Development of Stress Reactivity
Christian Caldji, Josie Diorio, and Michael J. Meaney
Naturally occurring variations in maternal care in early postnatal life are associated with the development of individual differences in behavioral and hypothalamic– pituitary–adrenal responses to stress in the rat. These effects appear to be mediated by the influence of maternal licking/grooming on the development of central systems that serve to activate (corticotropin-releasing factor) or inhibit (g-aminobutyric acid) the expression of behavioral and endocrine responses to stress through effects on forebrain noradrenergic systems. Importantly, individual differences in maternal care are transmitted from mother to daughter, providing a mechanism for the behavioral transmission of individual differences in stress reactivity across generations. Biol Psychiatry 2000;48:1164 –1174
© 2000 Society of Biological Psychiatry
Key Words: Maternal care, stress reactivity, GABAA
receptor, corticotropin-releasing factor
Introduction
S
tress is a risk factor for a variety of illnesses, ranging from metabolic and cardiovascular disorders to mental illness. The pathways by which stressful events can promote the development of such divergent forms of illness involve the same hormones that ensure survival during a period of stress (Brindley and Rolland 1989; Dallman et al 1995; De Kloet et al 1998; McEwen 1998; McEwen and Stellar 1993; Munck et al 1984). Stress-induced increases in sympathoadrenal release of cat-echolamines, adrenaline and noradrenaline, and adrenal glucocorticoids orchestrate a move to catabolism, increas-ing lipolysis and mobilizincreas-ing glucose reserves. These ac-tions serve to increase the availability and distribution of energy substrates. There are also emotional and cognitive responses to stressors that are mediated by adrenal hor-mones as well as the activation of central corticotropin-releasing factor (CRF) systems (Bakshi et al 2000;Nem-eroff 1996). During stress, feelings of apprehension and fear predominate; individuals become hypervigilant. The level of attention directed to the surrounding environment is increased at the expense of the ability to concentrate on tasks not related to the stressor (e.g., Arnsten 1998). Glucocorticoids act on brain structures such as the hip-pocampus and amygdala to disrupt episodic memory (Lupien et al 1998). At the same time, glucocorticoids and catecholamines act on areas of the brain, such as the amygdala, to enhance learning and memory for emotion-ally relevant stimuli (e.g., Cahill and McGaugh 1998; Davis et al 1997; Pitkanen et al 1998; Quirarte et al 1997). Although these responses are highly adaptive, chronic activation of these systems can promote the development of hyperlipidemia, hypertension, chronic immunosuppres-sion and decreased viral resistance, states of anxiety and dysphoria, sleep disorders, etc. (Chrousos and Gold 1992; McEwen 1998; Nemeroff 1996; Rosmond et al 1998).
Despite the profound risk associated with chronic stress, it is clear that measures of life events take us only so far in understanding why and when certain individuals de-velop chronic illness. Simply put, under comparable levels of environmental demand, some individuals fall ill and others do not. Recent findings on posttraumatic stress disorder (PTSD) underscore this point. There is consider-able variation in the rates of PTSD associated with such obvious trauma as violent assault (e.g., Breslau et al 1998) and evidence for high-risk populations (Yehuda et al 1998). These findings suggest the existence of a predis-position such that, when stress meets vulnerability, disease is a more likely outcome. The next and obvious question concerns the origin and nature of this predisposition.
The quality of the early family environment can serve as a major source of vulnerability in later life. Individuals who are the victims of physically or sexually abusive families are at considerably greater risk for mental illness in adulthood (e.g., Bifulco et al 1991; Brown and Ander-son 1993; McCauley et al 1997). Perhaps somewhat surprisingly, persistent emotional neglect or conditions of harsh, inconsistent discipline serve to increase the risk of depression and anxiety disorders to a level comparable to that observed in more obvious cases of abuse (Holmes and Robins 1987, 1988). Indeed, low scores on parental
From the Developmental Neuroendocrinology Laboratory, Douglas Hospital Re-search Center, McGill University, Montre´al, Canada.
Address reprint requests to Michael J. Meaney, Ph.D., McGill University, Douglas Hospital Research Centre, 6875 LaSalle Boulevard, Montre´al, Que´bec H4H 1R3, Canada.
Received April 10, 2000; revised October 19, 2000; accepted October 26, 2000.
bonding scales, reflecting cold and distant parent– child relationships, also significantly increase the risk of depres-sion in later life (e.g., Canetti et al 1997; Parker 1981). Children need not be beaten to be compromised. And the risk is not unique to mental health. Russak and Schwartz (1997), in a 35-year follow-up of populations in the Harvard Stress Mastery Study, found that by midlife those individuals who as undergraduate students rated their relationship with parents as cold and detached had a fourfold greater risk of chronic illness, including not only depression and alcoholism, but also heart disease and type II diabetes.
Thus, individual differences in parental care are related to the health of the offspring. We have argued that this effect is, in part at least, mediated by parental influences on the development of neural systems that underlie the expression of behavioral and endocrine responses to stress (Coplan et al 1996; DeBellis et al 1994; Francis and Meaney 1999; Helm et al 1977; Higley et al 1991; Kraemer et al 1989; Meaney et al 1996; Seckl and Meaney 1994). Parental rearing that results in enhanced reactivity to stress appears to increase the risk for illness in later life (e.g., Heim et al 1977). The cornerstone of this argument is the fact that increased levels of stress hormones, notably the glucocorticoids and catecholamines, can promote the development of multiple forms of chronic illness (see above and Chrousos and Gold 1992; McEwen 1998; Owens and Nemeroff 1994; Sapolsky 1994). Although the stress hormones have emerged as reasonable mediators of the relationship between stress and disease, the critical question here concerns the nature of the underlying pre-disposition and its relationship to early life events.
