Increased type II transforming growth factor-
b
receptor expression
in liver cells during cholesterol challenge
Giovanna Baccante
a, Gabriella Mincione
b, Concetta Di Febbo
a, Anna Coppa
c,
Domenico Angelucci
d, Domenico Lapenna
a, Franco Cuccurullo
a, Giulia Colletta
b,
Ettore Porreca
a,*
aDepartment of Internal Medicine,Uni
6ersity of Chieti,Medical School,Chieti, Italy bDepartment of Oncology and Neuroscience,Uni
6ersity of Chieti,Medical School,Chieti, Italy cExperimental Medicine and Pathology,Uni6ersity of Rome‘La Sapienza,Medical School’,Rome, Italy
dDepartment of Pathology,Uni6ersity of Chieti,Medical School,Chieti, Italy
Received 21 June 1999; received in revised form 13 October 1999; accepted 2 November 1999
Abstract
A large body of evidences implicates transforming growth factor-b (TGF-b) in the pathogenesis of atherosclerosis. In this context, TGF-b receptor dysfunction has been suggested to be relevant. We tested the effect of hypercholesterolemia, a well-known risk factor for atherosclerosis, on liver type II TGF-b receptor (TbR-II) expression in atherosclerosis-susceptible C57BL/6 mouse strain fed atherogenic diet. In addition, the relationship between cholesterol and TbR-II expression was verified by cholesterol challenge on human hepatoma cell (HepG2) cultures. The susceptible C57BL/6 mice fed atherogenic diet exhibited significant mRNA and immunohistochemical TbR-II liver expression at 2, 5, 9 and 15 weeks as compared to animals fed a regular diet. The TbR-II profile on HepG2 resulted in a time-dependent increased expression when the cells were incubated with soluble free cholesterol, associated with an increased TGF-b-dependent biological activity as detected by luciferase assay of reporter gene. These data provide evidence for a cholesterol-dependent TbR-II induction that may play a potentially relevant role in the development of hypercholesterolemia and atherogenesis. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cholesterol; Transforming growth factor-breceptors; Atherosclerosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
Transforming growth factor-b (TGF-b) is a multi-functional cytokine involved in many physiological and pathological processes, including atherogenesis [1 – 4]. Progress towards elucidating the mode of action of TGF-b has been made with identification of its type I and type II receptors (TbR-I and TbR-II), the expres-sion of which has been demonstrated to be necessary to TGF-bsignal transduction [5 – 7]. Previous studies have suggested a relationship between cholesterol and the TGF-b system. Up-regulation of TGF-b system in the vascular and extravascular tissues during
hypercholes-terolemia has been reported [8 – 11]. In addition, an up-regulatory effect of TGF-b has been demonstrated on low density lipoprotein (LDL) receptor activity, on LDL receptor-mediated cholesterol metabolism in hu-man liver [12], and in vascular smooth muscle cells [13], suggesting a possible role for TGF-b in the regulation of processes related to cholesterol delivery and processing.
The purpose of this study was to investigate the role of TbR-II in hypercholesterolemic (HC) C57BL/6 mouse strain fed atherogenic diet [14] as compared to normocholesterolemic (NC) animals. We first evaluated TbR-II expression in the liver, a tissue that exhibits, during hypercholesterolemic diet, a substantial lipid accumulation [15]. Then we verified a possible relation-ship between cholesterol and TbR-II expression, by testing the effect of a cholesterol challenge in human hepatoma cell cultures (HepG2), a TGF-b responsive * Corresponding author. Present address: Centro Servizi Biomedici,
Universita` ‘G. D’Annunzio’, Facolta` di Medicina e Chirurgia, Via dei Vestini, 66013, Chieti, Italy. Tel./fax: +39-871-355-6720.
E-mail address:[email protected] (E. Porreca).
cell line [16], and a well-known model for cholesterol metabolism studies [17].
