AMINO ACID AND FATTY ACID PROFILLING FROM MODIFIED
SORGHUM FLOUR
Yohanes Martono, Vellisya Puspaningsih, Sri Hartini Chemistry Department, Faculty of Science and Mathematics
Satya Wacana Christian University, Salatiga, Indonesia E-mail: [email protected].
ABSTRACT
Sorghum is potential food source that can be developed in Indonesia. Sorghum has high content of protein and rich in unsaturated fatty acid. The aim of this research was to identify and determine the content of amino acid and fatty acid from modified shorgum flour. Shorgum was modified by fermentation and fortification with peanuts. Identification of amino acid was done by using High Performance Liquid Chromatography (HPLC) while fatty acid was analysed by using Gas Chromatography – Mass Spektrometer (GC-MS). Amino acid and fatty acid from modified sorghum flour was compared with unmodified sorghum flour. Data were analysed descriptively. The results showed that amino acid in modified sorghum flour were aspartic acid, glutamic acid, serine, histidine, glysine, arginine, alanin, tyrosine, methionine, valine, phenylalanine, ileusine, leusin and lysine.The highest content of amino acid was glutamic acid, 148.98 ppm and the lowest or limitted amino acid in sorghum flour was methionine, 19.95 ppm. The main compound unsaturated fatty acid of modified sorghum flour were oleic acid and linoleic acid.The contents of both main unsaturated fatty acids were 35,64% and 32,41% respectively.
Key words: amino acid, fatty acid, sorghum, modified flour.
INTRODUCTION
One of the potential crop used as a functional food is sorghum. [24] states that the chemical, sorghum is an important potential source of functional food (nutraceuticals phenolic). This plant also has great potential to be developed commercially in Indonesia particularly dry area [18], because it is supported by ecological condition [19].
Problems faced currently is limited in sorghum cultivation for lack of information about the potential of sorghum. Over the years, sorghum only be used as animal feed [10][11]. According [19], one of factors that caused this limited was the high rate of anti-nutritional substances, tannins (about 0.40 to 3.60%) contained in sorghum. It is therefore necessary optimizations in the reduction of anti-nutritional tannins compounds.
through fortification of peanut (Arachis hypogaea). Peanuts not only have high levels of protein (25.3 g in 100 g) but also contains essential amino acids that can not be produced by the human body. Besides, unsaturated fatty acids of peanuts is high (42.8 g in 100 g) [25].
Food containing high protein may not have a good protein quality. Protein quality of a food is determined composition and availability of amino acids that can be absorbed by the body [6][1]. It is necessary to identify amino acids of protein beside determine protein content in food. The aim of this research was to identify and determine the content of amino acid and fatty acid from modified shorgum flour. Shorgum was modified by fermentation and
fortification with peanuts.
METHODS Materials
The sample used is a sorghum (Sorghum bicolor L.) varieties derived from Surakarta, peanuts (Arachis hypogaea) obtained from Kulon Progo in Ambarawa market, and tempeh
fermipan Raprima brand. All chemicals were pro-analysis grade and were purchased from Merck, Germany. Amino acids standard were purchased from Sigma Aldrich.
Instrumentations
Instruments used in analysis were spektrophotometer (Optizen UV 2120, Korea), waterbath (Memmert WNB 14, Germany), centrifuge (EBA 21 Hettich Zentrifugen, Germany), mass balanced (Mettler H-80, Germany), soxhlet, rotary evaporator (Buchi R-114, Switzerland), HPLC (Shimadzu LC 10, Japan), GCMS (Shimadzu QP2010S, Japan).
Procedure
Degradation of Tannins Content in Sorghum Seed [26].
Decreased levels of tannins in sorghum was done by immersion method in 0.3% Na2CO3 for
8 hours, followed by immersion in 0.2% Na2HPO4 for 2 hours. Method performed with 1:3
ratio (sorghum seed: solvent).
