www.elsevier.com / locate / bres
Research report
M100,907, a selective 5-HT
2Aantagonist, attenuates dopamine release
in the rat medial prefrontal cortex
a ,
*
a,b a a aE.A. Pehek
, H.G. McFarlane
, K. Maguschak , B. Price , C.P. Pluto
a
Department of Psychiatry, Case Western Reserve University School of Medicine, Kenyon College, Gambier, OH 43022, USA
b
Department of Psychology, Kenyon College, Gambier, OH 43022, USA Accepted 19 September 2000
Abstract
Previous research has suggested that serotonin 5-HT2Areceptors modulate the functioning of the mesocortical dopamine (DA) pathway. However, the specific role of 5-HT2A receptors localized within the medial prefrontal cortex (mPFC) is not known. The present study
1 21
employed in vivo microdialysis to examine the role of this receptor in the modulation of basal and K -stimulated (Ca -dependent) DA release. The selective 5-HT2A antagonist M100,907 was infused directly into the mPFC of conscious rats. This resulted in a
1
concentration-dependent blockade of K -stimulated DA release. Intracortical application of M100,907 also blocked increases in DA release produced by the systemic administration of the 5-HT2A / 2Cagonist, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI). These
21
findings demonstrate that local 5-HT2Aantagonism has an inhibitory effect on stimulated, Ca -dependent DA release. They suggest that cortical 5-HT2A receptors potentiate the phasic release of mesocortical DA. 2001 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters and receptors
Topic: Interactions between neurotransmitters
Keywords: Antipsychotic; In vivo; Mesocortical DA pathway; Microdialysis; Schizophrenia; Serotonin; DOI
1. Introduction receptors regulate DA function in the mPFC. For example,
administration of the atypical antipsychotic drug clozapine, The mesocortical dopamine (DA) system has been a potent 5-HT antagonist [20], increases extracellular DA2
implicated in a wide range of emotional, motivated, and concentrations in vivo when administered either sys-cognitive behaviors. Neurons in this pathway originate in temically or directly into the mPFC [15,21,28]. In addition, the ventral tegmental area of the midbrain and terminate in systemic administration of amperozide, a 5-HT2A antago-the medial prefrontal cortex (mPFC) [40]. Several in vivo nist [33,37], and intracortical administration of ritanserin, a studies have demonstrated that cortical DA neurochemistry 5-HT antagonist, were both found to elevate dialysate DA2
is modulated by serotonin (5-HT) receptors [5,10,12]. levels in the mPFC [15,24,29,30]. Thus, each of these Examination of the interactions between DA and 5-HT is potent 5-HT2A antagonists increased cortical DA efflux thus central to the understanding of psychotropic drug suggesting that 5-HT2A receptors inhibit DA release from action in the prefrontal cortex. the mesocortical DA system.
In vitro and in vivo neurochemical studies in the dorsal Although there are three known subtypes of the 5-HT2
and ventral striatum indicate that 5-HT receptors regulate2 receptor, the 2A, the 2B, and the 2C [32], many of the DA function [9,26]. There is also evidence that 5-HT2 studies that have attempted to examine the role of these receptors in mesocortical DA function have used ligands that are not subtype selective. Those experiments that have used selective ligands have generally employed the sys-*Corresponding author. VA Medical Center GMH(B), 10000
Breck-temic administration of such agents [7,10]. Since sys-sville Rd., Brecksys-sville, OH 44141, USA. Tel.:11-440-526-3030 ext.
temically administered drugs can have actions at multiple 6610; fax:11-440-546-2713.
E-mail address: [email protected] (E.A. Pehek). sites in the brain, the literature remains unclear as to the 0006-8993 / 01 / $ – see front matter 2001 Elsevier Science B.V. All rights reserved.
specific role of cortical 5-HT2A receptors in the modulation before the start of the experiments, microdialysis probes of mesocortical DA function. were lowered carefully through the guide cannulae into In the present study, we examined the role of pre- awake rats and secured in place with Krazy Glue GelE. frontocortical 5-HT2A receptors through the use of the Immediately following probe implantation, animals were highly selective antagonist M100,907 (R-(1)-a-(2,3- placed in clear Plexiglas test chambers and tethered to dimethoxyphenyl) - 1 - [2 - (4 - fluorophenylethyl)] - 4 - piper - counterbalance arms that permitted relatively free move-idine-methanol). This ligand has 100 fold selectivity for ment. They remained there with food and water until the the 5-HT2A (Ki50.36 nM) over the 5-HT2C(Ki5105 nM) start of the experiments.