Our results provide evidence for in vivo and in vitro cholesterol-dependent up-regulation of TbR-II expres-sion associated with an increased TGF-b biological activity. These findings may give insight to the relation-ship between hypercholesterolemia and atherogenesis.
2. Material and methods
2.1. Mice and diets
C57BL/6 mice were purchased from Piccioni Labora-tory (Milan, Italy). All the mice were female, 6 – 12 weeks of age at the time of experiment. The control diet was Purina Chow (Piccioni) containing 4% fat. The atherogenic diet (Piccioni) contained 15% fat, 1.25% cholesterol and 0.5% cholic acid. Animals were divided in two groups: (a) C57BL/6 female mice placed on the atherogenic diet (n=20) and (b) control C57BL/6 mice fed with standard chow (n=20). Groups of five ani-mals were killed after 2, 5, 9 and 15 weeks of hyperc-holesterolemic or control diet. For mRNA analysis, livers were collected, frozen in liquid nitrogen, and stored at −70°C. For immunohistochemical analysis, liver samples were fixed in 10% formalin and then routinely processed for paraffin embedding. Fasting total cholesterol was measured by standard colorimetric methods (Sigma, St Louis, MO). The experimental protocol was reviewed and approved by the University of Chieti Animal Care Committee.
2.2. Northern blot analysis
Total RNA was extracted from the frozen tissues using the guanidinium thiocyanate-phenol-chloroform method [18] and quantified spectrophotometrically at 260 nm. Total RNA (10 mg) was fractionated on a 1% formaldehyde agarose gel, transferred to nylon mem-branes (Hybond-N, Amersham, Little Chalfont, UK) and cross-linked by ultraviolet irradiation (254 nm, UV-Crosslinker, Amersham). The membranes were pre-hybridized and pre-hybridized at 42°C in a hybridization oven (Biotech, Rome, Italy) following the standard procedure. The blots were washed and signals were detected using autoradiography at −80°C with New RX films (Fuji Medical X-ray film). DNA probes were purified from agarose gel using the GeneClean method (BIO101, Vista, CA) and random primed with [32P]dCTP (10 mCi/ml, 370 MBq/ml; Amersham) at 2×108 cpm/mg with a Mega Prime Kit (Amersham). To evaluate the mRNA expression of TbR-II recep-tor, a 4.5-kb EcoRI fragment of H2-3FF plasmid [19] was used. The sizes of the transcripts were indicated as relative to 18S and 28S rRNA, which were assumed to
be 1.8 and 5.4 kb, respectively. Northern blots were scanned using a Molecular Imager System (BioRad Laboratories, Hercules, CA, USA) and the intensities of bands were estimated by peak area×mm relative to the GAPDH reporter gene (American Type Culture Collection, Rockville, MD).
2.3. Immunohistochemical analysis
Paraffin liver sections 3-mm thick were used for im-munohistochemical localization of TbR-II. Sections were deparaffinized and rehydrated in PBS (pH 7.2). Endogenous peroxidase activity was inactivated by in-cubation in 3% H2O2 for 5 min. Sections were then microwaved for 9 min in 10 mM sodium citrate (pH 6.0) to unmask the antigen sites. Normal goat serum was used to block non-specific sites. Slides were then incubated in a humidified chamber for 30 min at room temperature with L-21 rabbit anti human polyclonal antibody to TbR-II (1:250) (Santa Cruz Biotechnology, Santa Cruz, CA) which cross-reacts with mouse TbR-II. Antigen binding sites were visualized by incubation with biotinylated secondary antibody, followed by streptavidin-peroxidase complex, and peroxidase activ-ity was visualized with diaminobenzidine tetrachloride as cromogen. Negative control sections were processed in an identical manner by substitution of primary anti-body with normal rabbit IgG fraction. All negative control sections showed no color reactions.