Optimization of Fortification and Fermentation Condition Based on Sorghum Soluble Protein Content
Sorghum seeds were boiled for 30 minutes and then cooled. Then, sorghum seeds were inoculated with the microbes, Rhizopus oligosporus 2.5% (w / w), 5% (w / w) and 10% (w / w) and fortified with peanuts (Arachis hypogaea) in various concentrations 0%, 2.5%, 5%, 7.5%, 10% (w / w). Each was fermented with the time variation of 0 hours to 48 hours with an analysis time per 12 hours. The results then dried, pulverized and sifted. Soluble protein content was measured by Biuret method.
Analysis of Protein Content in Sorghum Seed by Biuret Method
One gram samples was added by 9 ml of distilled water and then added 1 ml 1 M NaOH. Then, the solution was heated in a water bath with a temperature of 90 ° C and cooled. Solutions was centrifuged on 3000 rpm for 10 minutes. 1 ml supernatant was taken and added by 4 ml Biuret reagent (0.15 g + 0.6 g KNaTartaric CuSO4.5H2O in 50 mL volumetric flask and added 30 ml of 10% NaOH and fulfilled with distilled water in a 100 ml flask). Solution was incubated for 30 min at room temperature. Absorbance of the solution was measured with a spectrophotometer at 550 nm. Protein solution (BSA) was used as a standard with various concentrations.
Hydrolysis Of Proteins
Protein hydrolysis using a reflux, which is 15 g of sample was added 70 ml 7.5 M HCl and heated in water bath with a temperature of 90 ° C. Hydrolyzed samples were filtered and the filtrate evaporated at a temperature of 90 °C to remove HCl.
Amino Acid Analysis
60 mg sample was added by 4 ml 6 N HCl and heated for 24 hours at 110 oC.
Subsequently, the solution was neutralized (pH = 7) with 6 N NaOH and filtered with Whatman filter paper 0.2 µm. Then, 30 mL of sample solution was derivated by 300 mL OPA
(o-phthalaldehyde) and stirred for 5 minutes. Then, 20 mL solution was injected into the HPLC injector.
Chromatography Conditions of HPLC
Identification of amino acids using HPLC method on the condition of stationary phase
column Licrospher 100 RP 18 (125 x 4.0 mm, 5 m). The elution was isocratic system with
6.8 and solvent B = 65% methanol. Flow rate used was 1.5 ml / min and detected using a Flourescence detector (420 E. WATERS)
Fatty Acid Extraction
10 g samples were grinded put in Soxhlet extraction tube, then the water flows through the condenser. Solvent extraction tube fitted with enough diethyl ether for 4 hours.
Then, again continued for 2 hours with the same solvent. Weigh heavy residue on the bottle net weight expressed as fats and oils.
Identification of Unsaturated Fatty Acids by GC-MS Method
Identify the building blocks of sorghum seed fatty acids were analyzed using Gas Chromatography Mass Spectrophotometer (Shimadzu QP2010S), in the Laboratory of Organic Chemistry, Faculty of Science, University of Gadjah Mada in operational conditions:
Column : EGILENT J&W DB-5 (30 m x 0,25 mm)
Carier gas : Helium
Temperature gradien : 70oC for 5 minutes and 10oC/min-300oC
ionization : Electron Impact (EI)
RESULTS AND DISCUSSION
Degradation of Tannins Content in Sorghum Seed
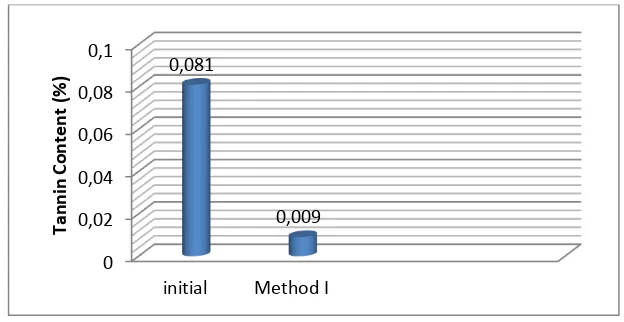
Quantitative assay of tannins in sorghum grains showed that the method used has significant impact on tannin degradation. Figure 1 shows that the levels of tannins in sorghum is still high at 0.08% (w / w), these results are consistent with studies [10][21] which mentioned that the phenol content in sorghum was still high and dominated by tannin.