receptor [25] and has negligible affinity for DA receptors A micro-infusion pump (PHD 2000E, Harvard Ap-(Ki.540 nM) [14]. M100,907 was infused directly into paratus) and liquid swivels were used to perfuse the buffer the mPFC through reverse microdialysis. Effects on both through the probes at a constant rate. Although each
1
basal and potassium (K )-stimulated DA release were experiment is self-contained with its own appropriate examined. Additionally, the effects of intracortical controls, perfusion flow rates differed between some M100,907 on alterations in DA release produced by the experiments. An initial experiment (Experiment 2) em-systemic administration of 1-(2,5-dimethoxy-4- ployed a flow rate of 1.5ml / min. In order to increase the iodophenyl)-2-aminopropane (DOI), a 5-HT2 agonist, recovery of DA, the flow rate was decreased to 1.0ml / min were investigated. We hypothesized that intracortical infu- in all subsequent experiments. Dialysate samples were sion of a selective 5-HT2A antagonist would increase DA collected every 30 min until basal DA concentrations were release in the mPFC. stable for at least 3 baseline samples. Drugs or artificial cerebrospinal fluid (aCSF) buffers with altered ionic compositions were then administered by manually
switch-2. Materials and methods ing tubing connections. This was performed rapidly and
flow rate and collection volumes were maintained. Sample 2.1. Animals and surgery collections then continued every 30 min for another 2.5 to
3.5 h depending on the experiment.
Male Sprague–Dawley rats (Zivic Miller, Hillson, PA, For experiments examining the effects of M100,907 on USA), weighing from 200 to 400 g at the time of surgery, basal or DOI-induced DA release (Experiments 2 and 4), a were used throughout this study. Rats were housed in pairs modified, commercially available, aCSF was employed: in a temperature-controlled room on a 12 / 12 light / dark Dulbecco’s phosphate buffered saline (137 mM NaCl, 2.7 cycle. Food and water were available ad libitum. Prior to mM KCl, 0.5 mM MgCl , 1.5 mM KH PO , 8.1 mM2 2 4
surgery, the rats were anesthetized with a mixture of Na HPO , pH: 7.4). CaCl2 4 2 (1.2 mM) and glucose (10 ketamine (70 mg / kg) and xylazine (6 mg / kg) injected mM) were added to this solution. All previous work in the i.m., and then mounted in a stereotaxic frame. After dura present laboratory has employed this buffer which has was removed, 21 gauge stainless steel guide cannulae were excellent pH stability and produces stable DA levels in
1
chronically implanted on the brain surface above the control animals. However, the high K studies (Experi-mPFC (AP 3.2, ML 0.8) [27]. The guide cannulae were ments 1 and 3) required an increased KCl concentration secured to the skull with three skull screws covered with (80 mM) and, to maintain solution osmolarity, a simulta-cranio-plastic cement. Animals were then housed indi- neous decrease in NaCl (to 60 mM). Both adjustments vidually for the 3–5 day period between surgery and were not possible using the Dulbecco’s buffer solution. microdialysis experiments. Thus, a laboratory prepared Krebs–Ringer buffer (137 mM Each rat was used once, and after the experiment was NaCl, 3 mM KCl, 1.2 mM MgSO , 0.4 mM KH PO , 1.24 2 4
concluded, probe placements were verified histologically. mM CaCl and 10 mM glucose, pH: 7.4) was employed.2
Only animals whose probe placements were verified to be The ionic concentrations of this normal Ringers was then 1 in the mPFC (see below) were used in the study. All modified (KCl 80 mM, NaCl 60 mM) to create a high K animal use procedures were in strict accordance with the Ringers solution.
1
NIH Guide for the Care and Use of Laboratory Animals For high K experiments, drug treated rats were pre-and were approved by the local animal care committee. treated for 30 min with the appropriate concentration of M100,907 dissolved in normal Ringer’s. This was fol-2.2. Microdialysis lowed by 30 min perfusion with the drug dissolved in the
1
high K Ringers solution. During these experiments, Microdialysis probes of a concentric flow design were control rats received infusions of normal Ringers for 30
1
used [43]. Probes were constructed to dialyze the mPFC min followed by high K Ringers for 30 min. from the dorsally located anterior cingulate cortex, through
the prelimbic cortex, and including the ventrally located 2.3. Drugs infralimbic subregion. The active dialyzing surface of the
were made by dissolving the drug in 2.5 ml of glacial h of perfusion and sample collection without drug. The acetic and 1 ml deionized water. aCSF was then utilized to vehicle group was perfused with aCSF throughout. dilute this solution to the appropriate concentrations (100
nM, 1.0 mM, 10 mM, and 100 mM, pH 7.4). (6)-DOI- 2.5.3. Experiment 3 hydrochloride (Research Biochemicals Incorporated, MA,
USA) was administered subcutaneously (s.c). DOI (2.5 2.5.3.1. Effects of lower concentrations of M100,907 on mg / kg) or vehicle (deionized water) were injected in 1.0 DA efflux. Experiment 3A examined the effects of 1.0mM
1
ml / kg volumes. M100,907 on K -stimulated DA efflux. Drug treated rats
1
were compared to high K controls. Procedures were the same as in Experiment 1. Experiment 3B determined the
2.4. Chromatography effects of lower concentrations of M100,907 on basal
dialysate DA. Following the collection of stable baselines, DA content of dialysate samples was measured by 100 nM concentrations were infused for 2 h. This was HPLC coupled with electrochemical detection. Twenty- followed by perfusion with 1.0mM concentrations for 2 h microliter dialysis samples were injected onto a 2 mm in the same rats.
