L
Journal of Experimental Marine Biology and Ecology 246 (2000) 259–279
www.elsevier.nl / locate / jembe
Changes in metallothionein concentrations in response to
variation in natural factors (salinity, sex, weight) and metal
contamination in crabs from a metal-rich estuary
a,b a,b ,* b,c b,c
S. Legras , C. Mouneyrac , J.C. Amiard , C. Amiard-Triquet ,
d
P.S. Rainbow a
´
IRFA, Departement des Sciences de la Vie et de la Terre, Laboratoire d’Ecologie Animale, 44, rue Rabelais, 49100 Angers, France
b
`
GDR1117 CNRS, Ecotoxicologie et Chimie Marines, 2, rue de la Houissiniere, BP 92208, 44322 Nantes Cedex 3, France
c
`
Service d’Ecotoxicologie, ISOMer, Facult de Pharmacie, 2, rue de la Houissiniere, BP 92208, 44322 Nantes Cedex 3, France
d
Department of Zoology, The Natural History Museum, Cromwell Rd., London SW7 5BD, UK Received 2 July 1999; received in revised form 25 November 1999; accepted 16 December 1999
Abstract
Intermoult male and female crabs Pachygrapsus marmoratus and Carcinus maenas were sampled from three sites between the mouth and 25 km upstream in the Gironde, the most Cd-contaminated estuary in France, in order to study the relative importance of natural factors (salinity, sex, weight) and accumulated metal concentrations on metallothionein (MT) con-centrations. In the two species studied, higher metal, total protein and MT concentrations were observed in the hepatopancreas than in the gills. In P. marmoratus, MT concentrations were mainly related to changes in the natural factors even if MT and Zn concentrations were positively correlated in the hepatopancreas whereas in C. maenas, the main relationships were with accumulated metal levels. In the case of the natural factors, the most important ones were weight in gills of both crab species, and salinity changes in both hepatopancreas and gills of P.
marmoratus. Cd and Cu concentrations in both organs of the two species were inversely related to
salinity. The same observation was found for Zn concentrations in C. maenas but not in P.
marmoratus. In the hepatopancreas of both species, the highest total protein concentrations were
found in crabs from the site with the highest salinity, whereas there were no such differences in the gills. It seems that changes in MT concentrations are linked more to changes in general protein metabolism than to changes in metal accumulation. Thus it was important to examine the storage of metals in other tissue compartments, particularly the insoluble fraction which includes mineral granules which is known to also contribute to trace metal detoxification in invertebrates. In the
*Corresponding author.
E-mail address: [email protected] (C. Mouneyrac)
gills of the crabs, Zn was present mainly in the insoluble fraction, whereas Cd was nearly equally distributed between soluble and insoluble fractions. In contrast, Cu in the gills and all three metals in the hepatopancreas of both species were mainly cytosolic, but this does not necessarily imply a predominant role for MT since the cytosolic fraction also includes other macromolecules which may be the target binding site for accumulated trace metals. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Crab; Heavy metals; Salinity; Metallothionein
1. Introduction
Trace metals, whether essential such as Cu and Zn or non-essential such as Cd, are taken up by aquatic invertebrates from both food and solutions. Uptake rates from solution can usually be interpreted in terms of the physicochemical form of the metal in the medium, but may, in some cases, also be affected by the implementation of some physiological control by the crustacean itself (Rainbow, 1997a,b). Trace metal uptake rates therefore vary with changes in salinity. Generally, decreases in salinity are associated with increased uptake rates of many trace metals by marine crustaceans (O’Hara, 1973a,b; Nugegoda and Rainbow, 1989a,b), the usual interpretation being that this increased uptake results from increases in free metal ion concentrations as chloride complexation decreases in lower salinities (Zirino and Yamamoto, 1972; Mantoura et al., 1978; Bruland, 1983). In euryhaline species, including the crab Carcinus maenas, however, there is a possibility that physiological adaptation, in particular osmoregulation processes, may also influence the uptake of dissolved metals (Chan et al., 1992; Rainbow et al., 1993; Rainbow, 1997a,b).
In metal-polluted environments, a high availability of a trace metal will promote a high rate of metal entry into the body. If the rate of uptake exceeds the rate at which the metal can be detoxified or excreted, then the metal is available internally to exert toxic effects. The survival of marine animals resident in polluted environments depends on the avoidance of this situation, which can be achieved by one or more different processes (Mason and Jenkins, 1995): (a) the limiting of metal uptake; (b) the enhancement of detoxification processes controlling internal metal speciation and (c) increased metal excretion.
The first of these processes has been considered in detail by Rainbow et al. (1999). Uptake rates of Zn and Cd from solution were measured in crabs (Carcinus maenas and Pachygrapsus marmoratus) from coastal sites in Britain and France (including the Gironde estuary) exposed to different degrees of trace metal enrichment. The mean metal uptake rates of both crab species did not show any consistent significant differences between the crustaceans from the metal-rich and control sites, suggesting that the chronic exposure to raised trace metal availabilities was not sufficient to select for a reduction in dissolved trace metal uptake rate among these estuarine populations (Rainbow et al., 1999).
Attention is therefore turned here to the second process, that of detoxification. Trace metal detoxification is typically achieved by binding of the metal to high affinity sites in insoluble granules, often phosphate-based, or in detoxificatory proteins such as metal-lothioneins (MTs) or ferritin (Mason and Jenkins, 1995). Metalmetal-lothioneins, a family of low-molecular-weight, cysteine-rich metal binding proteins have been shown to occur in most zoological taxa (Amiard and Cosson, 1997), including crustaceans (Rainbow, 1998 and literature cited therein), particularly in crabs (Pedersen et al., 1994, 1996). These proteins play a primary role in the homeostasis of the essential metals Cu and Zn, as well as being involved in the detoxification of non-essential metals, particularly Cd (Amiard and Cosson, 1997).