Tannins contained in the condensed tannin sorghum is known as proantosianin. Proantosianin are polymers of flavanoid-containing unit or flavan 3-ol catechin. These compounds include polyphenolic compounds that can form complexes with proteins resulting in lower quality and protein digestibility [24][26]. Tannins have the ability to precipitate proteins into complexes because of its ability to make copolymer crosslinking protein. Bonds between protein complexes with tannin sorghum causing protein is difficult to be digested or inhibit digestive enzymes work processes [26]
Table 1. Datapercent degradation (%) of tanin content
Method Initial (%) Final (%) % Degradation
Control 0.081 0.081 -
I 0.081 0.009 88.88
Note : Method I : Soaked by Na2CO3 and followed by Na2HPO4
Figure 1. Degradation tannin content by method used
Optimization of Protein Content in Modified Sorghum Seed
Results soluble protein content in sorghum that have not been processed and fermented reached 12.78%, but when processed or cooked dissolved protein levels decreased to 0.58% (Figure 2) Sorghum is a food that cannot be eaten, the need for processing to be consumed by humans. However, sorghum grain processing (boiling process) lead to lower levels of protein and protein digestibility in sorghum. This happens because protein polymers will be denatured enzimatically.
Figure 2. Soluble protein content of initial sorghum, boiled sorghum and peanuts.
Fortification by mixing two or more different sources of protein that have different limiting amino acids will be complementary of protein content [25]. Soluble protein content of
peanuts is high at 7.92%. In other words, peanuts can be used as another source of protein that is able to improve the nutrition and quality of food.
Optimization conditions of Peanut Fortification and Sorghum Fermentation
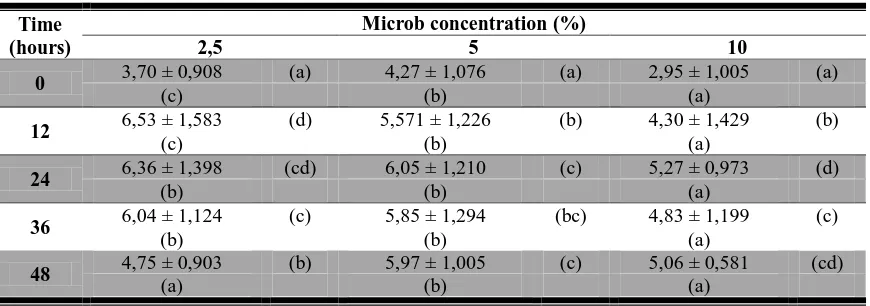
Optimization of conditions fortification followed by a review of the interaction of each variable, ie Rhyzopus oligosporus microbial levels, the addition of peanuts and fermentation time. Results of mathematical modeling interpolation method with matlab 6.5 programs produce fortification and fermentation conditions are optimal in terms of the levels of soluble protein content of microbes is 2.5% with the addition of peanuts by 9% and fermented for 49 hours (Figure 3).
Figure 3
. Mathematical modeling interpolation method with matlab 6.5 programs on sorghumflour modification
Table 2.
Soluble protein interaction between percentage peanut additions and various fermentation timeTable 3. Soluble protein interaction between percentage peanut additions and various
microb concentration
Soluble protein content increased with increasing peanuts. Consistent with research [9], the increase in soluble protein due to other sources of nitrogen derived from proteins other materials, in this study are peanuts (Arachis hypogaea).
Tabel 4. Soluble protein interaction between various microb concentration and
Increased levels of soluble protein during the process of fermentation can also be caused microbial hydrolysis of complex protein into free amino acids or peptides are simpler with the proteolytic enzyme activity [16][1]. This results is consistent with [7] that soluble protein and amino acid of fermented sorghum increased due to protease activity from microb.