Phenomenex column (UltracarbE, 3 mm particle size,
ODS 20). The mobile phase consisted of 32 mM citric 2.5.4. Experiment 4 acid, 54 mM sodium acetate, 0.074 mM EDTA, 0.215 mM
octylsulfonic acid, and 3% methanol (vol / vol), pH 4.2. To 2.5.4.1. Effects of DOI and M100,907 on cortical DA maintain separation of DA from its metabolites and 5- release. This study examined the effects of a systemic
hydroxyindoleacetic acid, the pH of the mobile phase and injection of DOI on DA efflux in the mPFC. The receptor the concentration of octylsulfonic acid were adjusted as specificity was examined by investigating the ability of needed. A BAS LC-4C electrochemical detector with a intracortical infusions of M100,907 (10 mM) to attenuate glassy carbon electrode, maintained at a potential of10.60 the effects of DOI. Four groups of rats were utilized in this V relative to an Ag /AgCl reference electrode, was em- study: DOI alone, vehicle, M100,907 alone, M100,9071 ployed. The limit of detection for dopamine was 0.1 pg / 20 DOI. The first group received a s.c. injection of DOI while
ml. the second group received vehicle injections. A third group
was perfused intracortically with 10.0mM M100,907 for 3 h. The last group received similar M100,907 infusions plus 2.5. Experimental design a systemic injection of DOI. DOI was administered 30 min
after the start of perfusion with M100,907.
2.5.1. Experiment 1 2.6. Data analysis
Data were expressed, analyzed, and graphed as the 1
2.5.1.1. Effects of M100,907 on K -stimulated cortical percentage of the last 3 baseline samples. Statistical
DA release. This experiment tested the ability of a high analyses were performed using repeated measures 1
K infusion to increase extracellular DA in the mPFC. ANOVAs. For two-way ANOVAs, time was the repeated
21
Furthermore, it tested the Ca -dependency of this effect measures factor and drug condition was the independent 1
and the ability of M100,907 to modulate the K -evoked factor. For one-way ANOVAs, time was the repeated release. Baseline samples were collected from all rats factor. Post-hoc comparisons employed Dunnett’s test for utilizing normal Ringer’s. One group of rats then received comparing treatment means with a control value.
1 21
infusions of a high K buffer with normal Ca (1.2 mM)
21
while a separate group was perfused with a Ca -free / high 1
K solution. Two other groups were pretreated with either 3. Results
1 10 or 100 mM M100,907 before infusions of high K
21
(both with normal Ca ). 3.1. Experiment 1
1
2.5.2. Experiment 2 3.1.1. K effects on DA concentrations
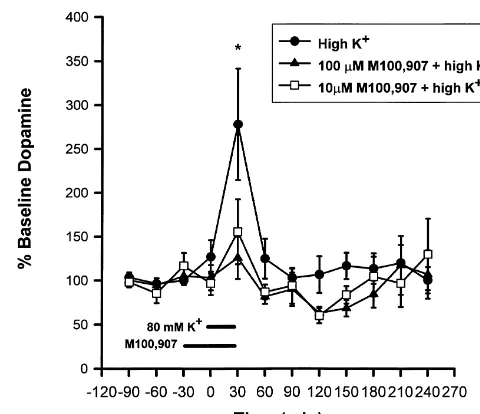
1
Fig. 1 shows that treatment with high K increased extracellular mPFC DA levels to 265% of baseline 2.5.2.1. M100,907 effects on basal DA. Microdialysis [F(9,81)54.54, P,0.001, one-way repeated measures samples were collected from three separate groups of rats: ANOVA]. Post hoc tests indicated that DA efflux was vehicle controls and those treated with either 10 or 100 significantly increased at the 30 min time point after
1 21
1 21 Fig. 2. The effects of intracortical infusions of M100,907 on high
Fig. 1. The effects of intracortical infusions of high K and Ca -free /
1
1 K -induced DA release. Values are expressed as the percentage of 3
high K Krebs–Ringer solution on extracellular DA concentrations.
pre-drug baselines and are the means6S.E.M. of the subjects. For the Values are expressed as the percentages of 3 pre-drug baselines and are
drug group, the bar indicates that M100,907 was infused for 60 min from the means6S.E.M. of the subjects. The bar indicates that the solutions
1
1 time230 to time 30, and high K was co-infused for 30 min from time 0
were infused for 30 min from time 0 to 30. * indicates that high K
1
to time 30. For the non-drug group, the high K solution was infused for significantly increased extracellular cortical DA concentrations (P,0.05).
1
21 30 min from time 0 to time 30. * indicates that high K significantly
This was blocked by removal of Ca from the perfusion medium (no
1 21 increased extracellular mPFC DA concentrations (P,0.05).
Co-adminis-significant increase in DA). Group ns were: high K 510; Ca -free / high
1 tration of M100,907 at either concentration blocked this increase. Group
K 55.