2.4. Cell cultures
HepG2, a liver cell derived from human hepatoblas-toma (ATCC) has been found to express a wide variety of liver-specific metabolic functions including those re-lated to cholesterol metabolism [17]. HepG2 cells were plated at a density of 5×106cell/75 cm2 and grown to confluency in minimum essential medium supplemented with Earle’s salts,L-glutamine (2 mmol/l), penicillin (50 mg/ml), streptomycin (50 mg/ml) and FCS 10% in 95% air/5% CO2at 37°C. Preconfluent cell monolayers were washed three times with phosphate-buffered saline (PBS), incubated for 72 h in serum free media, washed with PBS three times and incubated with serum-free media supplemented with selected concentrations of soluble free cholesterol (Sigma, St Louis, MO). To evaluate a possible effect of cholesterol challenge on cell growth behavior, at the intervals specified, [3H]thimidine-uptake was tested as previously described at 24, 48 and 72 h [20]. No significant differences in HepG2 DNA synthesis were observed after incubation of cell cultures in the presence or in the absence of soluble free cholesterol. In addition, the level of [35
2.5. Western blot analysis
Protein lysates (50 mg per sample; total protein concentration was determined by a bicinchoninic acid protein assay; Pierce, IL, USA) were heated at 100°C for 5 min in the presence of 50 mM Tris – HCl pH 6.8, 100 mM b-mercaptoethanol, 2% SDS, 0.1% bro-mophenol blue, and 10% glycerol. Lysates were sepa-rated by polyacrylamide gel electrophoresis in the presence of SDS for 120 min at 100 V using mini-gel vertical apparatus (Bio-Rad Laboratories, Hercules, CA, USA). An 8% separating gel and a 5% stacking gel were used. The resolved proteins were transferred electrophoretically to nitrocellulose membranes (Amersham). The membranes were blocked with a so-lution of 5% non-fat milk in phosphate buffered sa-line and 0.1% Tween-20 (PBS/T), followed by incubation with antibody for 1 h at room tempera-ture. L-21 polyclonal antibody (Santa Cruz
Biotech-nology, Santa Cruz, CA) was used against TbR-II (1:1000). As a positive control we used RIIK4, a clone of wt v-k-ras transformed rat thyroid epithelial cell line overexpressing the TbR-II gene [22]. The membrane was extensively washed with PBS-T and then incubated with a horseradish peroxidase labeled anti-rabbit IgG diluted 1:3000 for 1 h at room tem-perature. The immune complex was visualized using the ECL Western blot detection system (Amersham). Western blots were scanned using a Molecular Imager System (BioRad Laboratories, Hercules, CA, USA).
2.6. Cell transfection and functional assays
HepG2 cells were seeded at 50 – 60% confluence in 24-well plates and cultured in 10% FCS as described above. Then 24 h after plating the cells were switched to serum free medium for an additional 48 h. Plasmid p3TP-lux containing the luciferase reporter gene un-der the control of the TGF-b responsive modified promoter for plasminogen activator inhibitor type-1 (PAI-1) was used to evaluate TGF-b induced cell re-sponse [23]. 3TP-lux promoter activity was assayed by transient transfections using lipofectamine plus reagent (Life Technologies, GIBCO BRL, Italy). Briefly, the transfection mixture containing 1 mg of p3TP-lux and 0.1 mg of pCMV-b-gal was mixed with the lipofectamine reagent. The following day, cells were incubated either in absence or presence of TGF-b (10 ng/ml) and cholesterol (100 mg/ml) for addi-tional periods of 24 and 48 h. Cells were lysed and transduced enzymatic activities were assayed. Luci-ferase activity was measured using the luciLuci-ferase assay system (Promega, Italy) and normalized to b-galac-tosidase expression.
2.7. Statistical analysis
All data are reported as mean9S.D. The signifi-cance of differences between the two groups was eval-uated by the Mann – Whitney U-test or Student’s test for unpaired data. One-way ANOVA and the Stu-dent – Newman – Keul’s test were performed when ap-propriate. A value of PB0.05 was considered statistically significant.