The more levels of yeast were added apparently inversely proportional to the levels of protein produced. This result is consistent with research which states that the addition of inoculum impact on concentration decreased of soluble protein. This is caused by the increased levels of microbes causing Rhizopus oligosporus much more used amino acids as a source of N (nitrogen) for growth. Decrease in protein content can also be occured if the amino acids are metabolized into ammonia and flavor compound [17]. On these interactions was found that 24 hours is the most optimal fermentation time with soluble protein content of 6.36 ± 1.398 (% w / w).
Amino acid profilling of modified sorghum flour
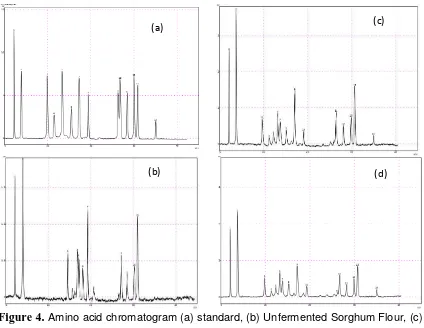
Protein quality of a food is determined composition and availability of amino acids that can be absorbed by the body [6][1]. Amino acid identification can be seen in Figure 4.
The results showed that there were change of amino acid variation in each sample. On the unfermented sorghum flour chromatogram is found only 8 kinds of amino acids, whereas the modified sorghum flour was found 14 types of amino acids. This indicates that sorghum is a food that contains high protein but not necessarily have a good quality protein.
Figure 4.
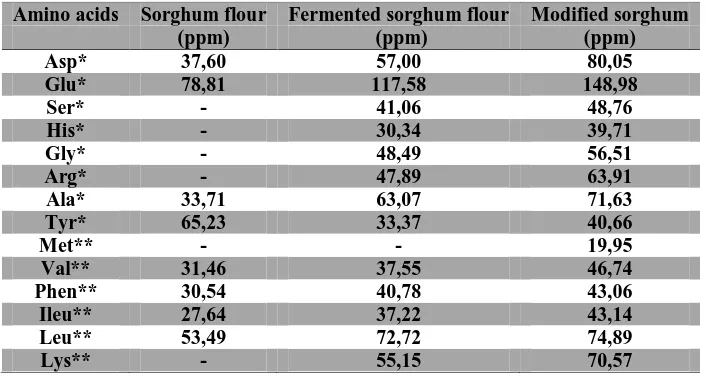
Amino acid chromatogram (a) standard, (b) Unfermented Sorghum Flour, (c) Fermented Sorghum Flour Without Fortification, (d) Modified Sorghum Flour.Table 5 shows that peanut fortification and fermentation processes are carried out apparently affect the variation of amino acids such as serine, histidine, glycine, arginine, methionine and lysine which appeared on a modified sorghum flour samples. The comparison between the amino acid identification of fermented sorghum flour without fortification and modified sorghum flour showed the essential amino acid change that appears methionine in sorghum modified. Methionine is one of the essential amino acids in peanuts [25]. [27] also describes when the two types of proteins that have a limiting amino acids are different from mixed or consumed together, the two proteins that will support each other (complementary) so that the nutritional quality of the protein mixture to be higher.
Fatty Acids Profilling of Modified Sorghum Flour
Figure 5a shows the results of fatty acids identification from modified sorghum flour. GC chromatogram shows the presence of 29 chemical components that appear as 29 peak (peak) on the modified sorghum flour. Meanwhile, Figure 5.b shows the analysis of fatty acids from fermented sorghum flour. GC chromatogram shows the presence of 22 chemical components that appear as 22 peak (peak) in the sample fermented sorghum flour.
Based on the mass spectra, obtained the identification of fatty acids components from modified sorghum flour by the dominant peak No. 15, 14, 10, 16, 26 (Table 6).
(a)
(b) (d)
Dominant peak results compared with the data base 229.LIB WILEY. Based on a comparison with a database WILEY 229.LIB, peak number 15 with a retention time of 23.175 min with molecular weight (MW) at m/z = 296 and a base peak at m/z = 55 refers to the 9-octadecenoic acid compound known as oleic acid (Figure 6). This compound is the most predominant compound in the oil-modified sorghum flour with 35.64% levels. Oleic acid is a type of unsaturated fatty acids are found naturally in plants. This acid is an omega-9 fatty acids and is a source of healthy fats in the diet. From the point of view of health, oleic acid capable of slowing the progression of heart disease and increase antioxidant [22]
Table 5.