1 1 1
ns were: high K 510; 10mM1high K 56; 100mM1high K 59.
1
high K group and 0.7560.14 pg / 20 ml (n55) for the
21
group that was subsequently treated with a Ca -free / high were 0.9760.17 pg / 20 ml (n59) for the 100 mM MDL 1
K solution. 100,907 group and 0.5460.08 pg / 20 ml (n56) for the 10
1
mM group. The high K group is from Fig. 1. 1
3.1.2. Effects of M100,907 on K -induced DA release 1
Treatment with M100,907 attenuated K -stimulated DA 3.2. Experiment 2 release in a concentration-dependent manner (see Fig. 2).
Dialysate DA only increased to 126% and 155% of 3.2.1. MDL effects on basal DA efflux
baseline values following infusions of 100mM or 10mM Fig. 3 demonstrates the effects of intracortical infusions M100,907, respectively. When the 100 mM concentration of 10 and 100mM M100,907 on basal DA outflow. There
1 1
plus high K was compared to the high K alone group, was a significant main effect for drug [F(2,23)53.41, there was a significant drug3time interaction [F(9, 153)5 P50.051). This was due to decreases in DA efflux after 2.38, P,0.015, two factor repeated measures ANOVA]. the drug perfusion was stopped (maximal decreases, 100 Post-hoc analyses of each condition over time revealed that mM: 55% of baseline at the 120 time point; 10mM: 63%
1
the high K infusion did NOT significantly increase DA of baseline at the 150 time point). Basal DA concentrations efflux when 100 mM M100,907 was co-infused [F(9, were 0.8260.21 pg / 20 ml (n56) for the 10 mM group, 72)51.99, P50.053, one-way, repeated measures 0.5560.09 pg / 20 ml (n511) for the 100 mM group, and ANOVA]. The trend towards a significant F value was due 0.6860.07 pg / 20 ml (n57) for the vehicle group. to decreases (maximal decrease563% of baseline) in DA
concentrations at the 120 and 150 min timepoints (i.e. 3.3. Experiment 3 90–120 min after the termination of the M100,907
perfu-sion). The 10mM concentration also blocked the effects of 3.3.1. Effects of lower concentrations of M100,907 on 1
high K , as demonstrated by the lack of a significant time DA efflux
Fig. 3. The effects of intracortical infusions of M100,907 at 10mM and 100mM concentrations on basal extracellular DA concentrations. Values are expressed as the percentages of 3 pre-drug baselines and are the means6S.E.M. of the subjects. The bar indicates that the solutions were infused for 1 h from time 0 to time 60. DA concentrations were significantly decreased (P50.05). Group ns were: vehicle57; 10mM58; 100mM511.
Fig. 4. The effects of intracortical administration of low concentrations of M100,907 on extracellular DA concentrations. Values are expressed as the percentages of 3 pre-drug baselines and are the means6S.E.M. of the
1
(Fig. 4B). Basal dialysate concentrations were 0.4660.09 subjects. A: Effects of 1.0mM M100,907 on K -stimulated DA efflux. 1
(n55) for the M100,9071high K group, 0.5560.05 (n5 For the drug group, the bar indicates that M100,907 was infused for 60
1
1 min from time
230 to time 30, and high K was co-infused for 30 min 6) for the high K controls, and 0.5260.11 (n58) for the
1
from time 0 to time 30. For the non-drug group, the high K solution was 100 nM / 1.0 mM group.
infused for 30 min from time 0 to time 30. This concentration of
1
M100,907 did not alter high K -induced DA release. Group ns were:
1 1
high K 56; M100,9071high K 55. B: Effects of 100 nM and 1.0mM 3.4. Experiment 4 concentrations on basal DA efflux. The arrows indicate the beginning of each drug infusion. 100 nM was infused first, for 2 h, followed by 1.0
mM, for 2 h. There were no significant effects on DA efflux. Group n was 8.
3.4.1. MDL effects on DOI-stimulated DA release Statistical analyses performed on the raw data (pg / 20 ml) demonstrated that systemic administration of 2.5 mg /
kg DOI increased dialysate DA concentrations [F(6,24)5 group, and 0.4060.03 (n55) for the DOI1M100,907 3.69, P50.01]. Post-hoc tests revealed a significant in- group.