Maternal Care and the Development of
Individual Differences in Stress Reactivity
We have attempted to address this issue with studies on the role of maternal care in neural development in the rat. Interestingly, there are substantial, naturally occurring variations in maternal licking/grooming in rat dams. Ma-ternal licking/grooming of pups occurs most frequently while the mother nurses in the arched-back position, so as you might imagine, the frequency of the two behaviors are closely correlated (r 5 1.91; 30) across mothers. The differences in maternal behavior in the high- and low-licking/grooming arched-back nursing mothers are not unique to the first litter (Francis et al 1999b). Across a group of 22 dams we found a correlation of1.87 between the licking/grooming of the first and second litters. These findings are comparable to those of primate studies in which individual differences in maternal behavior re-mained consistent across infants (e.g., Fairbanks 1996; Maestripieri 1999). In one series of studies, mothers were
divided into two groups: high- or low-licking/grooming arched-back nursing. Note there were no differences between these groups in the overall amount of time in contact with pups (Caldji et al 1998; Liu et al 1997). We then allowed the offspring to grow to adulthood and examined hypothalamic–pituitary–adrenal (HPA) and be-havioral responses to stress (Liu et al 1997). As adults, the offspring of high-licking/grooming arched-back nursing mothers showed reduced plasma adrenocorticotropic hor-mone and corticosterone responses to restraint stress. The offspring of the high-licking/grooming arched-back nurs-ing mothers showed a more modest HPA response to stress. In the adult rat, glucocorticoids act at specific receptor sites within selected brain regions, such as the hippocampus, to decrease CRF synthesis in paraventricu-lar nucleus of the hypothalamus (PVNh) neurons (De Kloet et al 1998; Jacobson and Sapolsky 1991). The high-licking/grooming arched-back nursing animals also showed significantly increased hippocampal glucocorti-coid receptor messenger RNA (mRNA) expression, en-hanced glucocorticoid negative feedback sensitivity, and decreased hypothalamic CRF mRNA levels. Moreover, the magnitude of the corticosterone response to acute stress was significantly correlated with the frequency of both maternal licking/grooming (r5 2.61) and arched-back nursing (r5 2.64) during the first 10 days of life, as was the level of hippocampal glucocorticoid receptor mRNA and hypothalamic CRF mRNA expression (all
rs . .70).
The offspring of the high- and low-licking/grooming arched-back nursing mothers also differed in behavioral responses to novelty (Caldji et al 1998; Francis et al 1999b). As adults, the offspring of the low-licking/groom-ing arched-back nurslow-licking/groom-ing rats showed increased startle responses, decreased open field exploration, and longer latencies to eat food provided in a novel environment, reflecting a greater fear of novelty. These findings are consistent with the results of the classic studies of Levine and Denenberg (Denenberg et al 1963; Hess et al 1969; Levine 1957, 1962; Levine et al 1967) showing that perhaps the greatest effect of early environmental events is on the development of responses to conditions of threat (implied danger).
neuronal firing and noradrenaline release (Gray and Bingaman 1996; Koegler-Muly et al 1993; Lavicky and Dunn 1993; Valentino et al., 1998) and behavioral states of fear (e.g., Butler et al 1990; Liang et al 1992). Predictably, stress-induced increases in PVNh levels of noradrenaline were significantly higher in the offspring of the low-licking/grooming arched-back nursing offspring (Caldji et al 1999; Liu et al 2000; for a recent review on the CRF–noradrenaline systems in these animals, see Francis et al 1999a).
Maternal Care and the
g
-Aminobutyric
Acid (GABA)
AReceptor Complex
The effects of maternal care are also apparent on systems that normally serve to inhibit emotional, behavioral, and endocrine responses to stress. The most potent source of such inhibitory regulation is the GABAergic system. In recent studies we have described the impressive effects of variations in maternal care on the development of the forebrain GABAergic system. One interesting feature of these studies is the regional specificity of these effects, which prominently feature the amygdala and the locus coeruleus.
The GABAAreceptor complex, which includes a
ben-zodiazepine-binding site, is comprised of multiple sub-units most commonly arranged in a pentameric structure (for a recent review, see Mehta and Ticku 1999). The GABAAreceptor complex is comprised ofa(at least six
variants),b(at least three variants), and g (at least three variants) subunits, with more recently identifiedd, e, r, and p subunits. In the rat forebrain the native GABAA
receptor complexes most often take the form of twoa, two b, and one g subunit or of two a, one b, and two g subunits. It is thought that the interface between theaand gsubunits forms the benzodiazepine receptor site. Thus, receptor complexes with twoaand twogsubunits would have two benzodiazepine-binding sites (e.g., Khan et al 1996). Subunit composition can be critical. Thea4 subunit does not appear to participate in the formation of a functional benzodiazepine receptor site. Likewise theg3 subunit does not confer benzodiazepine binding. In addi-tion, the a subunits confer varying levels of affinity for GABA upon the GABAAreceptor.
In earlier studies (Caldji et al 2000) we found that repeated periods of prolonged maternal separation resulted in decreased GABAAreceptor binding, as well as
dimin-ished central (type I) benzodiazepine receptor levels. These effects are associated with alterations in some of the GABAA receptor subunits noted above. Although
mater-nal separation studies provide an interesting insight into the potential role of mother–infant interactions in devel-opment, prolonged states of deprivation are highly
disrup-tive to normal physiology. The results of more recent studies have focused on the offspring of high- and low-licking/grooming arched-back nursing mothers to more clearly define the relationship between maternal care and the development of the GABAergic system.
We have examined GABAA receptor binding in the
adult offspring of high- and low-licking/grooming arched-back nursing mothers. The results revealed significantly higher levels of GABAAreceptor binding in the offspring
of high-licking/grooming arched-back nursing mothers. In earlier studies we found increased central benzodiazepine receptor (type I) binding in the basolateral and central nuclei of the amygdala as well as the locus coeruleus. These findings provide a mechanism for increased GABAergic inhibition of amygdala–locus coeruleus re-sponsivity to stress.
A series of situ hybridization studies have offered insight into the molecular mechanism for these differences in receptor binding and suggest that variations in maternal care might actually permanently alter the subunit compo-sition of the GABAA receptor complex in the offspring.