Given the role of MTs in the normal homeostasis of Zn and Cu, it is likely that MT concentrations will be affected by natural factors such as the moult cycle, sex and size, in addition to any response to increased metal uptake rates resulting from high external metal availabilities. Thus the moult cycle has an effect on concentrations of metals and MT in the blue crab Callinectes sapidus, via an interaction of the physiological cycling of body copper and the Cu-bearing respiratory pigment haemocyanin (Engel, 1987; Engel and Brouwer, 1991, 1993). Other natural factors that may affect accumulated metal and MT concentrations include season, size and sex. Seasonal variations occur in the metal burdens of aquatic invertebrates, due, for example, to the weight-related effects of feeding and / or reproductive state, particularly through the loss of metal-poor gametes during spawning (Langston and Spence, 1995). In krill, variations in Cu content are suggested to be the result of changes in Cu-binding proteins due to differences in physiological conditions between sexes and maturity stages (Yamamoto et al., 1990, cited by Langston and Spence, 1995). In the freshwater crab Oziotelphusa senex senex exposed to sublethal concentrations of Cd, the level of metal accumulation was greater in the organs of small specimens of both sexes, and was also higher in the organs of males than in those of females (Radhakrishnaiah et al., 1991).
the Gironde estuary, a site known from the biomonitoring of oysters (Crassostrea gigas) to contain elevated concentrations of Cd, Cu and Zn (RNO, 1995). Variation in MT concentrations may be correlated to a number of factors for the reasons detailed above. Relationships have therefore been investigated between hepatopancreas and gill MT concentrations and the natural factors salinity, sex and weight, and these MT con-centrations and the accumulated metal concon-centrations in the organs.
2. Materials and methods
2.1. Sampling sites
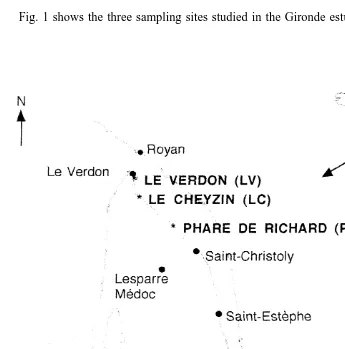
Fig. 1 shows the three sampling sites studied in the Gironde estuary, all of which are
located in a polyhaline area with salinities varying between 18 and 30‰ (Castel et al., 1976). In the main channel of the estuary, salinities varied from 7 to 17‰ at Phare de Richard, 13 to 25‰ at Le Cheyzin, and 18 to 30‰ at Le Verdon (Allen, 1972).
2.2. Specimens
The crabs P. marmoratus and C. maenas were collected on 23 June 1997 from indigenous populations at different locations in the Gironde estuary: (1) Le Verdon (salinity: 2468‰) and Phare de Richard (1267‰) for P. marmoratus males and (2) Le Verdon, Le Cheyzin (1968‰) and the Phare de Richard for C. maenas males. Females of the two species came only from Le Verdon.
Intermoult specimens (P. marmoratus, carapace width 1.35–2.90 cm; C. maenas, 1.67–9.37 cm) were selected, and each of these was characterized by the total weight of hepatopancreas (P. marmoratus, 0.03–0.5 g w.w.; C. maenas, 0.07–1.36 g w.w.) and gills (P. marmoratus, 0.02–0.27 g w.w.; C. maenas, 0.04–0.66 g w.w.). The samples were kept at 2808C until analysis.
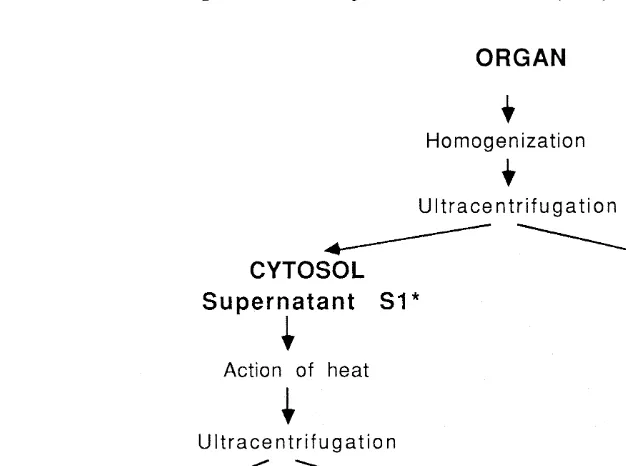
2.3. Metal compartmentation (Fig. 2)
The crabs were thawed and dissected to isolate the gills and hepatopancreas. Then,
25
each individual organ was homogenized at 48C in 20 mM Tris 10 mM b -mercap-toethanol, 0.1 mM phenylmethylsulfonylfluoride (PMSF), 150 mM NaCl solution adjusted to pH 8.6 (4 ml / 1 g soft tissue). The soluble and insoluble fractions were subsequently separated by centrifugation (21 250 g for 55 min at 48C). The heat-stable compounds including MT were isolated by centrifugation of the soluble fraction (12 000 g for 10 min) after heat-treatment (758C for 15 min).
2.4. Protein analysis
Total proteins were quantified in the soluble fraction (S1) according to the Lowry procedure (Lowry et al., 1951).
In the heat-denaturated cytosol (S2), the amount of MT was determined by differential pulse polarographic analysis (DPP), a technique based on –SH compound determination according to the Brdicka reaction (Brdicka, 1933) as described by Thompson and Cosson (1984). The PAR Model 174 analyser, the PAR / EG and G Model 303 static mercury drop electrod (SMDE) and an X–Y recorder (RE 0089) were used. The temperature of the cell was maintained at 58C. The standard addition method was used for calibration with rabbit liver MT (Sigma, St. Louis, MO, USA) in the absence of a crab MT standard.
MTLP measured here is considered to be a true MT and is referred to as such. Protein concentrations were expressed on a wet weight basis.
2.5. Metal analysis
Nalgene bottles were used to store all reagents. All glassware was soaked in 10% HCl, rinsed three times with deionized water and dried in a desiccator protected from atmospheric dust. The insoluble (P1) and soluble (S1) fractions were heated (758C, 12 h) with suprapure HNO acid (Carlo Erba). After digestion, metal concentrations in these3 acid solutions were determined after dilution with deionized water by flame atomic absorption spectrophotometry (AAS) (Cu, Zn) or electrothermal AAS (Cd) using the Zeeman effect (Hitachi Z 8200 spectrophotometer). The analytical method has been described previously by Amiard et al. (1987b).