crease 30 min post-injection (Fig. 5). DA concentrations in vehicle animals did not change over time [F(6,18)50.62,
n.s.]. Pretreatment with an intracortical infusion of 4. Discussion
M100,907 (10 mM) attenuated the increase in DA
pro-duced by DOI [no change over time: F(6,24)51.10, n.s.]. The present findings indicate that, contrary to our initial Infusions of M100,907 (10mM) alone for 3 h decreased hypothesis, 5-HT2A receptor antagonism does not increase basal DA efflux [F(6,24)54.63, P50.003]. Post hoc tests cortical DA release. In fact, the opposite was observed, demonstrated that this decrease was significant at all time namely that intracortical administration of the selective points except for the 90 min interval following drug 5-HT2A antagonist M100,907 blocked DA release induced
1
observed in the present study. These investigators found that systemic administration of SR 46349B blocked DA release in the nucleus accumbens that was induced by the stimulation of the dorsal raphe nucleus. They also found that local infusions of this drug blocked DA release in the striatum that was induced by treatment with haloperidol. Haloperidol induces impulse-dependent DA release by blocking somatodendritic DA autoreceptors that regulate DA cell firing [40]. These results are similar to ours in the
1
mPFC employing high K as the depolarizing agent. They indicate that the 5-HT2A receptor regulates phasic DA release in both the striatum and the mPFC. This agrees with the suggestion by Lucas and Spampinato [18] that 5-HT2Areceptors may modulate DA release only when DA neurons are activated. These investigators, as well as others [10,45], found no effect of selective 5-HT2A an-tagonism on basal DA efflux. In the present work, there were relatively slight decreases in dialysate DA following Fig. 5. The effects of intracortical M100,907 (10mM) administration on
DOI-induced DA release. Values are expressed as the percentages of 3 reverse dialysis with M100,907. These decreases may pre-drug baselines and are the means6S.E.M. of the subjects. DOI (2.5 reflect a neuromodulatory, conditional, role of 5-HT
2A
mg / kg s.c.) or vehicle were injected at the time indicated by the arrow.
receptors on DA release that is more apparent when the
The timing of M100,907 perfusion is indicated by the bar. Perfusion 1
mesocortical system is stimulated (e.g. with high K or began 30 min before the DOI injection and lasted to the end of the
haloperidol administration). experiment (another 2.5 h). DOI administration significantly increased
cortical DA efflux (P50.01) and this was blocked by intracortical Administration of M100,907 has also been shown to M100,907 (no significant increase in DA). Infusions of M100,907 alone attenuate striatal DA release induced by the administration decreased basal DA release (P50.003).
of the psychostimulant 3,4-methylenedioxymetham-phetamine (MDMA) [36]. In contrast, administration of DOI potentiated MDMA-induced DA release [11]. Local infusion of M100,907 also produced small but Schmidt and colleagues have proposed that these ‘‘results significant decreases in basal DA concentrations. These suggest a permissive role for 5-HT2 receptors in the results suggest that, under certain conditions, 5-HT2A activation of the dopamine system which occurs during receptors, localized in the mPFC, may function physiologi- states of high serotonergic activity . . . ’’ [35]. The present cally to augment mesoprefrontocortical DA release. results agree with this suggestion since infusions of high
1
Most previous work has employed the systemic adminis- K should increase the release of all transmitters, includ-tration of drugs, which does not elucidate the neuro- ing DA and 5-HT. In addition, it has recently been anatomical localization of the relevant receptors. Theoret- demonstrated that administration of M100,907 attenuates ically, 5-HT2A receptors could regulate mesocortical DA the increases in prefrontocortical DA observed following activity, and subsequent transmitter release, through ac- the systemic administration of fluoxetine [45]. Fluoxetine tions on the DA cell bodies in the ventral tegmental area blocks the 5-HT transporter and thereby increases synaptic and / or on neurons in the mPFC. Gobert and Millan [10] concentrations of 5-HT. Thus, considered together, the past have recently published microdialysis studies demonstra- and present results provide support for the suggestion that, ting that systemic administration of the 5-HT agonist DOI2 at least under conditions of activated dopaminergic and increased cortical DA release by 50% and this release was serotonergic systems, 5-HT2A receptors potentiate DA blocked by systemic administration of M100,907. These release in the mPFC.
authors suggested that 5-HT2A receptors are involved in Microdialysis studies utilizing the acute, systemic ad-facilitating the phasic release of frontocortical DA. Our ministration of nonselective 5-HT antagonists, have gen-2
1
results demonstrating a significant blockade of high K erally observed increases in cortical extracellular DA and DOI stimulated DA release by M100,907 agree with levels. For example, amperozide [24,29], ritanserin [30] this interpretation. The present data further suggest that and clozapine [21,28] have all been shown to increase systemically administered 5-HT2A ligands alter mesocorti- cortical dialysate DA levels. However, ritanserin could cal DA release by acting, at least in part, on 5-HT2A have actions on 5-HT2C receptors [33]. Amperozide is receptors localized in the mPFC, where they are abundant selective for the 5-HT2A over the 5-HT2Creceptor [33] but
[4,6,31,41]. also blocks the DA transporter [42]. As is well known,
as a consequence of selective 5-HT2A receptor blockade in 1000 mM and 0.25–4 mM concentrations of other 5-HT1
the cortex. agonists. In the present study, 10 mM M100,907
sig-1