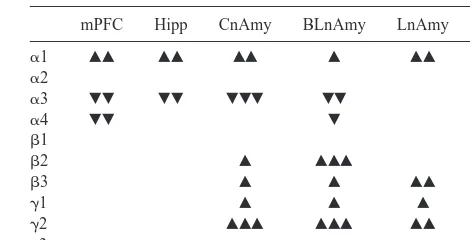
These findings are summarized in Table 1. The most obvious differences in relation to the receptor binding profile lie in the levels of the mRNAs for the g1 and g2 subunits. Both subunits actively contribute to the forma-tion of a funcforma-tional central benzodiazepine-binding site. Note that a1 and g2 mRNA levels are increased in the amygdala and the locus coeruleus of the adult offspring of high-licking/grooming arched-back nursing mothers. There are no differences in the mRNA levels for theg3 subunit, which does not appear to contribute to a func-tional central benzodiazepine-binding site. Note that we have not, as yet, examined the expression of the short and long variants of theg2 subunit. These findings provide a Table 1. A Summary of Differences ing-Aminobutyric Acid A Receptor Subunit Messenger RNA Expression across Multiple Brain Regions
mPFC Hipp CnAmy BLnAmy LnAmy LC
a1 ŒŒ ŒŒ ŒŒ Œ ŒŒ Œ
a2
a3
a4
b1
b2 Œ ŒŒŒ ŒŒŒ
b3 Œ Œ ŒŒ Œ
g1 Œ Œ Œ
g2 ŒŒŒ ŒŒŒ ŒŒ Œ
g3
The results are summarized from experiments comparing the adult offspring of high- and low-licking/grooming arched-back nursing mothers.
mPFC, medial prefrontal cortex; Hipp, hippocampus; CnAmy, central nucleus of the amygdala; BLnAmy, basolateral nucleus of the amygdala; LnAmy, lateral nucleus of the amygdala; LC, locus coeruleus;ŒŒ, high.low at p,.01;Œ, high.low at p,.05;, low.high at p,.01;, high,low at p,
mechanism for the increased central benzodiazepine re-ceptor binding in the high-licking/grooming arched-back nursing offspring. But such differences are not unique to thegsubunits.
Levels of a1 mRNA are significantly higher in the amygdala and locus coeruleus of high-licking/grooming arched-back nursing offspring. Similar differences are also apparent in the medial prefrontal cortex. Thea1 subunit appears to provide the most efficient form of the GABAA
receptor complex, through increased receptor affinity for GABA. The a6 subunit appears largely confined to the cerebellum. However, there are effects of maternal care on the expression of the remaining a subunits and this is where, in our minds, the story becomes most intriguing.
After viewing thea1 subunit data it was tempting for us to ascribe the decreased GABAAreceptor binding simply
to a decreased level of subunit expression. This is not the case. The adult offspring of the low-licking/grooming arched-back nursing mothers actually show increased expression of the mRNAs for thea3 anda4 subunits in the amygdala and the locus coeruleus. Two features of these subunits are noteworthy. First, they confer upon the GABAAreceptor complex a reduced affinity for GABA,
relative to thea1 subunit. Second, thea4 subunit does not contribute to the formation of a central benzodiazepine receptor site.
These differences in GABAA receptor subunit
expres-sion are also reflected in the central benzodiazepine receptor binding. Interestingly, though the a3 subunit contributes to the formation of a benzodiazepine receptor binding site, those sites are of the type II rather than type I variety. [3H]Zolpidem can be used to distinguish type I
and type II benzodiazepine receptor sites, since this radioligand has little affinity for the type II receptor (e.g., Arbilla et al 1986). The previously reported differences in benzodiazepine receptor binding capacity between the adult offspring of high- and low-licking/grooming arched-back nursing mothers lie in the density of type I sites: differences in [3H]zolpidem binding map onto and, in fact,
exceed those observed using [3H]flunitrazepam, which
labels both type I and type II receptor sites. It appears as though thea1 subunit alone produces a type I benzodiaz-epine receptor site. GABAAreceptor complexes
contain-ing a3 or a5 subunits display type II benzodiazepine receptor pharmacology, although thea5-containing recep-tors lack high-affinity zolpidem binding (type I benzodi-azepine receptor; e.g., Hadingham et al 1993). The a4 subunit, as mentioned above, does not produce a benzo-diazepine receptor type of either variety (e.g., Khan et al 1996).
In conclusion, the differences in type I benzodiazepine receptor binding in the adult offspring of the high- and low-licking/grooming arched-back nursing mothers
ap-pear to emerge from tissue-specific differences in GABAA
receptor complex subunit composition. In the offspring of high-licking/grooming arched-back nursing mothers there is increased expression of thea1,g1, andg2 subunits, all of which contribute to the formation of GABAAreceptor
complexes with type I benzodiazepine receptor binding. In contrast, there is increased expression of the a3 and a4 subunits in the offspring of the low-licking/grooming arched-back nursing mothers. Both subunits result in reduced affinity for GABA and neither of these subunits contributes to type I benzodiazepine receptor variants. These subunit profiles in both groups actively contribute to the differences in type I benzodiazepine receptor bind-ing observed in the offsprbind-ing of the high- and low-lickbind-ing/ grooming arched-back nursing mothers. This is not simply
the case of a deficit in subunit expression in the offspring of the low-licking/grooming arched-back nursing mothers, but of an apparently active attempt to maintain a specific benzodiazepine receptor profile in selected brain regions.
And it is important to also reflect on the tissue specificity of these effects. It is as if maternal care during the first week of life permanently alters subunit expression and thus GABAAreceptor complex composition in adulthood,
specifically in brain regions that regulate stress reactivity. It is important to note that, to date, such differences have been described only at the level of mRNA. We are currently in the midst of studies, including those using immunoprecipitation of native receptors, that would con-firm that differences in gene expression are, in fact, associated with differences in GABAA receptor
composition.