Standard addition analyses were performed in an iso-medium and added
con-21 centrations of each element were 1125, 1250 and 1500 ng Cu and Zn ml for
21
flame AAS and 10.25, 10.5 and 11 ng Cd ml for EAAS. The analytical methods were validated by external intercalibrations (Coquery and Horvat, 1996) (Table 1).
Total metal concentrations in each organ on a wet weight basis were recalculated from summation of quantities of trace elements in soluble and insoluble fractions determined previously.
2.6. Statistical treatment
Comparisons of two series (Students t-test) and regression analysis (linear or
1
multiple) were carried out using a standard statistical package (STATVIEW SE
GraphicsE).
When the MT concentration (dependent variable) was to be expressed as a function of natural factors (salinity, weight, sex) and metal concentrations (independent variables), a standardization of the independent variables was needed to overcome the problem of the presence of different units. The method used, therefore, was to transform the
in-Table 1
Results of external quality control (concentrations in mg / kg dry wt.) (Coquery and Horvat, 1996)
Cd Cu Zn
Fish homogenate MA-MEDPOL-1 / TM
Our value (Lab. 12) 0.0021 (0.0007) 0.66 (0.10) 17.3 (0.6)
Certified value 0.015 (0.012) 0.62 (0.12) 16.80 (0.48)
a
Z-scores 21.1 0.3 0.2
Marine sediment SD-MEDPOL-1 / TM
Our value (Lab. 12) 0.701 (0.06) 23.9 (1.4) 189.0 (2.3)
Certified value 0.59 (0.10) 25.1 (3.8) 191 (17)
a
Z-scores 1.5 20.4 20.1
a
dependent natural variables x (weight, salinity, sex, concentrations of Cd, Cu, Zn) intoi coded variables X .i
MT5a 1 Sao iXi (1)
Xi,max5 11 for the maximum value of variable and Xi,min5 21 for the minimum one. The transformation of the independent variables to coded variables is given by the following equations (Montgomery and Peck, 1982)
xi2(xi,max1xi,min) / 2 ]]]]]]
Xi5 (x 2x ) / 2 (2)
i,max i,min
where xi,min5the lowest value of the independent variable, and xi,max5the highest value of the independent variable.
The model coefficients (a0,a1,a2,a3,a4) were estimated by theSTATVIEWprogram.
These coefficients were tested by using Student’s t-test. A correlation matrix was used to test the linear independence between all the metal concentrations and natural factors examined in the multiple regression analyses.
3. Results
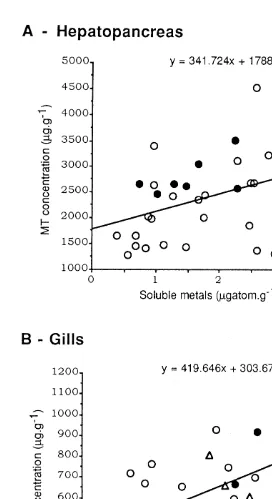
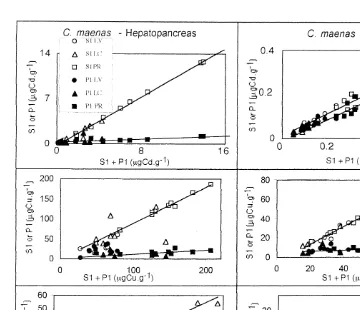
Because MT is a cytosolic heat-stable protein, it is logical to consider the relationship between MT concentrations in an organ and metal concentrations in the supernatant obtained after heat-denaturation of the cytosol (S2 in Fig. 2). However, during heating, the distribution of metals among cytosolic compounds may be modified since the analysis of Cd in fractions separated from either S1 or S2 by gel chromatography showed some discrepancies (Berthet, pers. comm.). Consequently, Figs. 3 and 4 depict the relationships between MT concentrations and metals contents in the supernatant before heat treatment (S1 in Fig. 2). All three of the metals studied (Zn, Cu, Cd) bind to MT and might therefore contribute concomitantly to MT induction (Amiard and Cosson, 1997), so soluble metal concentrations have been calculated by adding together soluble Cd, Cu and Zn concentrations expressed in mg atoms.
Fig. 3A and B depicts the results obtained for the hepatopancreas and gills of C. maenas. When all the three metals were considered together, significant relationships were observed between MT and metal concentrations in both organs (at the 95% level for hepatopancreas; at the 99% level for the gills) whereas, in P. marmoratus, no significant relationships were found (not shown).
A correlation matrix was then drawn up for all the regressors. No strong correlations 2
Fig. 2. Procedure of metal compartmentation and metallothionein determination in soft tissues of crabs. *Metal quantification by atomic absorption spectrophotometry; **MT quantification by differential pulse polarography.
MT5f(Cd, Cu, Zn, W, Se, Sa, Pr) (3)
The equations are shown hereafter for each species and organ examined. The significance of each coefficient (probability based on t-test) is shown in brackets. Independent variables are only reported if they make a significant contribution (P,0.05) to the explanation of the variance in MT concentration.