The cellular localization of cortical 5-HT2A receptors nificantly attenuated basal, K -stimulated, and DOI-stimu-regulating DA release remains to be determined. Evidence lated DA release. This concentration of M100,907 is 1 / 10 indicates that 5-HT2A receptors are not localized pre- of that employed in prior work with local infusions of synaptically on DA terminals [16,17]. Thus, 5-HT2A clozapine [28]. This ratio of 10:1 roughly corresponds to receptors likely modulate DA release indirectly, either the affinities of clozapine (4 nM) versus M100,907 (0.36 through neuronal circuits located within the mPFC, or nM) for the 5-HT2A receptor ([34,25], respectively). 100 through connections to other brain areas. Recent immuno- nM and 1.0mM concentrations M100,907 did not alter DA histochemical work has shown that prefrontocortical 5- efflux, suggesting that the present work reflects the left HT2A receptors appear to be located principally on the side of the concentration–response curve. This also agrees apical dendrites of pyramidal cells as well as on GABA with prior work demonstrating that 100 nM M100,907 did interneurons in the rat and monkey [13,39]. 5-HT could not alter 5-HT-stimulated cortical DA release [12]. theoretically act on 5-HT2A receptors localized to pyrami- In summary, local infusions of the selective 5-HT2A
1 dal cells or GABAergic interneurons that, in turn, regulate antagonist M100,907 into the mPFC attenuated K -in-mesocortical DA release. duced DA release in a concentration-dependent manner. One limitation of the present approach is that the Systemic administration of the 5-HT2 agonist DOI in-concentration of M100,907 entering the brain is unknown. creased cortical DA efflux. This increase was attenuated by The current study, like most microdialysis studies, em- intracortical administration of M100,907. These results ployedmM concentrations to produce effects equivalent to indicate that, under activated conditions, cortical 5-HT2A
low to moderate doses of systemically administered lig- receptors potentiate the phasic release of DA. They raise ands. In particular, the present work with 10 and 100mM questions as to whether 5-HT2A receptors modulate DA M100,907 agrees with the previous finding that systemic that is released physiologically (e.g., in response to novelty injections of a moderate dose (0.4 mg / kg) of this com- or stress) or in a non-impulse dependent manner (e.g., pound blocked DOI-stimulated cortical DA release [10]. amphetamine-induced). This, in turn, raises the issue of Empirical evidence suggests that the concentrations of whether 5-HT2A receptors regulate DA-mediated be-drugs crossing the dialysate membrane are extremely haviors. In fact, it has been demonstrated that M100,907 small. For example, the amounts of various drugs (DA blocks both cocaine [19] and amphetamine-mediated be-uptake blockers) that crossed the dialysate membrane in haviors [22]. The present data suggest that when cortical vitro range from 2.0 to 8.6% [23]. However, in vitro DA is released physiologically, 5-HT, via a 5-HT2A
studies of recovery fail to account for impediments to drug mechanism, may act to further stimulate DA release and diffusion that normally occur in vivo in the brain tissue. In thus increase the salience of the neurochemical signal. This fact, there is evidence that ‘‘the tissue is normally more corresponds with recent reports demonstrating that 5-HT important than the membrane in determining the per- may act as a paracrine transmitter [3].
formance of a microdialysis probe’’ [8]. One study that calculated the true in vivo diffusion of an antiviral
nucleoside demonstrated that the recovery was one-third of Acknowledgements that observed in vitro [38]. Furthermore, even if the drug
concentrations immediately surrounding the membrane are We wish to thank Dr. Christine Nocjar for her helpful relatively high, there is significant tissue damage in this insights and discussions in the preparation of this manu-vicinity [44]. Studies with smaller in vivo voltammetric script. This work was supported by grant MH52220 to electrodes have shown that pharmacological effects on DA E.A.P. The authors wish to thank Hoechst Marion Roussell efflux are absent in this zone [44]. Other work has shown for their kind donation of M100,907.
that there is a gradient of drug concentration around the probe and that DA measured by microdialysis is diffusing from areas close to, but away from, the probe [2]. Thus,
References
the changes in DA release in the present study are likely due to actions at sites slightly distal to the probe and not in
[1] S. Benloucif, M.P. Galloway, Facilitation of dopamine release in the vicinity ofmM concentrations. vivo by serotonin agonists: studies with microdialysis, Eur. J.
The concentrations of M100,907 employed in the pres- Pharmacol. 200 (1991) 1–8.
ent research were chosen on the basis of work by others [2] P.M. Bungay, P.F. Morrison, R.L. Dedrick, Steady-state theory for quantitative microdialysis of solutes and water in vivo and in vitro, employing reverse dialysis of serotonergic ligands. For
Life Sci. 46 (1990) 105–119.
example, 100–300 mM concentrations of 5-HT1B ligands [3] M.A. Bunin, R.M. Wightman, Paracrine neurotransmission in the altered DA release in the mPFC [12]. Perfusion with 50 CNS: involvement of 5-HT, Trends in Neurosci. 22 (1999) 377– mM 8-OH-DPAT, a 5-HT1A agonist, stimulated DA release 382.
dis-tribution of 5-HT1A and 5-HT2A receptor mRNA in human brain, cortex, nucleus accumbens, and striatum of the rat: An in vivo Brain Res. 676 (1995) 157–168. microdialysis study, J. Neurochem. 54 (1990) 1755–1760. [5] J. Chen, H.M. van Praag, E.L. Gardner, Activation of 5-HT3 [22] P.C. Moser, P.M. Moran, R.A. Frank, J.H. Kehne, Reversal of
receptor by 1-phenylbiguanide increases dopamine release in the rat amphetamine-induced behaviours by M100,907, a selective 5-HT2A
nucleus accumbens, Brain Res. 543 (1991) 354–357. antagonist, Beh. Brain Res. 73 (1996) 163–167.