Amino acids concentration (ppm) in sorghum samples, fermented sorghum, and modified sorghum* nonessential Amino acids, ** essential amino acids. Asp = aspartate, glutamate = Glu, Serine = Ser, His = hystidine, Gly = glycine, Arg = Arginine, Al = Alanine, Tyrosine = Tyr, Met = methionine, Val = Valine, Phe = phenilalanine, Ileu = isoleucine, Leu = Leusin, Lys = Lysine.
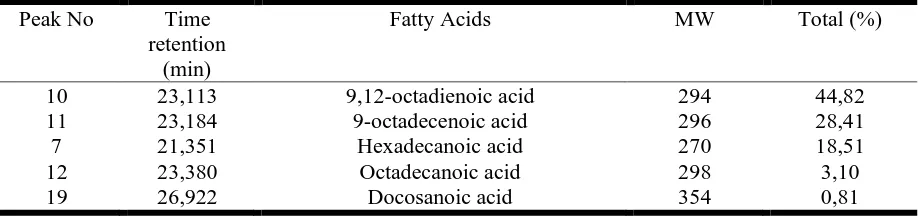
Table 7 shows the identification of fatty acids components from fermented sorghum flour by the dominant peak no 10, 11, 7, 12, 19. Dominant peak results compared with the data base 229.LIB WILEY. Based on a comparison with a database WILEY 229.LIB, peak number 10 with a retention time of 23.113 min with molecular weight (MW) at m/z = 294 and a base peak at m/z = 67 refers to the 9,12-octadienoic acid compound known as linoleic acid (Figure 7). This compound is the most predominant compound in the oil-fermented sorghum flour with 44.82% levels.
Amino acids Sorghum flour Fermented sorghum flour Modified sorghum
Table 6. Identification of fatty acid from modified sorghum flour based on dominant peak
Peak No Retention time (min)
Fatty Acids MW Total Content
(%)
15 23,175 9-octadecenoic acid 296 35,64
14 23,071 9,12-octadienoic acid 294 32,41
10 21,344 Hexadecanoic acid 270 18,55
16 23,373 Octadecanoic acid 298 3,64
26 26,928 Docosanoic acid 354 2,61
(a)
(a)
(b)
(b)
(c)
(c)
Figure 6. Mass spectra of 9-octadecenoic
acid (a) Peak no 15, (b) Data Base WILEY 229.LIB (c) Chemical structure
9-octadecenoic acid
Figure 7. Mass spectra of 9,12-octadienoic
acid (a) Peak no 10, (b) Data Base WILEY 229.LIB (c) Chemical structure
9,12-octadienoic acid
(a) (b)
Figure 5. GC analysis of fatty acid from (a) modified sorghum flour and (b) fermented
Table 7.
Identification of fatty acid from fermented sorghum flour based on dominant peak[20] mentions that linoleic acid is a type of essential fatty acid, is a fatty acid that can not be produced by the body. Linoleic acid is an important role in the prevention of coronary heart disease and healthy blood vessels.
Identification results showed that there is not change in the fatty acid variation between the modified and fermented sorghum oil shows that but there are changes in the levels of individual fatty acids. In research [2] found that fermentation can reduce fatty acid levels in seeds of sorghum, this is due to the reduction caused by biochemical activity and physiological changes during the immersion process, the process requires energy so that part of the fatty acids in the sample used for producing energy [2].