3. Results
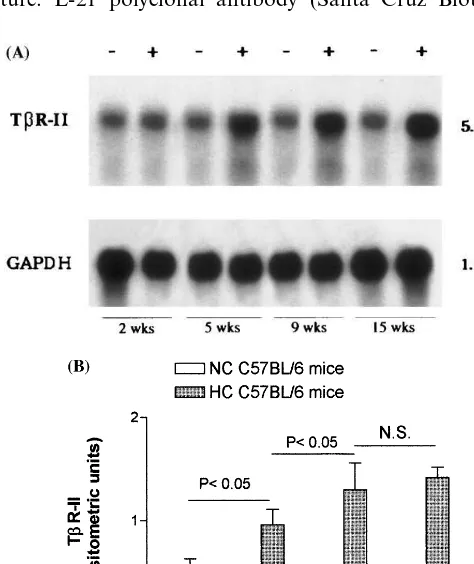
The average plasma cholesterol levels in C57BL/6 mice fed atherogenic diet were 197916, 184925, 200943, and 397990 mg/dl at 2, 5, 9, 15 weeks, respectively, compared to an average value of 6096 mg/dl in mice fed control diet at the same times. Fig. 1. Time course of liver TbR-II gene induction in HC C57BL/6
3.1. Li6er TbR-II expression
Fig. 1 (panel A) shows the time course of representa-tive Northern blot analysis of mice liver TbR-II mRNA. TbR-II mRNA expression was evaluated after 2, 5, 9, and 15 weeks of atherogenic and control diet. TbR-II transcripts were evident and significantly in-creased at 5 weeks, compared to control liver, and sustained up to 15 weeks. TbR-II contents in each HC and NC group (n=5) were normalized to that of GAPDH mRNA as described in Section 2 and ex-pressed as mean densitometric absorbance units (Fig. 1, panel B). The values of the densitometric ratios of TbR-II/GAPDH mRNA increased significantly at 5 and 9 weeks of atherogenic diet (2.4-fold and 3.2-fold, respectively, vs. controls) and no further significant increase at 15 weeks (3.4-fold) was observed as com-pared with liver from NC animals.
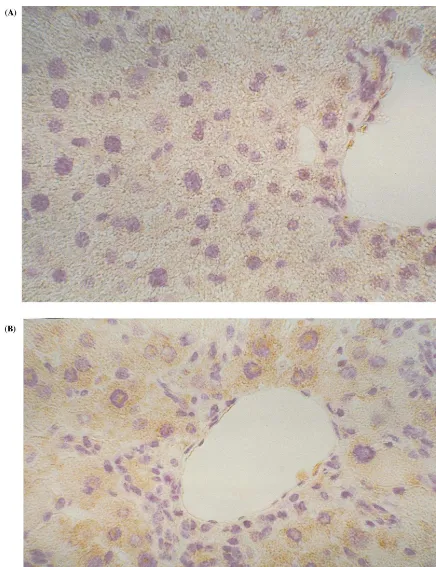
Because the mRNA expression of TbR-II appears increased in liver tissue of hypercholesterolemic mice, immunohistochemistry was also performed to verify the corresponding protein expression. The typical appearance of liver sections from HC animals im-munostained with antibody anti-TbR-II, after 15 weeks of atherogenic diet, is shown in Fig. 2. A significant immunostaining positivity for TbR-II was observed in the cytoplasm of liver cells after high-fat diet compared to a weak staining in the control hepato-cytes.