3.1. Pachygrapsus marmoratus
Hepatopancreas (n570; r50.616)
MT5 21.045 10.39 Zn 13.07 Sa
(4) (P50.0001) (P50.0001)
Gills (n570; r50.771)
MT50.042 10.481 Pr 21.837 Sa 20.323 Se 20.316 W
3.2. Carcinus maenas
Hepatopancreas (n532; r50.542)
MT5 2 0.459 20.565 Cd 10.714 Cu
(6) (P50.0133) (P50.002)
Gills (n532; r50.765)
MT5 2 0.125 10.399 Zn 10.363 Cu 20.338 W
(7) (P50.002) (P50.0039) (P50.0137)
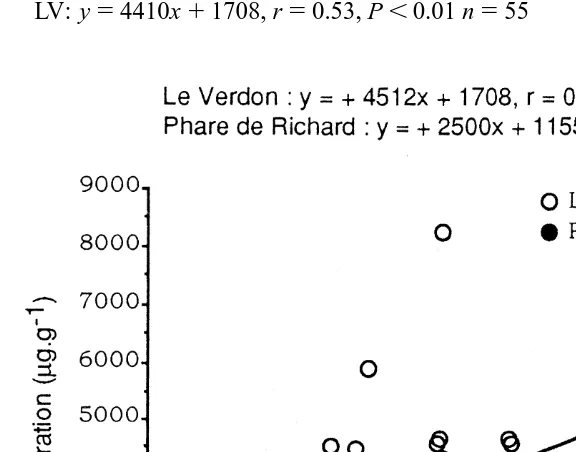
Soluble Zn concentrations and salinity appeared as the major factors correlated with MT concentrations in the hepatopancreas of P. marmoratus (Eq. (4)). Fig. 4 depicts the relationship between MT and soluble Zn concentrations for the hepatopancreas samples of P. marmoratus from Le Verdon (LV, where the highest salinity was registered) and from Phare de Richard (PR, the most upstream site). At both sites a significant correlation was present
LV: y54410x11708, r50.53, P,0.01 n555 (8)
PR: y52444x11155, r50.54, P,0.05 n515 (9)
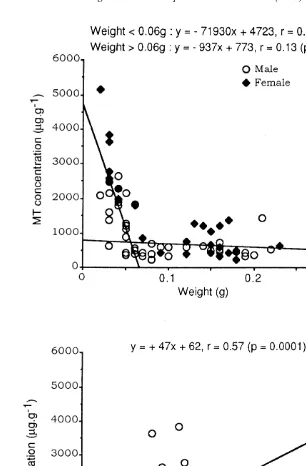
Over all the range of soluble Zn concentrations, specimens experiencing the lowest salinity (PR) in their environment showed the lowest hepatopancreas MT concentrations. In the gills of P. marmoratus, no significant relationship was shown between concentrations of MT and those of any of the three soluble metals studied (Eq. (5)). MT concentration decreased with increasing weight of the gill in small specimens then remained steady with weight .0.06 g (Fig. 5A). Considering either small (,0.06 g) or larger specimens (.0.06 g), MT levels were moderately but significantly (P50.0139) higher in females than in males. MT concentrations in the gills of both males and females were positively correlated (at the level of 99%) with total protein levels (Eq. (5) and Fig. 5B). MT and total protein concentrations in the gills of small specimens appeared to be higher than in medium and large specimens (P50.0001 and P50.0071, respectively) (Fig. 5B).
In C. maenas, on the other hand, the MRA has shown up the influence of soluble metal concentrations (Cd and Cu in the hepatopancreas, Eq. (6); Cu and Zn in the gills, Eq. (7)) on the MT concentrations in each organ. This finding is in agreement with the significant relationships shown above (Fig. 3A and B) when the concentrations of all three metals were considered.
In C. maenas a positive correlation was observed between MT and the sum of the three metal concentrations in both gills and hepatopancreas (Fig. 3A and B), whereas no significant relationships were observed in either the gills or hepatopancreas of P. marmoratus. This interspecific difference is particularly striking since the correlations were based upon a larger number of specimens in P. marmoratus. This unexpected lack of relationship between MT and metal concentrations, at least in one species, is the starting point for an examination of other physicochemical forms involved in the detoxified storage of accumulated trace metals.
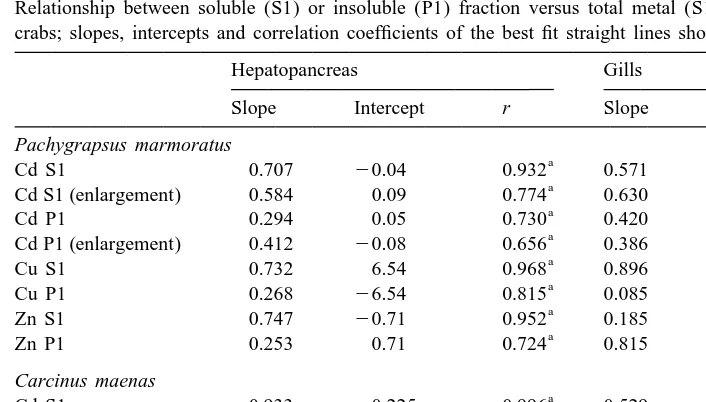
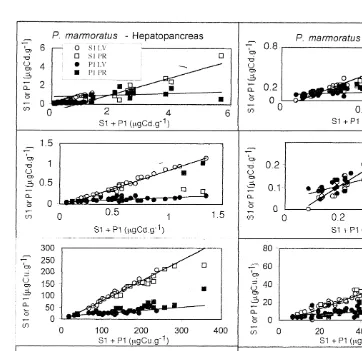
The distribution of Cd, Cu and Zn among soluble and insoluble fractions in the hepatopancreas (HP) and gills (G) of male crabs (P. marmoratus and C. maenas) is shown in Figs. 6 and 7. The highest Cd, Cu and Zn concentrations whatever the fraction considered were found in the hepatopancreas. In this organ, the soluble fraction was more important than the insoluble fraction for all three metals in both crab species as shown by the slopes of the best-fit straight lines (Table 2) and confirmed by a paired t-test (P,0.05). The same distribution was observed for Cu in the gills whereas Cd was nearly equally distributed between soluble and insoluble fractions at least for the lowest
21
concentrations of total Cd (,0.5 mg Cd S11P1 g , see enlargement in Fig. 6). In contrast gill Zn was predominantly present in the insoluble fraction (paired t-test, P,0.05). If females were considered (not shown), the same distribution was generally observed for Cd, Cu and Zn in both organs of the two species with the exception of Cd which was predominantly present in the soluble fraction in the gills of P. marmoratus females.
Fig. 5. MT levels in the gills of P. marmoratus (both sites, n570). (A) Relation with weight and sex; (B) relation with total protein concentrations.
