue of being able to accumulate and sequester metals. Over 400Figure 1
Predicted topology of a ZIP family member. The protein is predicted to cross the membrane eight times (TOP-PRED II). There is a variable region between transmembrane domains 3 and 4 that is histidine rich and predicted to lie in the cytoplasm.
Outside N
C
Inside
metal hyperaccumulating species of plants have been reported, of which about 16 are zinc hyperaccumulators (defined as containing more than 10,000 µg zinc g–1 in
shoot tissues [26,27]. Certain populations of Thlaspi caerulescenscan accumulate up to 40,000 µg zinc g–1tissue
in their shoots whereas optimal zinc concentration for most plants is between 20 and 100 µg g–1tissue. There is great
interest in hyperaccumulators because of their potential for use in extracting metals from soils, either in aid of phy-toremediation [28•] or in aid of phytomining [27].
Radiotracer studies with T. caerulescens and a non-hyperac-cumulating related species, T. arvense, have shown that the Vmax for the uptake of zinc was 4.5 fold greater for
T. caerulescens than for the non-hyperaccumulator whereas their Kmvalues were not significantly different. This suggests
that zinc uptake is controlled by regulating the number of active transporters in the membrane [29]. A T. caerulescens gene, ZNT1, has been identified by functional expression cloning in a zrt1 zrt2mutant yeast strain [30]. This gene encodes a presumptive zinc transporter that is 88% similar to the Arabidopsis ZIP4 transporter. Northern analysis shows that the ZNT1transcript is very abundant in roots and shoots regardless of zinc status. This is especially interesting in light of the fact that ZIP4 is also expressed in both roots and shoots of Arabidopsis but only when plants are starved for zinc [12••]. Constitutive expression
of a zinc transporter gene regardless of zinc status may, in part, explain the ability of T. caerulecensto accumulate zinc.
The zinc that is taken up by T. caerulescensalso appears to be more readily available for loading into the xylem, as
Figure 2
Dendrogram showing amino acid sequence relationships among the ZIP family members. Computer database comparisons were performed using BLAST and the multiple sequence alignment was performed using PILEUP and PRETTY (GCG).
Arabidopsis IRT1 Arabidopsis IRT2 Pea RIT1
Oryza sativa D49213 Arabidopsis ZIP3 Arabidopsis ZIP5 Arabidopsis ZIP4 Arabidopsis IRT3 Arabidopsis ZIP1 Arabidopsis ZIP6 S. cerevisiae ZRT1 S. cerevisiae ZRT2 T. brucei AA601838 Arabidopsis ZIP2 Homo sapiens H20615 Homo sapiens 998569 C. elegans U92784 C. elegans U42437 C. elegans Z70306 C. elegans U80447 C. elegans AL03268 C. elegans U28944 C. elegans Z81110 C. elegans Z81044
T. caerulescensxylem sap contained five-fold more zinc than xylem sap from T. arvense[31••]. Similar conclusions were
reached in a comparison of T. caerulescens and the non-hyper-accumulator T. ochroleucum[32]. Interestingly, T. caerulescens has a greater requirement for zinc than other plants, sug-gesting that constitutive sequestration mechanisms exist which decrease its physiological availability [32]. Once in the shoot, zinc is sequestered in a soluble form in the vac-uoles of leaf cells, preventing the buildup of toxic levels in the cytoplasm [33]. In the zinc tolerant plant Silene vulgaris, zinc transport across the tonoplast was about 2.5 times high-er than in zinc-sensitive plants of the same species [34].
Regulation by zinc
In all organisms, zinc uptake is tightly controlled to ensure that adequate levels of the metal are accumulated while potentially toxic overaccumulation is prevented. In yeast, this control is exerted at both the transcriptional and post-translational level. At the transcriptional level, expression of the ZRT1and ZRT2genes is induced more than 10-fold in zinc-limited cells [6,7]. Regulation of these genes in response to zinc is mediated by the product of the ZAP1gene [35,36]. ZAP1 is a zinc-responsive transcriptional activator protein that senses intracellular zinc levels, perhaps by direct bind-ing of zinc to the protein, and translates that signal into changes in gene expression. The mechanism of this regula-tion is currently unknown but may involve direct binding of zinc to ZAP1 which could inhibit DNA binding or activation domain activity. Post-translational regulation of ZRT1 occurs when cells are exposed to high levels of extracellular zinc [8••]. Under these conditions, ZRT1 uptake activity is
rapid-ly lost and this decrease is due to endocytosis of the ZRT1 protein and its subsequent degradation in the vacuole. Zinc-induced endocytosis of ZRT1 is a specific response to zinc and allows the rapid shutoff of zinc uptake activity thereby protecting cells from zinc overaccumulation.