3.2. Cholesterol challenge of HepG2 cells
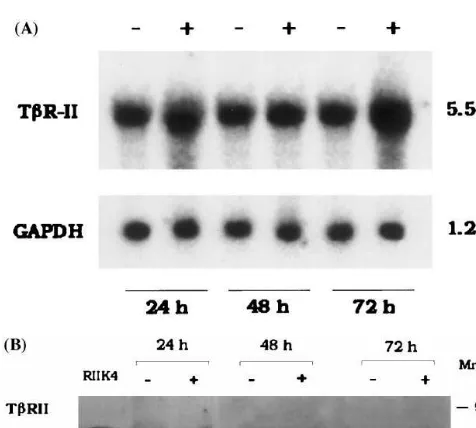
The increased TbR-II expression in liver as docu-mented by Northern blot and immunohistochemical analysis suggested further studies to determine whether TbR-II was expressed by liver cells in culture after exposure to cholesterol. HepG2 cells were assessed in unstimulated conditions and after continuous exposure to free cholesterol (100 mg/ml) at 24, 48 and 72 h of treatment. A representative time course experiment of changes in concentrations of TbR-II transcript and its relative protein as evaluated by Northern and Western blot analyses, respectively, is shown in Fig. 3 (panels A and B). After normalization with GAPDH reporter gene, a significant increase in TbR-II mRNA expression (3-fold) was found after 72 h of incubation time as compared to cells cultured in the absence of cholesterol. Densitometric analysis of Western blot showed a 2.5-fold increase in the amount of TbR-II in HepG2 cells cultured in the presence of cholesterol as compared to protein amount expressed in the absence of choles-terol (mean of three experiments). No significant changes were observed in mRNA and protein expres-sion by HepG2 cells at 24 and 48 h of cholesterol incubation compared to control cultures without cholesterol.
3.3. 3TP-lux reporter acti6ity in HepG2 cells
To evaluate a possible TGF-b-dependent biological effect for cholesterol-induced TbR-II changes, we used the TGF-bresponsive promoter-luciferase construct as-say, a highly sensitive and specific bioassay for TGF-b activity. A faint but significant increase in luciferase activity was found in cholesterol-treated cells after 24 h (3.5-fold); a further 1.5-fold increase was observed when HepG2 cells were co-incubated with TGF-b (10 ng/ml) and cholesterol (100 mg/ml) as compared to TGF-b alone (Fig. 4).
4. Discussion
The present study documents a novel effect of hyper-cholesterolemia on the upregulation of type II TGF-b receptor expression in dyslipidemic mice fed an athero-genic diet. We found a time-dependent increase in the levels of liver TbR-II transcripts during mouse choles-terol feeding as compared with liver of animals fed a control diet. Immunohistochemical staining of liver of HC mice showed a positive labeling pattern for TbR-II, compared to weak staining of NC animals. The in vivo findings were confirmed in HepG2 cultures, which showed an induction of type II TGF-breceptor mRNA and protein expression and an increased TGF-b-depen-dent activity after incubation with soluble free cholesterol.
Our findings are in line with previous observed up-regulation of TGF-b during experimental hypercholes-terolemia. Increased mRNA expression of TGF-b has been demonstrated in the aorta of Watanabe rabbit, in non-human primates during cholesterol feeding [8,9], and in the kidney of hypercholesterolemic rats [10,11]. In this context, the upregulated gene expression for TGF-b1 has been found to be associated with an increased extracellular matrix protein expression, sug-gesting a link between hypercholesterolemia and TGF-b-dependent activity. We found in HepG2 cultures an increase in TbR-II expression and TGF-b-dependent biological activity as evaluated by luciferase assay after cholesterol incubation. In fact, the increase in TbR-II gene expression, found after 24 – 72 h of cholesterol treatment, could account for the increase in luciferase activity, observed in the same conditions in hepatic cells. Thus, according to other studies in human myeloid leukemia cell lines HL-60 and U-936 [24,25], our findings seem to suggest that cholesterol challenge enhances liver cell sensitivity to TGF-band this may be involved in the regulation of the number of its receptors.