[2] mentions that the fatty acid content in sorghum is palmitic acid (0.43 to 0.56%), oleic acid (48.99%), linoleic acid (27.59% ) and linolenic acid (1.71%). Whereas the fatty acid content in peanuts is palmitic acid (10.50%), stearic acid (3.10%), arachidat acid (14.80%), and oleic acid (53.80). If the results of the GC-MS analysis in comparison with the literature fatty acid content of sorghum and peanuts, it was found that the levels of linoleic fatty acids on modified sorghum increased. This is due to the hydrolysis of fats by lipase enzymes during fermentation. Consistent with research [20], which states that are capable of producing Rhizopus lipase during fermentation. Lipase activity serves to hydrolysis, but at the same time make the process of synthesis. Oleic acid is found in sorghum will undergo denaturation to linoleic acid. Oleic acid is converted into fatty acids linoleic with the help of
Δ12
desaturase enzyme [20]. Therefore, an increase in the percentage levels of linoleic acid and decreased levels of oleic acid percentage in the fermented sorghum. The results also showed that the addition of peanuts affect levels of oleic and linoleic acid on modified sorghum flour.
CONCLUSION
glysine, arginine, alanin, tyrosine, methionine, valine, phenylalanine, ileusine, leusin and lysine.The highest content of amino acid was glutamic acid, 148.98 ppm and the lowest or limitted amino acid in sorghum flour was methionine, 19.95 ppm. The main compound unsaturated fatty acid of modified sorghum flour were oleic acid and linoleic acid.The contents of both main unsaturated fatty acids were 35,64% and 32,41% respectively.
REFERENCES
[1] Amodou I, Mohamed T, Kamara, Tidjani A, Foh MBK, Guo-Wei L. 2010. Physichochemical and Nutritional Analysis of Fermented Soybean Protein Meal by Lactobacillus plantarum Lp6.World J Dairy & Food Sci 5: 14-118. http://www.idosi.org/wjdfs/wjdfs5(2)/3
[2] Afify, A, E, M., Hossam S. El Beltabi., Samiha M. ABD EL-Salam & Azza A. Omran. 2012. Oil and Fatty Acid Contents of White Sorghum Varieties Under Soaking, Cooking, Germination and Fermenetation Processing for Improving Cereal Quality. Not Bot Horti Agrobo 40 (1) : 86-92 ISSN 0255-965X.
[3] Andayani, P. 2008. Isolasi dan Identifikasi Mikrob dari Tempe Sorghum Coklat (Sorghum bicolor) Serta Potensinya Dalam Mendegradasi Pati dan
Protein. Universitas Brawijaya, Malang.
[4] Boonnop K, Wanapat M, Nontaso N, Wanapat S. 2009. Enriching Nutritive Value Of Cassava Root By Yeast Fermentation. Sci Agric (Piracicaba, Braz.) 6: 629-633. http://dx.doi.org/10.1590/S0103-90162009000500007
[5] Cervantes-Pahm SK, Stein HH. 2010. Ileal digestibility of amino acids in conventional, fermented, and enzyme-treated soybean meal and in soy protein isolate, fish meal, and casein fed to weanling pigs. J Anim Sci 8: 2674-2683. doi:10.2527/jas.2009-2677
[6] Chen CC, Shih YC, Chiou PWS, Yu B. 2010. Evaluating Nutritional Quality of Single Stage- and Two Stage-fermented Soybean Meal. Asian-Aust. J Anim Sci 23: 598 – 606
[7] Correia, I., Nunes, A., Guedes, S., Barros, A.S., and Delgadillo. 2010. Screening of lactic acid bacteria potentially useful for sorghum fermentation. Journal of Cereal Science, 52: 9-15. doi:10.1016/j.jcs.2010.02.011
9: 187-194. DOI: 10.5897/AJB09.620
[9] Martono, Y., Gunawan, I.W., and Hartini, S. 2012. Analisis Protein dan Identifikasi asam Amino pada Tepung Gaplek Terfortifikasi Tepung Kedelasi (Glycine max (L)). Prosiding Seminar Nasional Sains dan Pendidikan Sains VII. Vol 3(1): 109-116
[10] Mella Onesmo. N. O. 2011. Effect of Malting and Fermenetation on The Composition and Functionality of Sorghum Flour. University of Nebraska.
Lincoln.