In plants, we also have evidence that expression of the zinc transporters is metal responsive — ZIP1, ZIP3, and ZIP4 mRNAs are all induced in zinc-limited plants. Furthermore, ZIP1 shows zinc-induced inactivation when expressed in yeast. That is, ZIP1-dependent zinc uptake is rapidly lost when cells are exposed to high levels of zinc (ML Guerinot, D Eide, unpublished data). We are cur-rently testing transgenic plants carrying a CaMV 35S-ZIP1 construct to see if a zinc-induced endocytosis mechanism like that affecting ZRT1 activity is also operating in plants.
Intracellular zinc transport
Once zinc is transported into a eukaryotic cell, it must be delivered to intracellular compartments to supply zinc-dependent proteins within those compartments. Moreover, it is likely that excess zinc is stored in intracel-lular compartments such as the vacuole. Two potential intracellular zinc transporters have been identified in S. cerevisiae. These transporters are encoded by the ZRC1and COT1genes. ZRC1was isolated as a suppressor of zinc tox-icity, that is overexpression of ZRC1 results in zinc
resistance [37]. The COT1 gene was isolated in a similar fashion to ZRC1, that is as a suppressor of cobalt toxicity, but was later found to confer zinc resistance as well [38,39]. Disruption of either ZRC1 and COT1 resulted in greater sensitivity to zinc, further supporting the potential role of these genes in zinc compartmentalization.
ZRC1 and COT1 are closely related proteins (60% identi-ty) of approximately 440 amino acids and six–seven potential transmembrane domains. The physiological roles of these transporters are still unclear. Neither ZRC1 nor COT1 are essential genes, and a zrc1 cot1 mutant is also viable. Neither protein appears to catalyze zinc efflux from the cell. Although COT1 was originally thought to be localized in the mitochondria [38], both COT1 and ZRC1 have recently been demonstrated to be vacuolar proteins [40]. These observations suggest that COT1 and ZRC1 play roles in transporting zinc into the vacuole.
ZRC1 and COT1 belong to yet another family of metal transport proteins, referred to as the CDF (cation diffusion facilitator) family [41]. In all, over 16 members of this fam-ily have been identified in prokaryotes and eukaryotes. Analyses of other CDF proteins lend support to the role of the yeast proteins in metal transport. For example, one member of this family, the CzcD protein of the prokaryote Alcaligenes eutrophus, confers resistance to zinc as well as cobalt and cadmium [42]. Four mammalian CDF proteins, ZnT-1-4, are zinc transporters [43]. ZnT-1 is a zinc efflux transporter in the plasma membrane [44]. ZnT-2 is found in the membrane of an acidic endosomal/lysosomal com-partment and may play a role in zinc sequestration [45]. ZnT-3 is expressed only in the brain and testis and is most abundant in the neurons of the hippocampus and the cere-bral cortex [46]. ZnT-3 is localized to synaptic vesicle membranes, suggesting that this protein transports zinc into this compartment. ZnT-4 has been identified as the lethal-milk gene and is thus implicated in the deposition of zinc into the maternal milk supply [47]. The similarity of these proteins with ZRC1 and COT1 supports the hypoth-esis that the yeast proteins detoxify excess zinc by transporting the metal into an intracellular vesicular com-partment. In an intriguing point of similarity with ZIP proteins, the eukaryotic CDF proteins contain a histidine-rich region with the sequence (HX)nwhere n = 3–6. As is
the case of the ZIP proteins, this domain is predicted to be cytoplasmic and its function is still unknown. There are currently two CDF Arabidopsis homologs in the database (accession numbers AC005310 and AC004561). One of these, now called ZAT1, leads to increased zinc accumula-tion and increased zinc tolerance when overexpressed in Arabidopsis [48••]. In these overexpressing lines, zinc
accu-mulates in the roots but not in the shoots. Expression of ZAT1 in yeast did not lead to increased resistance to zinc.