Another critical question concerns the link between these differences in GABAA/benzodiazepine receptor
binding and the functional differences in stress reactivity that we observe between the offspring of high- and low-licking/grooming arched-back nursing mothers. Re-sults of knockout/knockdown models as well as pharma-cologic interventions suggest that such differences might indeed be associated with underlying differences in stress reactivity and, perhaps, with a vulnerability to stress-related anxiety disorders. This idea is certainly consistent with a wealth of studies on GABAA/benzodiazepine
the central nuclei of the amygdala. The results of recent studies have indicated that the CRF neurons within the amygdala might serve as a specific target for benzodiaz-epine effects. The benzodiazbenzodiaz-epine receptor agonist alpra-zolam has been shown to reduce CRF content in the locus coeruleus (Owens et al 1991). The amygdala is a signifi-cant source of the CRF in the region of the locus coeruleus (Gray et al 1989; Koegler-Muly et al 1993; van Bockstaele et al 1996). de Boer et al (1992) have shown that benzodiazepine administration attenuates the effects of intracerebroventricular CRF treatment, suggesting that the CRF system is a target for the anxiolytic effects of the benzodiazepines (also see Owens et al 1991; Swerdlow et al 1986). We have recently reported significantly elevated CRF mRNA in amygdalae of MS and, to a lesser extent, NH rats, as compared with H rats (P.M. Plotsky et al, unpublished observations). The same pattern was observed for CRF immunoreactivity in the locus coeruleus (also see Ladd et al 1996). Considering the importance of this amygdaloid CRF system in mediating behavioral re-sponses to novelty (Hitchcock and Davis 1986; Krahn et al 1988; Liang et al 1992), we propose that benzodiazepine– CRF interactions within the amygdala serve as a critical neural substrate for the the behavioral differences in response to novelty observed among H, NH, and MS rats. In addition to the amygdala, there is also evidence for the importance of benzodiazepine action at the level of the frontal cortex (Lippa et al 1979) for anxiolytic effects. It appears likely that a variety of structures are involved in mediating the anxiolytic effects of benzodiazepines de-pending upon the nature of the stressor (Gonzalez et al 1996). In this respect, since we have found alterations at several sites, the differences in GABAA/central
benzodi-azepine receptor binding as a function of early rearing condition could serve to influence a wide range of behav-ioral and endocrine responses.
These findings are also interesting in light of the substantial differences in HPA responses to stress in the adult offspring of high- and low-licking/grooming arched-back nursing mothers (Liu et al 1997). Hypothalamic– pituitary–adrenal responses to stress are mediated by the release of CRF from the PVNh. Interestingly, we found no differences in GABAA/central benzodiazepine receptor
binding in the PVNh. However, there were highly signif-icant differences in the noradrenergic cell body regions, including the locus coeruleus and the nucleus tractus solitarius. Noradrenergic input to the PVNh provides the major known source of activation for CRF synthesis and release (Pacak et al 1995; Plotsky 1991; Plotsky et al 1989). Thus, the increased GABAA/central
benzodiaz-epine receptor binding observed in these regions in the high-licking/grooming arched-back nursing offspring may also serve to dampen HPA responses to stress by
decreas-ing the magnitude of the noradrenergic signal. Precisely the same logic can be applied in consideration of the differences in behavioral responses to stress, considering the importance of the CRF–noradrenaline system in the expression of fear (Bakshi et al 2000; Dunn and Berridge 1990; Koob et al 1994).
Perhaps the most interesting data on this point emerge from human studies of benzodiazepine type I receptors. Malizia et al (1995) reported a significant decrease in forebrain type I benzodiazepine receptor levels using positron emission tomography with unmedicated patients with a history of panic disorder. Although these studies are preliminary, the findings are certainly consistent with those using pharmacologic measures of benzodiazepine receptor sensitivity. Glue et al (1995) found that subjects who were high on measures of neuroticism showed re-duced sensitivity to the benzodiazepine midazolam. In a series of studies Roy-Byrne and colleagues (1990, 1996) found reduced sensitivity to diazepam in patients with panic disorders and obsessive– compulsive disorders and proposed that the reduced central benzodiazepine sensitiv-ity was related to the phenomenon of anxiety. Although these studies have not directly shown decreased central benzodiazepine receptor levels, the findings are certainly consistent with the idea that decreased receptor levels in humans could be related to increased vulnerability to anxiety disorders. The hypothesis here is that there exists an endogenous anxiolytic ligand for the central benzodi-azepine receptor and that decreased central benzodiaz-epine-binding sites would thus result in enhanced fearful-ness in the face of threat. The results of at least one study directly relate the effects of rearing condition on central benzodiazepine receptor levels with those on behavior. Escorihuela et al (1992) found that postnatal handling improved performance in a test of a two-way avoidance task. The effect of handling was significantly reversed in animals treated acutely with the central benzodiazepine receptor antagonist RO 15-1788.
certainly very preliminary. Nevertheless, the differences in GAD expression suggest the possibility of differential GABA inhibition in the offspring of high- and low-licking/grooming arched-back nursing mothers.
Transmission of Individual Differences in
Stress Reactivity
Such effects of maternal behavior suggest that variations in maternal care might serve as a possible mechanism by which selected traits might be transmitted from one generation to the next. Interestingly, individual differences in maternal behavior show intergenerational transmission. The female offspring of high-licking/grooming arched-back nursing mothers showed significantly more licking/ grooming and arched-back nursing than did the female offspring of low-licking/grooming arched-back nursing mothers. The intergenerational transmission of parental behavior has also been reported in primates. In rhesus monkeys there is clear evidence for family lineages expressing child abuse (Maestripieri 1999). There is also evidence for transmission of individual differences in parental styles falling within the normal range. Fairbanks (1989, 1996) found that daughters who were reared by mothers who consistently spent a higher amount of time in physical contact with their offspring became mothers who were similarly more attentive to their offspring. In rhesus monkeys, Berman (1990) found that the rate of mothers rejecting their infants was correlated with the rejection rate of the mothers’ mothers. In primates, such individual differences in maternal behavior may be revealed in juvenile, nulliparous females. In all cases these findings were independent of the social rank of the mother. In humans, Miller et al (1997) found that scores on parental bonding measures between a mother and her daughter were highly correlated with the same measures of bonding between the daughter and her child. These findings sug-gest a perhaps common process of intergenerational trans-mission of maternal behavior. The critical question here concerns the mechanism underlying this intergenerational transmission of individual differences in behavior.
We (Francis et al 1999b) have provided evidence for a nongenomic transmission of individual differences in maternal behavior. In one study, we performed reciprocal cross-fostering of the offspring of low- and high-licking/ grooming arched-back nursing mothers. The primary con-cern here was that the wholesale fostering of litters between mothers is known to affect maternal behavior (Maccari et al 1995). To avert this problem and maintain the original character of the host litter, no more than two of 12 pups were fostered into or from any one litter. The critical groups of interest are the biological offspring of low-licking/grooming arched-back nursing mothers
fos-tered onto high-licking/grooming arched-back nursing dams, and vice versa. The control groups included 1) the offspring of low-licking/grooming arched-back nursing mothers fostered onto other low-licking/grooming arched-back nursing mothers as well as offspring of high-licking/ grooming arched-back nursing dams fostered onto other high-licking/grooming arched-back nursing mothers, 2) sham-adoption animals that were simply removed from the nest and fostered back to their biological mothers, and 3) unmanipulated pups. The limited cross-fostering design did not result in any effect on group differences in maternal behavior. Hence, the frequency of pup licking/ grooming and arched-back nursing across all groups of high-licking/grooming arched-back nursing mothers was significantly higher than that for any of the low-licking/ grooming arched-back nursing dams, regardless of litter composition.