Table 2
Relationship between soluble (S1) or insoluble (P1) fraction versus total metal (S11P1) in soft tissues of crabs; slopes, intercepts and correlation coefficients of the best fit straight lines shown in Figs. 6 and 7
Hepatopancreas Gills
Slope Intercept r Slope Intercept r
Pachygrapsus marmoratus
a a
Cd S1 0.707 20.04 0.932 0.571 20.01 0.905
a a
Cd S1 (enlargement) 0.584 0.09 0.774 0.630 20.03 0.888
a a
Cd P1 0.294 0.05 0.730 0.420 0.02 0.848
a a
Cd P1 (enlargement) 0.412 20.08 0.656 0.386 0.03 0.799
a a
Cu S1 0.732 6.54 0.968 0.896 24.95 0.935
a
Cu P1 0.268 26.54 0.815 0.085 4.92 0.322
a a
Zn S1 0.747 20.71 0.952 0.185 0.81 0.531
a a
Zn P1 0.253 0.71 0.724 0.815 20.81 0.941
Carcinus maenas
a a
Cd S1 0.933 20.225 0.996 0.529 0.003 0.958
a a
Cd P1 0.075 0.21 0.775 0.458 0.002 0.938
a a
Cu S1 0.903 29.2 0.897 0.971 26.3 0.988
Cu P1 20.026 19.9 0.152 0.030 6.4 0.187
a a
Zn S1 0.849 24.11 0.946 0.396 20.52 0.818
a a
Zn P1 0.151 4.11 0.462 0.604 0.52 0.908
a
Correlations significant at the 99% level.
concentrations were observed in both organs at the site with the lowest salinity (Phare de Richard), whereas the soluble concentration of Zn was not significantly affected by salinity. In C. maenas, when the same sites were compared, the highest values were shown in both organs at Phare de Richard for all the three metals (except Cu in gills). However, the differences were not always significant when the intermediate site (Le Cheyzin) was compared with the sites showing extreme salinities (Phare de Richard and Le Verdon) (Table 3). The changes according to salinity are summarized in Table 4.
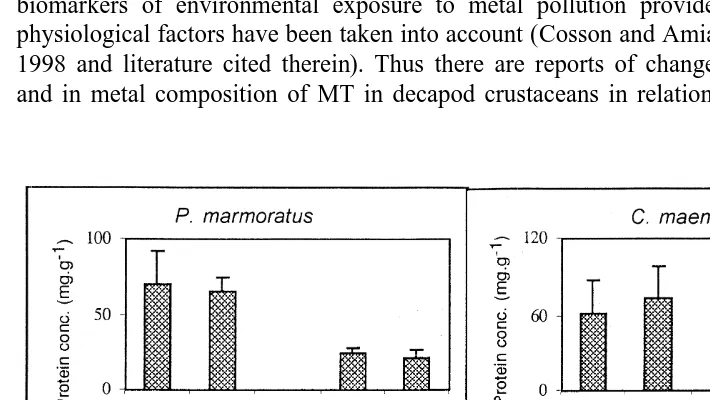
In the case of MT and total proteins in the soluble fraction of specimens originating from Le Verdon, the highest concentrations were found in the hepatopancreas whatever the sex and the species (Fig. 8). The same pattern was found for P. marmoratus or C. maenas originating from the Phare de Richard or Le Cheyzin (not shown). Contrary to what was observed for metals, MT levels increased with increasing salinity in both organs of P. marmoratus, whereas no difference was observed in C. maenas. Total protein levels increased with increasing salinity in hepatopancreas of both species whereas no difference occurred in gills (Table 4).
3.3. Discussion
Fig. 6. Relationship between soluble (S1) or insoluble (P1) fraction versus total metal (S11P1) in the soft tissues of hepatopancreas or gills of male P. marmoratus (n532). See enlargement for total Cd concentrations which were between 0.07 and 1.39 in the hepatopancreas and between 0.09 and 0.49 in gills.
Pedersen et al., 1997), and in Callinectes sapidus from two Connecticut estuaries (USA, Jop et al., 1997). In contrast, Cd concentrations were more than ten times higher in the
21
hepatopancreas of C. maenas from the Gironde estuary (3.9 mg kg wet weight)
21
Fig. 7. Relationship between soluble (S1) or insoluble (P1) fraction versus total metal (S11P1) in the soft tissues of hepatopancreas or gills of male C. maenas (n525).
Table 3
Intersite comparisons between metal levels in hepatopancreas and gills of P. marmoratus and C. maenas (probabilities obtained with unpaired Student’s t-test) LV5Le Verdon; LC5Le Cheyzin; PR5Phare de Richard
Hepatopancreas Gills
Pachygrapsus marmoratus
CV–PR LV–PR
Cd P50.003 P50.0001
Cu P50.0121 P50.0016
Zn P50.8192 P50.1564
Carcinus maenas
LV–PR LV–LC PR–LC LV–PR LV–LC PR–LC
Table 4
Changes in cytosolic metal and protein concentrations in hepatopancreas (HP) and gills (G) of crabs with increasing salinity. Comparisons carried out using Student’s t-test between males caught at Le Verdon and Phare de Richard and selected within the same range of weight;pincrease orodecrease significant at least at the 95% level; –: NS
Pachygrapsus marmoratus Carcinus maenas
HP G HP G
Cd o o o o
Cu o o o o
Zn – – o o
MT p p – –
Total proteins p – p –
than in the gills. A comparable distribution has been shown in equivalent organs in several zoological groups (Brown et al., 1984; Bebianno et al., 1993; Hamza-Chaffai et al., 1995; Mouneyrac et al., 1998) and in C. maenas originating from different sites (Pedersen et al., 1997).
In field studies, metallothioneins in marine organisms have been proposed as potential biomarkers of environmental exposure to metal pollution provided that natural and physiological factors have been taken into account (Cosson and Amiard, 1998; Rainbow, 1998 and literature cited therein). Thus there are reports of changes in concentrations and in metal composition of MT in decapod crustaceans in relation to the moult cycle
Fig. 8. MT and total protein concentrations (means and confidence intervals at 95% level) in hepatopancreas (HP) or gills (G) of P. marmoratus (M5male, n532; F5female, n524) or C. maenas (male: n58; female:
(Engel and Brouwer, 1991, 1993), justifying the choice of intermoult (C4 stage) specimens in this work.