Conclusions
metal will have two or more relatively specific transport systems: high affinity systems that are active in metal lim-iting conditions and low affinity systems that function when substrates are more abundant [5•]. We also know that
zinc, like other metals, is transported from the soil into the root and then must cross both cellular and organellar mem-branes as it is distributed throughout the plant. Specific zinc transporters may play different roles in this distribu-tion process. Various molecular approaches ultimately can tell us not only in what tissue and cell type certain trans-porters are expressed but where within a cell each is expressed. We are also now in a position to identify plant mutants carrying insertions in particular transporter genes; this will greatly help in assigning functions. Having cloned genes in hand is also allowing us to undertake structure–function studies on the encoded proteins them-selves. Finally, moving beyond how any one transporter functions, we need to keep in mind that we want to under-stand zinc transport at the whole plant level and to use such knowledge to create plants with enhanced mineral content as well as plants that bioaccumulate or exclude this potentially toxic cation. Such understanding will require knowledge of how zinc levels are sensed by plants and how gene expression is controlled by zinc. Studies in yeast are providing important information about zinc regulation that we believe will be directly applicable to plants. Over the next five years, the relationship between high and low affinity zinc transporters, between transporters responsible for zinc influx and those responsible for zinc efflux, and between transporters and the system(s) responsible for sensing zinc levels in cells should become clearer.
Note added in proof
A new paper on zinc finger proteins in plants has recently been published [49].
Acknowledgements
We wish to thank members of our laboratories for their contributions. Work in the Guerinot lab is supported by grants from the National Science Foundation and the Department of Energy and work in the Eide lab by grants from the National Science Foundation, the Department of Energy and the National Institutes of Health.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Clarke ND, Berg JM: Zinc fingers in Caenorhabditis elegans: finding families and probing pathways.Science1998, 282 :2018-2022.
2. Marschner H: Mineral Nutrition of Higher Plants, edn 2. Boston: Academic Press; 1995.
3. Berg JM, Shi Y: The galvanization of biology: a growing appreciation for the roles of zinc.Science1996, 271:1081-1085.
4. Ruel MT, Bouis HE: Plant breeding: a long term strategy for the
• control of zinc deficiency in vulnerable populations.Amer J Clin Nutr1998, 68:488S-494S.
This article describes ongoing plant breeding strategies that could increase the intake of bioavailable zinc from staple food crops. The three most promis-ing breedpromis-ing strategies include increaspromis-ing the concentration of zinc, reduc-ing the amount of phytic acid and raisreduc-ing the concentration of sulfur
containing amino acids. Phytic acid decreases zinc absorption in humans, whereas addition of sulfur-containing amino acids to the diet has been shown to increase the bioavailability of zinc.
5. Eide DJ: The molecular biology of metal ion transport in
• Saccharomyces cerevisiae.Annu Rev Nutr1998, 18:441-469. This is an overview of the genes involved in transport of the transition metals Cu, Fe, Mn and Zn in S. cerevisiae.
6. Zhao H, Eide D: The yeast ZRT1gene encodes the zinc transporter of a high affinity uptake system induced by zinc limitation.Proc Natl Acad Sci USA1996, 93:2454-2458.
7. Zhao H, Eide D: The ZRT2gene encodes the low affinity zinc transporter in Saccharomyces cerevisiae.J Biol Chem1996, 271:23203-23210.
8. Gitan RS, Luo H, Rodgers J, Broderius M, Eide D: Zinc-induced
•• inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation.J Biol Chem1998, 273:28617-28624.
Regulated endocytosis of plasma membrane proteins plays an important role in controlling nutrient uptake and responses to extracellular signals. The authors demonstrate that zinc-induced ZRT1 inactivation is a specific regu-latory mechanism to shut off zinc uptake in cells exposed to high zinc levels, thereby preventing overaccumulation of this potentially toxic metal. It is clear that we will need to understand such feed-back mechanisms if we wish to design plants that have altered metal content.
9. Eide D, Broderius M, Fett J, Guerinot ML: A novel iron-regulated metal transporter from plants identified by functional expression in yeast.Proc Natl Acad Sci USA1996, 93:5624-5628.
10. Korshunova YO, Eide D, Clark WG, Guerinot ML, Pakrasi HB: The IRT1 protein from Arabidopsis thalianais a metal transporter with broad specificity.Plant Mol Biol1999, 40:37-44.