Fig. 2. Immunohistochemical analysis of TbR-II expression in the liver of NC and HC mice after 15 weeks of atherogenic diet. The cytoplasm of liver cells of NC animals displayed faint TbR-II immunolabeling (panel A). Conversely, hepatocytes of hypercholesterolemic mouse show more evident TbR-II expression (panel B). Representative sections are shown at high magnification (×25).
of the plasma cholesterol levels [26], the marked hepatic expression of TbR-II observed during hypercholes-terolemic status could suggest a TGF-b-dependent con-trol of cholesterol uptake. In this context, it has been
proliferation as well as in the synthesis of extracellular matrix proteins is well known [1,2,4,27]; consequently a
possible TGF-b-dependent antiproliferative and/or profibrotic effect could be suggested during hyperc-holesterolemia. Finally, since the liver plays a crucial role in the biosynthesis and secretion of hemostatic factors [28] and TGF-b may be involved in regulation of hepatic function [29,30], implications in hemostatic balance can be hypothesized as a consequence of cholesterol-dependent induction of TGF-b receptors. In conclusion, this report is the first to address the effect of cholesterol on in vivo and in vitro TbR-II expression. The effect on the cellular protein expression was associated with an increased mRNA gene expres-sion. This response may have relevant pathophysiologi-cal implications during hypercholesterolemia and atherogenesis.
Acknowledgements
This work was partially supported by the Italian National Research Council (CNR contract no. 97.04081.CT04) (E.P.), by MURST ex 40% and AIRC 1998 grants (G.C.) We thank Tommaso D’Antuono for technical assistance in immunohistochemical analysis and Alessandro Piccirelli for the densitometric analysis.
References
[1] Border WA, Ruoslahti E. Transforming growth factor-b in disease: the dark side of tissue repair. J Clin Invest 1992;90:1 – 7. [2] Border WA, Noble NA. Transforming growth factorbin tissue
fibrosis. New Engl J Med 1994;331:1286 – 92.
[3] Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801 – 9.
[4] Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. New Engl J Med 1994;330:1431 – 8.
[5] Massague´ J. Transforming growth factor-beta family. Annu Rev Cell Biol 1990;6:597 – 641.
[6] Dijke P, Miyazono K, Heldin CH. Signaling via hetero-oligomeric complexes of type I and type II serine/threonine kinase receptors. Curr Opin Cell Biol 1996;8:139 – 45.
[7] Graff JM, Bansal A, Melton DA. Xenopus MAD proteins transduce distinct subsets of signals for the TGF-bsuperfamily. Cell 1996;85:479 – 87.
[8] Lo`pez-Candales A, Scott MJ, Wickline SA. Cholesterol feeding modulates spatial expression of TGF-b1 and b2 in aortas of Watanabe rabbits. Cytokine 1995;7:554 – 61.
[9] Ross R, Masuda J, Raines EW, Gown AM, Katsuda S, Sasa-hara M, et al. Localization of PDGF-B protein in macrophages in all stages of atherogenesis. Science 1990;248:1009 – 12. [10] Ding G, Pesek-Diamond I, Diamond JR. Cholesterol,
macrophages, and gene expression of TGF-b1 and fibronectin during nephrosis. Am J Physiol 1993;264:577 – 84.
[11] Eddy AA. Interstitial inflammation and fibrosis in rats with diet-induced hypercholesterolemia. Kidney Int 1996;50:1139 – 49. [12] Moorby CD, Gherardi E, Dovey L, Godliman C, Bowyer DE. Transforming growth factor-b1 and interleukin-1b stimulate LDL receptor activity in HepG2 cells. Atherosclerosis 1992;97:21 – 8.
Fig. 3. Panel A: Representative Northern blot analysis of TbR-II mRNA in cholesterol-laden HepG2 cultures. Total RNA was ex-tracted from HepG2 cells grown in serum-free media (−) or media containing soluble free cholesterol 100mg/ml for 24, 48 and 72 h (+). Northern blots were hybridized with 32P-labeled TbR-II cDNA probe. mRNA was normalized by comparison to GAPDH. Panel B: Representative Western blot analysis of TbR-II protein expression in HepG2 cells. HepG2 cells were incubated for 24, 48 and 72 h in serum-free media (−) or media containing soluble free cholesterol 100mg/ml (+). RIIK4 represents control cell line positive for TbR-II expression as indicated in Section 2. After electrophoresis bound antibody to TbR-II was detected with a horseradish peroxidase-la-beled conjugated anti-rabbit IgG. Arrows indicate positions of spe-cific immunoreactive proteins.