Salinity is a natural factor influencing metal uptake (Rainbow, 1995, 1997b and literature cited by this author) and thus the amount of incoming metal that potentially needs binding to MT somewhere in the animal. There is therefore a clear potential mechanism to link variations in salinity and MT concentrations. In the Gironde estuary, Cd and Cu concentrations in the two studied crabs were inversely related to salinity. The same observation was found for Zn concentrations in C. maenas but not in P. marmoratus (Table 3). The influence of salinity may be direct via physicochemical processes, and / or by interaction between these and physiological processes (Wright and Zamuda, 1987; Rainbow et al., 1993; Campbell, 1995; Rainbow, 1997a). Physiological changes due to salinity may, for example, act via changes in epidermal permeability or may also involve other processes including total protein concentrations (Chan et al. 1992, Rainbow et al., 1993). In this context, in the hepatopancreas of the two species studied in the Gironde estuary, the highest total protein concentrations were found at the site with the highest salinity whereas there were no such differences in the gills (Table 3). Metallothionein levels were also influenced by salinity at least in P. marmoratus (Eqs. (3) and (4), Table 3), and it seems that changes in MT levels were more linked to changes in general protein metabolism than to changes in metal concentrations (Table 3). As for the observed interspecific differences, it is possible that P. marmoratus could have developed physiological adaptations to low salinity more marked than those of C. maenas. It is not unreasonable that a warmer water / intertidal crab with a more southerly distribution (like P. marmoratus) might experience a higher range of physical variables including salinity and temperature than a temperate crab like C. maenas.
The influence of weight has been extensively reported in bivalves as an important factor affecting metal and MT levels (NRC, 1980; Phelps and Hetzel, 1987; Amiard-Triquet et al., 1998; Mouneyrac et al., 1998). In the present study, weight did not show up among the factors statistically related to MT concentrations in the hepatopancreas of both crabs, whereas it did so in the case of the gills.
When all the three metals were considered together, no relationship between MT and metal concentrations were shown in P. marmoratus (Fig. 3A and B). However when MRA was performed, a significant relationship was shown between MT and Zn concentrations (Eq. (3) and Fig. 5) in the hepatopancreas but not in gills (Eq. (4)). On the other hand, in C. maenas, accumulated metal concentrations were among the major factors statistically related to MT levels by both simple (Fig. 4A and B) and multiple regression analysis (Eqs. (5) and (6)). Therefore in this species at least, MT does have some potential for use as a biomarker of metal pollution. This finding is reinforced by the comparative study of impacted and non-impacted sites (Pedersen et al., 1997), but the selection of crabs as biomonitor organisms is inconsistent with the conventional practice of selecting sedentary test species (such as bivalve molluscs). Moreover, the complexities of the inter-relationships between metals bound to MT and other com-pounds, particularly haemocyanin (the respiratory pigment in decapod crustaceans), it is probably necessary to be cautious when using it as a biomarker of environmental metal exposure (George and Olsson, 1994).
Cd were mainly associated with the cytosolic fraction. The same distribution has been observed for Cu in the gills. However, this does not necessarily imply a role for MT in metal detoxification since, as mentioned above, the cytosolic fraction includes both metals bound to MT and / or high-molecular-weight compounds. For Cu, glutathione which inhibits free radical formation from Cu (I) and H O through chelation has been2 2 shown to play an important detoxificatory function (Mason and Jenkins, 1995). Brouwer and Hoexum-Brouwer (1998) found that blue crabs use glutathione as the primary Cu chelator, before the synthesis of the second copper chelator, metallothionein. Moreover, superoxide dismutase and glutathione peroxidase constitute a second line of defence to help prevent or repair damage from copper not sequestered by the cellular chelators.
In the case of Cd and Zn distributions in the gills, our results have shown that Cd was nearly equally distributed between the soluble and insoluble fraction, and Zn was predominantly accumulated in the insoluble fraction, a feature which has also been shown in C. maenas originating from clean and metal-rich sites along the Devon coast, UK (Pedersen et al., 1997). It has been shown that, in the case of a bivalve mollusc, at the beginning of the Cd exposure the metal might be associated to metal-binding proteins, but after a longer exposure, the Cd might be displaced to the particulate fraction (Romeo and Gnassia-Barelli, 1995). Insoluble metal-rich deposits can be derived from the lysosomal breakdown of metallothioneins (Dallinger, 1992; Rainbow, 1997a; Nassiri et al., in press), and metallothionein-bound Cd might well be the source of the insoluble Cd in the gills as it is broken down in lysosomes. In the case of Zn, orthophosphate granules binding zinc have been identified in the hepatopancreas of C. maenas (Hopkin and Nott, 1980, Simkiss and Taylor, 1989).
Although it remains true that a statistical correlation does not necessarily have a causal basis, it is notable that MT concentrations were related to metal concentrations in both hepatopancreas and gills of C. maenas whereas in P. marmoratus such relationships were poor. Thus this study also illustrates specific variability in the role of MT in metal detoxification. [RW]
References
´ ` ´
Allen, G.P., 1972. Etude des processus sedimentaires dans l’ estuaire de la Gironde. These de Doctorat d’etat ´
es Sciences, Universite de Bordeaux I. ´
Amiard, J.C., Amiard-Triquet, C., Berthet, B., Metayer, C., 1987a. Comparative study on the patterns of bioaccumulation of essential (Cu, Zn) and non-essential (Cd, Pb) trace metals in various estuarine and coastal organisms. J. Exp. Mar. Biol. Ecol. 106, 73–89.
´
Amiard, J.C., Pineau, A., Boiteau, H.L., Metayer, C., Amiard-Triquet, C., 1987b. Application of atomic absorption spectrophotometry using Zeeman effect to the determination of eight trace elements (Ag, Cd, Cr, Cu, Mn, Ni, Pb and Se) in biological materials. Wat. Res. 21 (6), 693–697.