11. Eng BH, Guerinot ML, Eide D, Saier MHJ: Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins.J Membr Biol1998, 166:1-7.
12. Grotz N, Fox T, Connolly EL, Park W, Guerinot ML, Eide D: •• Identification of a family of zinc transporter genes from
Arabidopsis that respond to zinc deficiency.Proc Natl Acad Sci USA 1998, 95:7220-7224.
This paper reports the identification of the first zinc transporter genes, ZIP1-4, cloned from plants. The genes belong to a family of transporters called the ZIP gene family. ZIP1and ZIP3are expressed in roots in response to zinc deficiency, suggesting that they transport zinc from the soil into the plant.
ZIP4is induced in both roots and shoots of zinc-limited plants.
13. Norvell WA, Welch RM: Growth and nutrient uptake by barley (Hordeum vulgareL. cv. Herta): studies using an N-(2-hydroxyethyl)ethylenedinitrilotriacetic acid-buffered nutrient solution technique. I. Zinc ion requirements.Plant Physiol1993, 101:619-625.
14. Rengel Z, Hawkesford MJ: Biosynthesis of a 34-kDa polypeptide in the root-cell plasma membrane of a Zn-efficient wheat genotype increases upon Zn deficiency.Aust J Plant Physiol
1997, 24:307-315.
15. Belouchi A, Cellier M, Kwan T, Saini HS, Leroux G, Gros P: The macrophage specific membrane protein Nramp controlling natural resistance in mice has homologues expressed in the root systems of plants.Plant Mol Biol1995, 29:1181-1196.
16. Belouchi A, Kwan T, Gros P: Cloning and characterization of the
OsNrampfamily from Oryza sativa, a new family of membrane proteins possibly implicated in the transport of metal ions.Plant Mol Biol1997, 33:1085-1092.
17. Cellier M, Privé G, Belouchi A, Kwan T, Rodrigues V, Chia W, Gros P: Nramp defines a family of membrane proteins.Proc Natl Acad Sci USA1995, 92:10089-10093.
18. Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollon JL, Hediger MA: Cloning and characterization of a mammalian proton-coupled metal-ion transporter.Nature1997, 388:482-488.
19. Fleming MD, Trenor CC, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC: Microcytic anaemia mice have a mutation in
Nramp2, a candidate iron transporter gene.Nature Genet1997, 16:383-386.
21. Cakmak I, Gülüt KY, Marschner H, Graham RD: Effect of zinc and iron deficiency on phytosiderophore release in wheat genotypes differing in zinc efficiency.J Plant Nutr1994, 17:1-17.
22. Walter A, Römheld V, Marschner H, Mori S: Is the release of phytosiderophores in zinc-deficient wheat plants a response to impaired iron utilization?Physiol Plantarum1994, 92:493-500.
23. Fox TC, Guerinot ML: Molecular biology of cation transport in
•• plants.Annu Rev Plant Physiol Plant Mol Biol1998, 49:669-696. This review summarizes current knowledge about genes whose products function in the transport of various cationic macronutrients (K, Ca) and micronutrients (Cu, Fe, Mn and Zn) in plants.
24. Rengel Z, Römheld V, Marschner H: Uptake of zinc and iron by wheat genotypes differing in tolerance to zinc deficiency.J Plant Physiol1998, 152:433-438.
25. von Wiren N, Marschner H, Römheld V: Roots of iron-efficient maize also absorb phytosiderophore-chelated zinc.Plant Physiol1996, 111:1119-1125.
26. Baker AJM, Brooks RR: Terrestrial higher plants which
hyperaccumulate metallic elements — a review of their distribution, ecology and phytochemistry.Biorecovery1989, 1:81-126.
27. Brooks RR, Chambers MF, Nicks LJ, Robinson BH: Phytomining.
Trends Plant Sci1998, 3:359-362.
28. Salt DE, Smith RD, Raskin I: Phytoremediation.Annu Rev Plant
• Physiol Plant Mol Biol1998, 49:643-668.
An excellent overview of the current information on using plants to clean up the environment. Toxic heavy metals and organic pollutants are the major tar-gets for phytoremediation.
29. Lasat MM, Baker AJM, Kochian LV: Physiological characterization of root Zn2+absorption and translocation to shoots in Zn
hyperaccumulator and nonaccumulator species of Thlaspi.Plant Physiol1996, 112:1715-1722.