[6] V. Cornea-Hebert, M. Riad, F. Zerari, S. Garcia, L. Descarries, C. [23] G.G. Nomikos, G. Damsma, D. Wenkstern, H.C. Fibiger, In vivo Wu, S. Singh, Cellular and subcellular distribution of the serotonin characterization of locally applied dopamine uptake inhibitors by 5-HT2Areceptor in the central nervous system of adult rat, J. Comp. striatal microdialysis, Synapse 6 (1990) 106–112.
Neurol. 409 (1999) 187–209. [24] G.G. Nomikos, M. Iurlo, J.L. Andersson, K. Kimura, T.H. Svensson, [7] P. De Deurwaerdere, U. Spampinato, Role of serotonin 2A and Systemic administration of amperozide, a new atypical antipsychotic serotonin (2B / 2C) receptor subtypes in the control of accumbal and drug, preferentially increases dopamine release in the rat medial striatal dopamine release elicited in vivo by dorsal raphe nucleus prefrontal cortex, Psychopharmacology 115 (1994) 146–147. electrical stimulation, J. Neurochem. 73 (1999) 1033–1042. [25] M.G. Palfreyman, C.J. Schmidt, S. M Sorensen, M.W. Dudley, J.H. [8] K.H. Dykstra, J.K. Hsiao, P.F. Morrison, P.M. Bungay, I.N. Mefford, Kehne, P. Moser, M.W. Gittos, A.A. Carr, Electrophysiological, M.M. Scully, R.L. Dedrick, Quantitative examination of tissue biochemical and behavioral evidence for 5-HT and 5-HT mediated2 3
concentration profiles associated with microdialysis, J. Neurochem. control of dopaminergic function, Psychopharmacology 112 (1993)
58 (1992) 931–940. S60–S67.
[9] C. Ennis, J.D. Kemp, B. Cox, Characterization of inhibitory 5- [26] L.H. Parsons, J.B. Justice, Perfusate serotonin increases extracellular hydroxytryptamine receptors that modulate dopamine release in the dopamine in the nucleus accumbens as measured by in vivo striatum, J. Neurochem. 36 (1981) 1515–1520. microdialysis, Brain Res. 606 (1993) 195–199.
[10] A. Gobert, M.J. Millan, Serotonin (5-HT)2A receptor activation [27] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, enhances dialysate levels of dopamine and noradrenaline, but not Academic Press, New York, 1998.
5-HT, in the frontal cortex of freely-moving rats, Neuropharmacol. [28] E.A. Pehek, B.K. Yamamoto, Differential effects of locally adminis-38 (1999) 315–317. tered clozapine and haloperidol on dopamine efflux in the rat [11] G.A. Gudelsky, B.K. Yamamoto, J.F. Nash, Potentiation of 3,4- prefrontal cortex and caudate-putamen, J. Neurochem. 63 (1994)
methylenedioxymethamphetamine-induced dopamine release and 2118–2124.
serotonin neurotoxicity by 5-HT2 receptor agonists, Eur. J. Phar- [29] E.A. Pehek, H.Y. Meltzer, B.K. Yamamoto, The atypical antipsy-macol. 264 (1994) 325–330. chotic drug amperozide enhances rat cortical and striatal dopamine [12] R.N. Iyer, C.W. Bradberry, Serotonin-mediated increase in prefrontal efflux, Eur. J. Pharmacol. 240 (1993) 107–109.
cortex dopamine release: pharmacological characterization, J. Phar- [30] E.A. Pehek, Local infusion of the serotonin antagonists ritanserin or macol. Exp. Ther. 277 (1996) 40–47. ICS 205,930 increases in vivo dopamine release in the rat medial [13] R.L. Jakab, P.S. Goldman-Rakic, 5-Hydroxytryptamine2Aserotonin prefrontal cortex, Synapse 24 (1996) 12–18.
receptors in the primate cerebral cortex: Possible site of action of [31] M. Pompeiano, J.M. Palacios, G. Mengod, Distribution of the hallucinogenic and antipsychotic drugs in pyramidal cell apical serotonin HT2 receptor family mRNAs: comparison between 5-dendrites, Proc. Natl. Acad. Sci. USA 95 (1998) 735–740. HT2Aand 5-HT2Creceptors, Mol. Brain Res. 23 (1994) 163–178. [14] J.H. Kehne, B.M. Baron, A.A. Carr, S.F. Chaney, J. Elands, D.J. [32] B.L. Roth, Multiple serotonin receptors: Clinical and experimental
Feldman, R. A Frank, P.L.M. van Giersbergen, T.C. McCloskey, aspects, Ann. Clin. Psych. 6 (1994) 61–78.