[13] Nicholson AC, Hajjar DP. Transforming growth factor-beta up-regulates low density lipoprotein receptor-mediated choles-terol metabolism in vascular smooth muscle cells. J Biol Chem 1992;267:25982 – 7.
[14] Breslow JL. Mouse model of atherosclerosis. Science 1996;272:685 – 8.
[15] Liao F, Andalibi A, deBeer FC, Fogelman AM, Lusis AJ. Genetic control of inflammatory gene induction and NF-kB-like transcription factor activation in response to an atherogenic diet in mice. J Clin Invest 1993;91:2572 – 9.
[16] Hayashi H, Abdollah S, Qui Y, Cai J, Xu YY, Grinnell BW, et al. The MAD-related protein Smad7 associates with the TGF-beta receptor and functions as an antagonist of TGFTGF-beta signal-ing. Cell 1997;89:1165 – 73.
[17] Javitt NB. Hep G2 cells as a resource for metabolic studies: lipoprotein, cholesterol, and bile acids. FASEB J 1990;4:161 – 8. [18] Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocynate-phenol-chloroform extraction. Anal Biochem 1987;162:156 – 9.
[19] Lin HY, Wang XF, Ng-Eaton E, Weinberg RA, Lodish HF. Expression cloning of the TGF-btype II receptor, a functional transmembrane serine/threonine kinase. Cell 1992;68:775 – 85. [20] Porreca E, Ucchino S, Di Febbo C, Di Bartolomeo N, Angelucci
D, Napolitano AM, et al. Antiproliferative effect of desferriox-amine on vascular smooth muscle cells in vitro and in vivo. Arterioscler Thromb 1994;14:294 – 9.
[21] Binsack R, Stegmeier K, Dorge L, Volkl A. Bezafibrate down regulates fibrinogen biosynthesis in human hepatoma HepG2 cells. Eur J Clin Invest 1998;2:151 – 63.
[22] Turco A, Coppa A, Scarpa S, Palumbo C, Baccheschi G, Colletta G. Overexpression of TbRII receptor in neuroblastoma cells reduces growth rate and induces neuronal differentiate phenotype. Int J Cancer 1998;80:85 – 91.
[23] Ca`rcamo J, Weis FMB, Ventura F, Weiser R, Wrana JL, Attisano L, et al. Type I receptor specifity growth inhibitory and transcriptional responses to TGF-band activin. Mol Cell Biol 1994;14:3810 – 21.
[24] Taipale J, Matikainen S, Hurme M, Keski-Oja J. Induction of transforming growth factorb1 and its receptor expression during myeloid leukemia cell differentiation. Cell Growth Differ 1994;5:1309 – 19.
[25] Wells RC, Yankelev H, Lin HY, Lodish HF. Biosynthesis of the type I and type II TGF-b receptors. Implication for complex formation. J Biol Chem 1997;272:11444 – 51.
[26] Brown MS, Goldstein JL. Lipoprotein receptors in the liver: control signals for plasma cholesterol traffic. J Clin Invest 1983;72:743 – 7.
[27] Carey DJ. Control of growth and differentiation of vascular cells by extracellular matrix proteins. Annu Rev Physiol 1991;53:161 – 77.
[28] Fair DS, Marlar RA. Biosynthesis and secretion of factor VII, protein C, protein S, and protein C inhibitor from a human hepatoma cell line. Blood 1986;67:64 – 8.
[29] Hopkins WE, Fujii S, Sobel BE. Synergistic induction of plasmi-nogen activator inhibitor type-1 in HEP G2 cells by thrombin and transforming growth factor-beta. Blood 1992;79:75 – 81. [30] Hassan J, Chelucci C, Peschle C, Sorrentino V. Transforming
growth factor-b(TGF-b) inhibits expression of fibrinogen and factor VII in a hepatoma cell line. Thromb Haemost 1992;67(4):478 – 83.