The results of the behavioral studies are consistent with the idea that the variations in maternal care are causally related to individual differences in the behavior of the offspring. The biological offspring of low-licking/groom-ing arched-back nurslow-licking/groom-ing dams reared by high-licklow-licking/groom-ing/ grooming arched-back nursing mothers were significantly less fearful under conditions of novelty than were any of the offspring reared by low-licking/grooming arched-back nursing mothers, including the biological offspring of high-licking/grooming arched-back nursing mothers. A separate group of female offspring were then mated, allowed to give birth, and observed for differences in maternal behavior. The effect on maternal behavior fol-lowed the same pattern as that for differences for fearful-ness. As adults, the female offspring of low-licking/ grooming arched-back nursing dams reared by high-licking/grooming arched-back nursing mothers did not differ from normal, high-licking/grooming arched-back nursing offspring in the frequency of pup licking/groom-ing or arched-back nurslicking/groom-ing. The frequency of licklicking/groom-ing/ grooming and arched-back nursing in animals reared by high-licking/grooming arched-back nursing mothers was significantly higher than in any of the low-licking/groom-ing arched-back nurslow-licking/groom-ing groups, and again this included female pups originally born to high-licking/grooming arched-back nursing mothers but reared by low-licking/ grooming arched-back nursing dams. Individual differ-ences in fearfulness or maternal behavior mapped onto those of the rearing mother, rather than the biological mother.
As adults, the handled F2 offspring of such mothers resemble the offspring of high-licking/grooming arched-back nursing mothers, a finding that is consistent with the nongenomic transmission hypothesis. We then studied the F3 generation, focusing on the offspring of the handled and nonhandled F2 offspring of low-licking/grooming arched-back nursing mothers. Bear in mind, we refer to these mothers as low-licking/grooming arched-back nurs-ing because they are derived from low-licknurs-ing/groomnurs-ing arched-back nursing mothers themselves. The low-licking/ grooming arched-back nursing mothers with handled pups behave in a manner that is indistinguishable from high-licking/grooming arched-back nursing dams, as do their female offspring. We (Francis et al 1999b) found that the handled offspring of low-licking/grooming arched-back nursing mothers resemble the offspring of high-licking/ grooming arched-back nursing dams on measures of be-havioral and endocrine responses to stress, hypothalamic CRF, and hippocampal glucocorticoid receptor mRNA expression, as well as central benzodiazepine receptor binding. These findings suggest individual differences in gene expression can also be transmitted across generations via a nongenomic mechanism. Moreover, an environmen-tal manipulation that alters maternal behavior can then serve to affect neural development in subsequent genera-tions through the transmission of these alteragenera-tions in parental care.
These findings are consistent with the results of studies using the cross-fostering technique as a test for maternal-mediation hypotheses. For example, the spontaneously hypertensive rat (SHR) is a strain bred for hypertension, which appears in adolescence. Although the selective breeding suggests a genetic background, the expression of the hypertensive trait is also influenced by epigenetic factors (McCarty et al 1992). Spontaneously hypertensive rat pups reared by wild-type, WKY mothers do exhibit hypertension to the extent of kin reared by SHR dams. When borderline hypertensive rats, a hybrid formed by SHR–WKY matings, are reared by WKY mothers, they do not express the spontaneous hypertensive phenotype.
The potential effects of maternal behavior on the devel-opment of behavior and endocrine responses to stress in BALBc mice have been examined. The BALBc strain mice are normally very fearful and show elevated HPA responses to stress. However, BALBc mice cross-fostered to C57 mothers are significantly less fearful, with lower HPA responses to stress (Zaharia et al 1996). Importantly, C57 mothers normally lick and groom their pups about twice as frequently as BALBc mothers (Anisman et al 1998). Comparable findings have emerged with rat strains. Typically, Fisher 344 rats are more responsive to novelty and have increased HPA responses to acute stress relative to Long–Evans rats. Moore and Lux (1998) reported that
Long–Evans dams lick/groom their offspring significantly more often than do Fisher 344 mothers.
Under normal circumstances, of course, BALBc mice are reared by BALBc mothers. The genetic and environ-mental factors conspire to produce an excessively fearful animal. This is usually the reality of development. Be-cause parents provide both genes and environment for their biological offspring, the offspring’s environment is therefore, in part, correlated with their genes (e.g., West and King 1987). The reason why many epidemiologic studies based on linear regression models often find that the epigenetic factors, such as parental care, do not add predictive value above that of genetic inheritance is because of this correlation. The environment the parent provides commonly serves to enhance the genetic differ-ences—they are redundant mechanisms. The knowledge of an animal’s BALBc pedigree is sufficient to predict a high level of timidity in adulthood. Additional information on maternal care would statistically add little to the predictability—the two factors work in the same direction. But this is clearly different from concluding that the maternal care is not relevant, and the results of the cross-fostering studies attest to the importance of such epigenetic influences.
The value of this process is that it can provide for variation. If the genetically determined trajectory is not adaptive for the animal, then development moving in the direction of the current environmental signal would be of adaptive value. Hence, environmental events can alter the path of the genetically established trajectory in favor of more adaptive outcomes. This, of course, is the adaptive value of plasticity and the essence of epigenesis.
advan-tages of increased stress reactivity. In this environment, the males that were most successful in avoiding the pitfalls associated with such a “criminogenic” environment were those who were shy and somewhat timid. Under such conditions a parental rearing style that favored the devel-opment of a greater level of reactivity to threat would be adaptive. The obvious conclusion is that there is no single ideal form of parenting: different environments demand different traits in the offspring. A final issue here concerns the cost of such increased stress reactivity. The shy and timid child in the urban slum may be at an advantage with respect to the demands of the immediate environment. The question is whether such traits would later also confer an increased risk for stress-induced illness. We argue that they would and that this risk reflects the cost of adaptation to a high level of environmental demand, such as a low–socioeconomic status environment, in early life. This level of plasticity also suggests a certain degree of reversibility.