30. Pence N, Larsen P, Ebbs S, Garvin D, Eide D, Kochian L: Cloning and characterization of a heavy metal transporter (ZNT1) from the Zn/Cd hyperaccumulator Thlaspi caerulescens.Plant Physiol1998, [Abstract 656 http://www.sheridan.com/aspp98/abs/45/ 0650.html]
31. Lasat MM, Baker AJM, Kochian LV: Altered Zn compartmentation in
•• the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens.Plant Physiol1998, 118:875-883.
The hyperaccumulator Thlaspi caerulescensdiffers in a number of respects from the related non-hyperaccumulating species Thalspi arvense. The authors show the zinc that is taken up by T. caerulescensis more readily available for loading into the xylem.
32. Shen ZG, Zhao FJ, McGrath P: Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescensand the
non-hyperaccumulator Thlaspi ochroleucum.Plant Cell Environ1997, 20:898-906.
33. Küpper H, Zhao FJ, McGrath SP: Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens.Plant Physiol1999, 119:305-311.
34. Verkleij JAC, Koevoets PLM, Blake-Kalff MMA, Chardonnens AN: Evidence for an important role of the tonoplast in the mechanism
of naturally selected zinc tolerance in Silene vulgaris.J Plant Physiol1998, in press.
35. Zhao H, Eide D: Zap1p, a metalloregulatory protein involved in zinc-responsive transcriptional regulation in Saccharomyces cerevisiae.Mol Cell Biol1997, 17:5044-5052.
36. Zhao H, Butler E, Rodgers J, Spizzo T, Duesterhoeft S, Eide D: Regulation of zinc homeostasis in yeast by binding of the ZAP1 transcriptional activator to zinc-responsive promoter elements.
J Biol Chem1998, 273:28713-28720.
37. Kamizono A, Nishizawa M, Teranishi Y, Murata K, Kimura A: Identification of a gene conferring resistance to zinc and cadmium ions in the yeast Saccharomyces cerevisiae.Mol Gen Genet1989, 219:161-167 38. Conklin DS, McMaster JA, Culbertson MR, Kung C: COT1, a gene
involved in cobalt accumulation in Saccharomyces cerevisiae.Mol Cell Biol1992, 12:3678-3688.
39. Conklin DS, Culbertson MR, Kung C: Interactions between gene products involved in divalent cation transport in Saccharomyces cerevisiae.Mol Gen Genet1994, 244:303-311.
40. Li L, Kaplan J: Defects in the yeast high affinity iron transport system result in increased metal sensitivity because of the increased expression of transporters with a broad transition metal specificity.J Biol Chem1998, 273:22181-22187.
41. Paulsen LT, Saier MH: A novel family of ubiquitous heavy metal ion transport proteins.J Membr Biol1997, 156:99-103.
42. Nies DH: CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc and cadmium (czc system) in
Alcaligenes eutrophus.J Bacteriol1992, 174:8102-8110. 43. McMahon RJ, Cousins RJ: Mammalian zinc transporters.J Nutr
1998, 128:667-670.
44. Palmiter RD, Findley SD: Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc.
EMBO J1995, 14:639-649.
45. Palmiter RD, Cole TB, Findley SD: ZnT-2, a mammalian protein that confers resistance to zinc by facilitating vesicular sequestration.
EMBO J1996, 15:1784-1791.
46. Palmiter RD, Cole TB, Quaife CJ, Findley SD: ZnT-3, a putative transporter of zinc into synaptic vesicles.Proc Natl Acad Sci USA
1996, 93:14934-14939.
47. Huang L, Gitschier J: A novel gene involved in zinc transport is deficient in the lethal milk mouse.Nature Genetics1997, 17:292-295.
48. van der Zaal EJ, Neuteboom LW, Pinas JE, Schat H, Verkleij J, •• Hooykaas PJJ: Overexpression of a zinc transporter gene from
Arabidopsis can lead to enhanced zinc resistance and zinc accumulation.Plant Physiol1999, 119:1-9.
A homolog of mammalian zinc effluxers has been identified in Arabidopsis. The authors show that overexpression of the homolog leads to a modest increase in zinc resistance and to an increase in the zinc content of roots. They speculate that ZAT1 may be involved in vacuolar sequestration of zinc.