M.P. Johnson, D.R. McCarty, M. Poirot, Y. Senyah, B.W. Siegel, C. [33] B.L. Roth, R.D. Ciarnello, H.Y. Meltzer, Binding of typical and Widmaier, Preclinical characterization of the potential of the puta- atypical antipsychotic agents to transiently expressed 5-HT1C re-tive atypical antipsychotic M100,907 as a potent 5-HT2Aantagonist ceptors, J. Pharmacol. Exp. Ther. 260 (1992) 1361–1365. with a favorable CNS safety profile, J. Pharmacol. Exp. Ther. 277 [34] B.L. Roth, H.Y. Meltzer, The role of serotonin in schizophrenia, in: (1996) 968–981. F.E. Bloom, D.J. Kupfer (Eds.), Psychopharmacology: The Fourth [15] T. Kuroki, H.Y. Meltzer, J. Ichikawa, Effects of antipsychotic drugs Generation of Progress, Raven Press, New York, 1995, pp. 1215–
on extracellular dopamine levels in rat medial prefrontal cortex and 1226.
nucleus accumbens, J. Pharmacol. Exp. Ther. 288 (1999) 774–781. [35] C.J. Schmidt, G.M. Fayadel, C.K. Sullivan, V.L. Taylor, 5-HT2
[16] J.E. Leysen, R. Geerts, W. Gommeren, M. Verwimp, P. Van Gompel, receptors exert a state-dependent regulation of dopaminergic func-Regional distribution of serotonin-2 receptor binding sites in the tion: studies with MDL 100,907 and the amphetamine analog, brain and effects of neuronal lesions, Arch. Int. Pharmacodyn. 256 3,4-methylenedioxymethamphetamine, Eur. J. Pharmacol. 223
(1982) 301–305. (1992) 65–74.
[17] J.E. Leysen, P. Van Gompel, M. Verwimp, C.J.E. Niemegeers, Role [36] C.J. Schmidt, C.K. Sullivan, G.M. Fayadel, Blockade of striatal and localization of serotonin (S )-receptor-binding sites: Effects of2 2 5-hydroxytryptamine receptors reduces the increase in extracellular2
neuronal lesions, in: P. Mandel, F.V. DeFeudis (Eds.), CNS Re- concentrations of dopamine produced by the amphetamine analogue ceptors — From Molecular Pharmacology to Behavior, Raven Press, 3,4-methylenedioxymethamphetamine, J. Neurochem. 62 (1994)
New York, 1983, pp. 373–383. 1382–1389.
[18] G. Lucas, U. Spampinato, Role of striatal serotonin2A and [37] J. Svartengren, P. Simonsson, Receptor binding properties of am-serotonin2C receptor subtypes in the control of in vivo dopamine perozide, Pharmacol. Toxicol. Suppl. 1 (1990) 8–11.
outflow in the rat striatum, J. Neurochem. 74 (2000) 693–701. [38] Y. Wang, S.L. Wong, R.J. Sawchuk, Microdialysis calibration using [19] L.R. McMahon, K.A. Cunningham, Attenuation of the locomotor retrodialysis and zero-net flux: application to a study of the stimulant and discriminative stimulus effects of cocaine in rats by distribution of zidovudine to rabbit cerebrospinal fluid and thalamus, the 5-HT2A antagonist MDL 100,907, Soc. Neurosci. Abstr. 25 Pharm. Res. 10 (1993) 1411–1419.
(1999) 561. [39] D.L. Willins, A.Y. Deutch, B.L. Roth, Serotonin 5-HT2A receptors [20] H.Y. Meltzer, S. Matsubara, J.-C. Lee, Classification of typical and are expressed on pyramidal cells and interneurons in the rat cortex,
atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and Synapse 27 (1997) 79–82.
serotonin pKi values, J. Pharmacol. Exp. Ther. 251 (1989) 238–2 [40] M.E. Wolf, A.Y. Deutch, R.H. Roth, Pharmacology of central
246. dopaminergic neurons, in: F.A. Henn, L.E. DeLisa (Eds.),
[41] D.E. Wright, K.B. Seroogy, K.H. Lundgren, B.M. Davis, L. Jennes, tion of extracellular dopamine: a study by combined voltammetry Comparative localization of serotonin 1A, 1C, and 2 receptor and microdialysis, in: H. Rollema, E. Abercrombie, D. Sulzer, J. subtype mRNAs in rat brain, J. Comp. Neurol. 351 (1995) 357–373. Zackheim (Eds.), Monitoring Molecules in Neuroscience, Proceed-[42] B.K. Yamamoto, H.Y. Meltzer, The effect of the atypical antipsy- ings of the 8th International Conference on In Vivo Methods, chotic drug, amperozide, on carrier-mediated striatal dopamine Rutgers, The State University of New Jersey, Newark, NJ, 1999, pp. release measured in vivo, J. Pharmacol. Exp. Ther. 263 (1992) 79–80.
180–185. [45] W. Zhang, K.W. Perry, D.T. Wong, B.D. Potts, J. Bao, G.D.