Conclusions
It may be surprising that rather subtle variations in maternal behavior have such profound impact on devel-opment. However, for a rat pup, the first weeks of life do not hold a great deal of stimulus diversity. Stability is the theme of the burrow, and the social environment in the first days of life is defined by the mother and littermates. The mother serves as a primary link between the environ-ment and the developing animal (Levine 1975). It seems reasonable that variations in mother–pup interaction would serve to have so much importance in development. Indeed, it appears that the mother serves to transduce information pertaining to the environment to her offspring. This situation may be more common than we imagine. In humans, the influence of poverty on child development appears to be mediated by effects on parental care: if the effects on parental care are statistically neutralized, there is no significant effect of socioeconomic status on child development (Conger et al 1994; Eisenberg and Earls 1975; McLoyd 1998). Hence, environmental effects on neural development during infancy, including systems that regulate stress reactivity in later life, may be commonly mediated through alterations in parental care.
Aspects of this work were presented at the conference “Genetics and Brain Function: Implications for the Treatment of Anxiety,” March 22–23, 2000, Washington, DC. The conference was jointly sponsored by the Anxiety Disorders Association of America (ADAA), the ADAA Scientific Advisory Board, and the National Institute of Mental Health.
References
Anisman H, Zaharia MD, Meaney MJ, Merali Z (1998): Do early life events permanently alter behavioral and hormonal re-sponses to stressors? Int J Dev Neurosci 16:149 –164. Arbilla S, Allen J, Wick A, Langer SZ (1986): High affinity
[3H]Zolpidem binding in the rat brain: An inidazopyridine with agonist properties at central benzodiazepine receptors.
Eur J Pharmacol 130:257–263.
Arnsten AF (1998): The biology of being frazzled. Science 280:1711–1712.
Bakshi VP, Shelton SE, Kalin NH (2000): Neurobiological correlates of defensive behaviors. Prog Brain Res 122:105– 115.
Berman CM (1990): Intergenerational transmission of maternal rejection rates among free-ranging rhesus monkeys on Cayo Santiago. Anim Behav 44:247–258.
Bifulco A, Brown GW, Adler Z (1991): Early sexual abuse and clinical depression in adult life. Br J Psychiatry 159:115–122. Breslau N, Kessler RC, Chilcoat HC, Schultz LR, Davis GC, Andreski P (1998): Trauma and posttraumatic stress disorder in the community. Arch Gen Psychiatry 55:626 – 632. Brindley DN, Rolland Y (1989): Possible connections between
stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clin Sci 77: 453– 461.
Brown GR, Anderson B (1993): Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. Am J Psychiatry 148:55– 61.
Butler PD, Weiss JM, Stout JC, Nemeroff CB (1990): Cortico-tropin-releasing factor produces fear-enhancing and behav-ioral activating effects following infusion into the locus coeruleus. J Neurosci 10:176 –183.
Cahill L, McGaugh JL (1998): Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci 21:294 – 299.
Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ (2000): The effects of early rearing environment on the development of GABAAand central benzodiazepine receptor levels and
novelty-induced fearfulness in the rat.
Neuropsychopharma-cology 22:219 –229.
Caldji C, Plotsky PM, Meaney MJ (1999): Maternal behavior in infancy regulates the in vivo release of noradrenaline in the paraventricular nucleus of the hypothalamus. Soc Neurosci
Abstr 25:616.
Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998): Maternal care during infancy regulates the development of neural systems mediating the expression of behavioral fearfulness in adulthood in the rat. Proc Natl
Acad Sci 95:5335–5340.
Canetti L, Bachar E, Galili-Weisstub E, De-Nour AK, Shalev AY (1997): Parental bonding and mental health in adoles-cence. Adolescence 32:381–394.
Chrousos GP, Gold PW (1992): The concepts of stress and stress system disorders. JAMA 267:1244 –1252.
Conger R, Ge X, Elder G, Lorenz F, Simons R (1994): Economic stress, coercive family process and developmental problems of adolescents. Child Dev 65:541–561.
S, Gorman JM, Nemeroff CB (1996): Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci U S A 93:1619 –1623. Dallman MF, Akana SF, Strack AM, Hanson ES, Sebastian RJ (1995): The neural network that regulates energy balance is responsive to glucocorticoids and insulin and also regulates HPA axis responsivity at a site proximal to CRF neurons. Ann
NY Acad Sci 771:730 –742.
Davis M, Walker DL, Lee Y (1997): Amygdala and bed nucleus of the stria terminalis: Different roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc
Lond B Biol Sci 352:1675–1687.
DeBellis MD, Chrousos GP, Dom LD, Burke L, Helmers K, Kling MA, et al (1994): Hypothalamic pituitary adrenal dysregulation in sexually abused girls. J Clin Endocrinol
Metab 78:249 –255.
de Boer SF, Katz JL, Valentino RJ (1992): Common mechanism underlying the proconflict effects of corticotropin-releasing factor, a benzodiazepine inverse agonist and electric foot-shock. J Pharmacol Exp Ther 262:335–342.
De Kloet ER, Vregdenhil E, Oitzl MS, Joels M (1998): Brain corticosteroid receptor balance in health and disease. Endocr
Rev 19:269 –301.
Denenberg VH, Zarrow MX, Kalberer WD, Farooq A, Ross S, Sawin PB (1963): Maternal behavior in the rabbit: Effects of environmental variation. Nature 197:161–162.
Dunn AJ, Berridge CW (1990): Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res Rev 15:71–100.
Eisenberg L, Earls FJ (1975): Poverty, social depreciation and child development. In: Hamburg DA, editor. American
Hand-book of Psychiatry. Vol. 6. New York: Basic Books, 275–291.
Escorihuela RM, Fernandez-Teruel A, Nunez FJ, Zapata A, Tobena A (1992): Infantile stimulation and the role of the benzodiazepine receptor system in adult acquisition of two-avoidance behavior. Psychopharmacology 106:282–284. Fairbanks LM (1996): Individual differences in maternal style.
Adv Stud Behav 25:579 – 611.