[11] Murtini, Erni S. 2009. Peningkatan Bioavailabilitas Protein Sorghum Lokal Varietas Coklat Dengan Solid State Fermentation Untuk Produksi tepung
Berfungsional Tinggi. Universitas Brawijaya, Malang.
[12] Mursyid M, Ali. 2005. Solid State-Fermentation on Cassava Pomace with Aspergillus oryzae: The Evaluation of Proteins and Amino Acids Content,
Digestibility, and Bioavailability Energy For Broiler Roasters. Buletin Peternakan 29: 72-77.
[13] Nisa, Fitri Choirun. 2010. Ekstraksi Antioksidan Alami dari Sorghum Lokal Varietas Cokelat Serta Peningkatan Aktivitasnya Dengan Perkecambahan Dan Gelombang Mikro. Jurnal Teknologi Pertanian Vol 11. No. 3 (184-195).
[14] Oboh G and Elusiyan CA. 2007. Changes in the nutrient and anti nutrient content of micro-fungi fermented cassava flour produced from low- and medium-cyanide variety of cassava tubers. African Journal of Biotechnology Vol. 6 (18): 2150-2157.
[15] Omafuvbe BO. 2006. Effect of salt on the fermentation of soybean (Glycine max) into daddawa using Bacillus subtilis as starter culture. Afr J Biotechnol 5: 1001-1005. DOI: 10.3923/ajft.2008.33.41
[16] Onweluzo JC and Nwabugwu CC. 2009. Fermentation of Millet (Pennisetum americanum) and Pigeon Pea (Cajanus cajan) Seeds for Flour
Production: Effects on Composition and Selected Functional Properties. Pakistan Journal of Nutrition 8: 737-744. DOI: 10.3923/pjn.2009.737.744
[17] Pranoto, Y., Anggrahinia, S., and Zulman Efendi. 2013. Effect of natural and Lactobacillus plantarum fermentation on in-vitro protein and starch
digestibilities of sorghum flour. Food Biosicence. http://dx.doi.org/10.1016/j.fbio.2013.04.001
Litbang Pertanian 22 (4): 133-140.
[19] Suarni. 2004. Pemanfaatan Tepung Sorghum Untuk Produk Olahan. Jurnal Litbang Pertanian 23 (4):145-151.
[20] Sudaryatiningsih & Supyani. 2010. Analisis Kandungan Asam Linoleat dan Asam Linolenat Tahu Kedelai dengan Rhyzopus oryzae dan Rhyzopus oligosporus Sebagai Koagulan. Bioteknologi 7 (1) : 10-18, ISSN : 0216-6887.
[21] Sujatmiko, B., A. Sutrisno, & E. S. Murtini. 2008. Degradasi Senyawa Tanin, Asam Fitat, Antri tripsin dan Peningkatan Daya Cerna Protein Secara In Vitro pada Sorghum Coklat (Sorghum bicolor L Moench) dengan Metode Fermentasi Ampok. Universitas Brawijaya, Malang.
[22] Sulistyowati. 2009. Efek Asam Lemak Jenuh dan Asam Lemak Tak Jenuh
“Trans” Terhadap Kesehatan. Media Penelitian dan Pengembangan Kesehatan Vol. XIX, Suplemen II.
[23] Supriyono, M. 2008. Faktor-Faktor Resiko Yang Berpengaruh Terhadap Kejadian Penyakit Jantung Koroner Pada Kelompok Usia ≤ 45 Tahun. Universitas Diponegoro, Semarang.
[24] Taylor, John, R, N. 2006. Novel food and non food uses for sorghum and millets. University of Pretoria, South Africa.
[25] Tiommanisyah. 2010. Analisa Kadar Protein Kasar Dalam Kacang Kedelai, Kacang Tanah dan Kacang Hijau Menggunakan Metode Makro Kjeldhal
Sebagai Bahan Makanan Campuran. Universitas Sumatera Utara, Medan.
[26] Widowati, S. Rahmawati Nurjanah, & Wiwit A. 2010. Proses Pembuatan dan Karakterisasi Nasi Sorghum Instan. IPB, Prosiding pekan Serealia Nasional:ISBN978-979-8940-29-3.