´ ´
Amiard, J.C., Cosson, R.P., 1997. Les metallothioneines. In: Lagadic, L., Caquet, T., Amiard, J.C., Ramade, F. ´
(Eds.), Biomarqueurs en ecotoxicologie: Aspects Fondamentaux, Masson, Paris, pp. 53–66.
Amiard-Triquet, C., Rainglet, F., Larroux, C., Regoli, F., Hummel, H., 1998. Metallothioneins in arctic bivalves. Ecotox. Environ. Saf. 41, 96–102.
Bebianno, M.J., Nott, J.A., Langston, W.J., 1993. Cadmium metabolism in the clam Ruditapes decussata: the role of metallothioneins. Aquat. Toxicol. 27, 315–334.
Brouwer, M., Hoexum-Brouwer, T.H., 1998. Biochemical defense mechanisms against copper-induced oxidative damage in the blue crab, Callinectes sapidus. Arch. Biochem. Biophys. 351 (2), 257–264. Brown, D.A., Bay, S.M., Alfafara, J.T., Hershelman, G.P., Ward, C.F., Rosenthal, K.D., 1984. Detoxication /
toxication of cadmium in scorpiofish (Scorpaena gutata): Acute exposure. Aquat. Toxicol. 5, 93–107. Bruland, K.W., 1983. Trace elements in seawater. In: Riley, J.P., Chester, R. (Eds.), Chemical Oceanography,
Vol. 8, Academic Press, London, p. 157.
Campbell, P.G.C., 1995. Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier, A., Turner, D.R. (Eds.), Metal Speciation and Bioavailability in Aquatic Systems, Wiley, Chichester, pp. 45–102.
´
Castel, J., Arzul, G., Lissalde, J.P., 1976. Etude preliminaire du plancton de l’estuaire de la Gironde. J. Rech. ´
Oceanogr. 1 (2), 17–24.
Chan, H.M., Bjerregaard, P., Rainbow, P.S., Depledge, M.H., 1992. Uptake of zinc and cadmium by two populations of shore crabs Carcinus maenas at different salinities. Mar. Ecol. Prog. Ser. 86, 91–97. Coquery, M., Horvat, M., 1996. The analytical performance study for the MED POL area: determination of
trace-elements in marine sediment SD-MEDPOL-1 / TM and fish homogenate MA-MEDPOL-1 / TM. Report IAEA, Monaco.
´ ´
Cosson, R.P., Amiard, J.C., 1998. Utilisation des metallothioneines comme biomarqueurs d’exposition aux ´
metaux. In: Lagadic, L., Caquet, T., Amiard, J.C., Ramade, F. (Eds.), Utilisation Des Biomarqueurs Pour La ´
Surveillance De La Qualite De L’Environnement, Lavoisier Tec and Doc, Paris, pp. 77–109.
Cuadras, J., Gimeno, A., Flos, R., Crespo, S., 1981. Levels of copper and zinc in tissues of the hermit crab
Dardanus arrosor (Herbst) from the Barcelona coast (Decapoda Anomura). Crustaceana 40 (1), 79–87.
Dallinger, R., 1992. Strategies of metal detoxification in terrestrial invertebrates. In: Dallinger, R., Rainbow, P.S. (Eds.), Ecotoxicology of Metals in Invertebrates, Lewis, Boca Raton, FL, pp. 245–289.
Eisler, R., 1981. Trace Metal Concentrations in Marine Organisms, Pergamon Press, New York.
Engel, D.W., 1987. Metal regulation and molting in the blue crab, Callinectes sapidus: copper, zinc and metallothionein. Biol. Bull. 172, 69–82.
Engel, D.W., Brouwer, M., 1991. Short-term metallothionein and copper changes in blue crabs at ecdysis. Biol. Bull. 180, 447–452.
Engel, D.W., Brouwer, M., 1993. Crustaceans as models for metal metabolism: I. Effects of the molt cycle on blue crab metal metabolism and metallothionein. Mar. Environ. Res. 35, 1–5.
George, S.G., Olsson, P.E., 1994. Metallothioneins as indicators of trace metal pollution. In: Kramer, K.J.M. (Ed.), Biomonitoring of Coastal Waters and Estuaries, CRC Press, Boca Raton, FL, pp. 151–178. Hamza-Chaffai, A., Cosson, R.P., Amiard-Triquet, C., El Abed, A., 1995. Physicochemical forms of storage of
metals (Cd, Cu and Zn) and metallothionein-like proteins in gills and liver of marine fish from the Tunisian coast: ecotoxicological consequences. Comp. Biochem. Physiol. 111C (2), 329–341.
Hopkin, S.P., Nott, J.A., 1980. Studies on the digestive cycle of the shore crab Carcinus maenas (L.) with special reference to the B cells in the hepatopancreas. J. Mar. Biol. Ass. UK 60, 891–907.
Jennings, J.R., Rainbow, P.S., 1979. Studies on the uptake of cadmium by the crab Carcinus maenas in the laboratory. I. Accumulation from seawater and a food source. Mar. Biol. 50, 131–139.
Jop, K.M., Biever, R.C., Hoberg, J.R., Sheperd, S.P., 1997. Analysis of metals in blue crabs, Callinectes
sapidus, from two Connecticut estuaries. Bull. Environ. Contam. Toxicol. 58, 311–317.
Langston, W.J., Spence, S.K., 1995. Biological factors involved in metal concentrations observed in aquatic organisms. In: Tessier, A., Turner, D.R. (Eds.), Metal Speciation and Bioavailability in Aquatic Systems, Wiley, Chichester, pp. 407–467.
Lowry, O.H., Rosenbrough, N.J., Farr, A.L., Landall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 262–275.
Mantoura, R.F.C., Dickson, A., Riley, J.P., 1978. The complexation of metals with humic materials in natural waters. Estuar. Coast. Mar. Sci. 6, 387–408.
Mason, A.Z., Jenkins, K.D., 1995. Metal detoxication in aquatic organisms. In: Tessier, A., Turner, D.R. (Eds.), Metal Speciation and Bioavailability in Aquatic Systems, Vol. 3, Wiley, Chichester, pp. 479–608. Montgomery, D.C., Peck, E.A., 1982. In: Introduction To Linear Regression Analysis, Wiley, New York, pp.