Fairbanks LY (1989): Early experience and cross-generational continuity of mother-infant contact in vervet monkeys. Dev
Psychobiol 22:669 – 681.
Farrington DA, Gallagher B, Morley L, St Ledger RJ, West DJ (1988): Are there any successful men from criminogenic backgrounds? Psychiatry 51:116 –130.
Francis D, Meaney MJ (1999): Maternal care and the develop-ment of stress responses. Curr Opin Neurobiol 9:128 –134. Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ
(1999a): The role of corticotropin-releasing factor–norepi-nephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry 46:1153–1166.
Francis DD, Diorio J, Liu D, Meaney MJ (1999b): Nongenomic transmission across generations in maternal behavior and stress responses in the rat. Science 286:1155–1158. Glue P, Wilson S, Coupland N, Ball D, Nutt D (1995): The
relationship between benzodiazepine receptor sensitivity and neuroticism. J Anxiety Disord 9:33– 45.
Gonzalez LE, Andrews N, File SE (1996): 5-HT1A and benzo-diazepine receptors in the basolateral amygdala modulate anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res 732:145–153.
Gray JA (1987): The Psychology of Fear and Stress. Cambridge, UK: Cambridge Publications.
Gray TS, Bingaman EW (1996): The amygdala: Corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol 10:155–168.
Gray TS, Carney ME, Magnuson DJ (1989): Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: Possible role in stress-induced adre-nocorticotropin release. Neuroendocrinology 50:433– 446. Haapasap J, Temblay RE (1994): Physically aggressive boys
from ages 6 to 12: Family background, parenting behavior, and prediction of delinquency. J Consult Clin Psychol 62: 1044 –52.
Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whitiing PJ (1993): Cloning of cDNA sequences encoding humana2 and a3g-aminobutyric acidAreceptor
sub-units and characterization of the benzodiazepine pharma-cology of recombinant a1-, a2-, a3- and a5-containing humang-aminobutyric acidAreceptors. Mol Pharmacol 43:
970 –975.
Heim C, Owens MJ, Plotsky PM, Nemeroff CH (1977): The role of early adverse life events in the etiology of depression and posttraumatic stress disorder: Focus on corticotropin-releas-ing factor. Ann N Y Acad Sci 821:194 –207.
Hess JL, Denenberg VH, Zarrow MX, Pfeifer WD (1969): Modification of the corticosterone response curve as a func-tion of handling in infancy. Physiol Behav 4:109 –112. Higley JD, Haser MF, Suomi SJ, Linnoila M (1991): Nonhuman
primate model of alcohol abuse: Effects of early experience, personality and stress on alcohol consumption. Proc Natl
Acad Sci U S A 88:7261–7265.
Hitchcock JM, Davis M (1986): Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav
Neu-rosci 100:11–22.
Hodges H, Green S, Glenn B (1987): Evidence that the amygdala is involved in benzodiazepine and serotonergic effects on punished responding, but not discrimination.
Psychopharma-cology 92:491–504.
Holmes SJ, Robins LN (1987): The influence of childhood disciplinary experience on the development of alcoholism and depression. J Child Psychol Psychiatry 28:399 – 415. Holmes SJ, Robins LN (1988): The role of parental disciplinary
practices in the development of depression and alcoholism.
Psychiatry 51:24 –36.
Jacobson L, Sapolsky RM (1991): The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenal axis. Endocr Rev 12:118 –134.
Khan ZU, Gutierrez A, De Blas AL (1996): The a1 and a6 sub-units can co-exist in the same cerebellar GABAA recep-tor maintaining their individual benzodiazepine-binding spec-ificities. J Neurochem 66:685– 691.
of the central amygdaloid nucleus and paraventricular nucleus of the hypothalamus. J Neuroendocrinol 5:95–98.
Koob GF, Heinrichs SC, Menzaghi F, Pich EM, Britton KT (1994): Corticotropin-releasing factor, stress and behavior.
Semin Neurosci 6:221–229.
Kraemer GW, Ebert MH, Schmidt DE, McKinney WT (1989): A longitudinal study of the effect of different social rearing conditions on cerebrospinal fluid norepinephrine and bio-genic amine metabolites in rhesus monkeys.
Neuropsycho-pharmacology 2:175–189.
Krahn DD, Gosnell BA, Levine AS, Morley JE (1988): Behav-ioral effects of corticotropin-releasing factor: Localization and characterization of central effects. Brain Res 443:63– 69. Ladd CO, Owens MJ, Nemeroff CB (1996): Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal depriviation. Endocrinology 137:1212–1218. Lavicky J, Dunn AJ (1993): Corticotropin-releasing factor
stim-ulates catecholamine release in hypothalamus and prefrontal cortex in freely moving rats as assessed by microdialysis.
J Neurochem 60:602– 612.
Levine S (1957): Infantile experience and resistance to physio-logical stress. Science 126:405– 406.
Levine S (1962): Plasma-free corticosteroid response to electric shock in rats stimulated in infancy. Science 135:795–796. Levine S (1975): Psychosocial factors in growth and
develop-ment. In: Levi L, editor. Society, Stress and Disease. London: Oxford University Press, 43–50.
Levine S, Haltmeyer GC, Karas GG, Denenberg VH (1967): Physiological and behavioral effects of infantile stimulation.
Physiol Behav 2:55– 63.
Liang KC, Melia KR, Miserendino MJ, Fails WA, Campeau S, Davis NI (1992): Corticotropin releasing factor: Long-lasting facilitation of the acoustic startle reflex. J Neurosci 12:2303– 2312.
Lippa AS, Critchett D, Sano MC, Klepner CA, Greenblat EN, Coupet J, Beer B (1979): Benzodiazepine receptors: Cellular and behavioral characteristics. Pharmacol Biochem Behav 10:831– 843.
Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ (2000): The effects of early life events on in vivo release of norepineph-erine in the paraventricular nucleus of the hypothalamus and hypothalamic-pituitary-adrenal responses during stress.
J Neuroendocrinol 12:5–12.
Liu D, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, et al (1997): Maternal care, hippocampal glucocorticoid receptor gene expression and hypothalamic-pituitary-adrenal responses to stress. Science 277:1659 –1662.
Lupien S, Sharma S, Nair NPV, Hauger R, McEwen BS, de Leon M, Meaney MJ (1998): Glucocorticoids and human brain aging. Nat Neurosci 1:69 –73.
Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M (1995): Adoption reverses the long-term impair-ments in glucocorticoid feedback induced by prenatal stress.
J Neurosci 15:110 –116.
Maestripieri D (1999): The biology of human parenting: Insights from nonhuman primates. Neurosci Biobehav Rev 23:411– 422.
Malizia AL, Coupland NJ, Nutt DJ (1995): Benzodiazepine receptor function in anxiety disorders. In: Biggo E, Sanna F,
Costa E, (editors). GABAA Receptors and Anxiety: From
Neurobiology to Treatment. New York: Ravin, 115–133.
McCarty R, Cierpial MA, Murphy CA, Lee JH (1992): Maternal involvement in the development of cardiovascular phenotype.
Experientia 48:315–322.
McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al (1997): Clinical characteristics of women with a history of childhood abuse: Unhealed wounds. JAMA 277:1362–1368.
McEwen BS (1998): Protective and damaging effects of stress mediators. New Engl J Med 338:171–179.
McEwen BS, Stellar E (1993): Stress and the individual: Mech-anisms leading to disease. Arch Intern Med 153:2093–2101. McLloyd VC (1998): Socioeconomic disadvantage and child
development. Am Psychol 53:185–204.
Meaney MJ, Diorio J, Widdowson J, LaPlante P, Caldji C, Seckl JR, Plotsky PM (1996): Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implica-tions for adrenocortical responses to stress. Semin Neurosci 18:49 –72.
Mehta AK, Ticku MJ (1999): An update on GABAA receptors.
Brain Res Rev 29:196 –217.
Miller L, Kramer R, Warner V, Wickramaratne P, Weissman M (1997): Intergenerational transmission of parental bonding among women. J Am Acad Child Adolesc Psychiatry 36: 1134 –1139.
Moore CL, Lux BA (1998): Effects of lactation on sodium intake in fischer-344 and Long-Evans rats. Dev Psychobiol 32:51– 56.
Munck A, Guyre PM, Holbrook NJ (1984): Physiological func-tions of glucocorticoids in stress and their relafunc-tions to pharmacological actions. Endocr Rev 5:25– 44.
Nemeroff CB (1996): The corticotropin-releasing factor (CRF) hypothesis of depression: New findings and new directions.
Mol Psychiatry 1:336 –342.
Owens MJ, Nemeroff CB (1994): Role of serotonin in the pathophysiology of depression: Focus on the serotonin trans-porter. Clin Chem 40:288 –295.
Owens MJ, Vargas MA, Knight DL, Nemeroff CB (1991): The effects of alprazoplam on corticotropin-releasing factor neu-rons in the rat brain: Acute time course, chronic treatment and abrupt withdrawal. J Pharmacol Exp Ther 258:349 –356. Pacak K, Palkovits M, Kopin I, Goldstein DS (1995):
Stress-induced norepinephrine release in hypothalamic paraventricu-lar nucleus and pituitary-adrenocortical and sympathoadrenal activity: In vivo microdialysis studies. Front
Neuroendocri-nol 16:89 –150.
Parker G (1981): Parental representations of patients with anxi-ety neurosis. Acta Psychiatr Scand 63:33–36.
Persold C, Treit D (1995): The central and basolateral amygdala differentially mediate the anxiolytic effects of benzodiaz-epines. Brain Res 671:213–221.
Pitkanen A, Savander V, LeDoux JE (1998): Organization of intra-amygdaloid circuitries in the rat: An emerging frame-work for understanding functions of the amygdala. Trends
Neurosci 20:517–523.
Plotsky PM, Cunningham ET, Widmaier EP (1989): Cat-echolaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev 10:437– 458. Quirarte GL, Roozendaal B, McGaugh JL (1997):
Glucocorti-coid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci
U S A 94:14048 –14053.
Rosmond R, Dallman MF, Bjorntorp P (1998): Stress-related cortisol secretion in men: Relationship with abdominal obe-sity and endocrine, emtabolic and hemodynamic abnormali-ties. J Clin Endocrinol Metabol 83:1853–1859.
Roy-Byrne P, Cowley DS, Greenblatt DJ, Shader RI, Hommer D (1990): Reduced benzodiazepine sensitivity in panic disorder.
Arch Gen Psychiatry 47:534 –538.
Roy-Byrne P, Wingerson DK, Radant A, Greenblatt DJ, Cowley DS (1996): Reduced benzodiazepine sensitivity in patients with panic disorder: Comparison with patients with obses-sive-compulsive disorder and normal subjects. Am J
Psychi-atry 153:1444 –1449.
Russak LG, Schwartz GE (1997): Feelings of parental care predict health status in midlife: A 35 year follow-up of the Harvard Mastery of Stress Study. J Behav Med 20:1–11. Sapolsky RM (1994): The physiological relevance of
glucocor-ticoid endangerment of the hippocampus. Ann NY Acad Sci 746:294 –304.
Seckl JR, Meaney MJ (1994): Early life events and later development of ischaemic heart disease. Lancet 342:1236. Swerdlow NR, Geyer MA, Vale WW, Koog GF (1986):
Corti-cotropin-releasing factor potentiates acoustic startle in
rats: Blockade by chlordiazepoxide. Psychopharmacology 88:147–152.
Thomas SR, Lewis ME, Iversen SD (1985): Correlation of [3H]diazepam binding density with anxiolytic locus in the amygdala complex of the rat. Brain Res 342:85–90.
Valentino RJ, Curtis AL, Page ME, Pavcovich LA, Florin-Lechner SM (1998): Activation of the locus ceruleus brain noradrenergic system during stress: Circuitry, consequences, and regulation. Adv Pharmacol 42:781–784.
van Bockstaele EJ, Colago EEO, Valentino RJ (1996): Cortico-tropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically mod-ulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol 364:523–534.
Van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE (1984): The organization of projections from the cortex, amygdala and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol 224:1–24.
West MJ, King AP (1987): Settling nature and nurture into an ontogenetic niche. Dev Psychol 20:549 –562.
Yehuda R, Schmeidler J, Wainberg M, Binder-Brynes K, Du-vdevani K (1998): Vulnerability to post traumatic stress disorder in adult offspring of Holocaust survivors. Am J
Psychiatry 155:1163–1171.