167–171.
Mouneyrac, C., Amiard, J.C., Amiard-Triquet, C., 1998. Effects of natural factors (salinity and body weight) on cadmium, copper, zinc and metallothionein-like protein levels in resident populations of oysters
Nassiri, Y., Rainbow, P. S., Amiard-Triquet, C., Rainglet, F., Smith, B.D. Trace metal detoxification in the ventral caeca of Orchestia gammarellus (Crustacea: Amphipoda). Mar. Biol., in press.
Neter, J., Kutner, M.H., Nachtsheim, C.J., Wasserman, W., 1996. Applied Linear Statistical Models, 4th Edition, Richard D. Irwin, Burr Ridge, Il.
Nimmo, D.W.R., Lightner, D.V., Bahner, L.H., 1977. Effects of cadmium on the shrimps Penaeus duorarum,
Palaemonetes pugio and P. vulgaris. In: Vernberg, F.J. et al. (Ed.), Physiological Responses of Marine Biota to Pollutants, Academic Press, New York, pp. 131–183.
NRC, 1980. The international mussel watch. Report of a workshop sponsored by the environmental studies board. Commission Natural Resources.
Nugegoda, D., Rainbow, P.S., 1989a. Effects of salinity changes on zinc uptake and regulation by the decapod crustaceans Palaemon elegans and Palaemonetes varians. Mar. Ecol. Prog. Ser. 51, 57–75.
Nugegoda, D., Rainbow, P.S., 1989b. Salinity, osmolality and zinc uptake in Palaemon elegans (Crustacea: Decapoda). Mar. Ecol. Prog. Ser. 55, 149–157.
O’Hara, J., 1973a. The influence of temperature and salinity on the toxicity of cadmium to the fiddler crab,
Uca pugilator. Fish. Bull. US 71, 149–153.
O’Hara, J., 1973b. Cadmium uptake by fiddler crabs exposed to temperature and salinity stress. J. Fish. Res. Bd Can. 30, 846–848.
Pedersen, K.L., Pedersen, S.N., Hojrup, P., Andersen, J.S., Roepstorff, P., Knudsen, J., Depledge, M.H., 1994. Purification and characterization of a cadmium-induced metallothionein from the shore crab Carcinus
maenas (L.). Biochem. J. 297, 609–614.
Pedersen, S.N., Pedersen, K.L., Hojrup, P., Depledge, M.H., Knudsen, J., 1996. Primary structures of decapod crustacean metallothioneins with special emphasis on freshwater and semi-terrestrial species. Biochem. J. 319 (3), 999–1003.
Pedersen, S.N., Lundebye, A.K., Depledge, M.H., 1997. Field application of metallothionein and stress protein biomarkers in the shore crab (Carcinus maenas) exposed to trace metals. Aquat. Toxicol. 37, 183–200. Phelps, H.L., Hetzel, E.W., 1987. Oysters size, age, and copper and zinc accumulation. J. Shellfish Res. 6 (2),
67–70.
Radhakrishnaiah, K., Suresh, A., Sivaramakrishna, B., 1991. Size and sex related study on cadmium accumulation in different organs of the freshwater field crab, Oziotelphusa senex senex (Fabricius). Proc. Ind. Natl. Sci. Acad. (B. Biol. Sci.) 57 (5), 347–352.
Rainbow, P.S., 1985. Accumulation of Zn, Cu and Cd by crabs and barnacles. Estuar. Coastal Shelf Sci. 21, 669–686.
Rainbow, P.S., 1995. Physiology, physicochemistry and metal uptake. A crustacean perspective. Mar. Pollut. Bull. 31 (1–3), 55–59.
Rainbow, P.S., 1997a. Trace metal accumulation in marine invertebrates: marine biology or marine chemistry? J. Mar. Biol. Ass. UK 77, 195–210.
Rainbow, P.S., 1997b. Ecophysiology of trace metal uptake in crustaceans. Estuar. Coastal Shelf Sci. 44, 169–175.
Rainbow, P.S., 1998. Phylogeny of trace metal accumulation in crustaceans. In: Langston, W.J., Bebianno, M.J. (Eds.), Metal Metabolism in Aquatic Environments, Chapman and Hall, London, pp. 285–319.
Rainbow, P.S., Malik, I., O’Brien, P., 1993. Physicochemical and physiological effects on the uptake of dissolved zinc and cadmium by the amphipod crustacean Orchestia gammarellus. Aquat. Toxicol. 25, 15–30.
Rainbow, P.S., Amiard-Triquet, C., Amiard, J.C., Smith, B.D., Best, S.L., Nassiri, Y., Langston, W.J., 1999. Trace metal uptake rates in crustaceans (amphipods and crabs) from coastal sites in NW Europe differentially enriched with trace metals. Mar. Ecol. Prog. Ser. 183, 189–203.
Ray, S., McLeese, D.W., Waiwood, B.A., Pezzack, D., 1980. The disposition of cadmium and zinc in Pandalus
montagui. Arch. Environm. Contam. Toxicol. 9, 675–681.
`
RNO, 1995. Les contaminants dans la matiere vivante. In: Anonymous (Ed.), Surveillance du milieu marin. Min Environ, Paris and IFREMER, Nantes, pp. 9–24
Simkiss, K., Taylor, M.G., 1989. Convergence of cellular systems of metal detoxification. Mar. Environ. Res. 28, 211–214.
Thompson, J.A.J., Cosson, R.P., 1984. An improved electrochemical method for the quantification of metallothionein in marine organisms. Mar. Environ. Res. 11, 137–152.
Wright, D.A., Zamuda, C.D., 1987. Copper accumulation by two bivalve molluscs: salinity effects is independent of cupric ion activity. Mar. Environ. Res. 23, 1–14.
21 21 21
Zatta, P., 1985. Interaction between Zn , Co , Mn with hemocyanin from Carcinus maenas. Cah. Biol. Mar. 26 (3), 241–249.