Cloning of an auxin-responsive 1-aminocyclopropane-1-carboxylate
synthase gene (
CMe
-
ACS
2) from melon and the expression of
ACS
genes in etiolated melon seedlings and melon fruits
Yasushi Ishiki
a, Akiko Oda
a, Yuka Yaegashi
a, Yoshikazu Orihara
a, Tomoe Arai
a,
Tetsuo Hirabayashi
c, Hiroki Nakagawa
a, Takahide Sato
a,b,*
aFaculty of Horticulture,Chiba Uni6ersity,648Matsudo,Chiba271,Japan
bGraduate School of Science and Technology,Chiba Uni6ersity,1Yayoi-cyo,Inage,Chiba263,Japan cThe Japan Horticultural Producti6ity Institute,207Shinbashidai,Kamishiki,Matsudo,Chiba271,Japan
Received 7 February 2000; received in revised form 18 May 2000; accepted 18 May 2000
Abstract
Two cDNA fragments (pCMe-ACS2 and 3) encoding auxin-responsive 1-aminocyclopropane-1-carboxylate synthase (ACS; EC.4.4.1.14) have been isolated from melon, and the expression patterns of the genes in etiolated melon seedlings and melon fruit have been determined by RT-PCR analysis. The deduced amino acid sequences of pCMe-ACS2 and 3 were homologous to those ofAT-ACS6 and 4, which were auxin-responsiveACSgenes ofArabidopsis. BothCMe-ACS2 and 3 were auxin-responsiveACS
genes and their expressions in roots and hypocotyls were induced by treatment with indole acetic acid (IAA, 100mM). The mRNA level of CMe-ACS2 in the fruit increased after pollination. Those of both CMe-ACS2 and 3 temporarily increased in the mesocarp tissues at the preclimacteric stage (from day 3 to day 5 after harvest) during ripening, while that ofCMe-ACS3 was lower than that ofCMe-ACS2. The increase in the mRNA level ofCMe-ACS1 (wound- and ripening-induced gene, T. Miki, M. Yamamoto, N. Nakagawa, O. Ogura, H. Mori, H. Imaseki, T. Sato, Nucleotide sequence of a cDNA for 1-aminocyclopropane-1-carboxylate synthase from melon fruits, Plant Physiol. 107 (1995) 297 – 298.) in the mesocarp tissue was not observed until 5 days after harvest. A genomic DNA encoding CMe-ACS2 was isolated and its nucleotide sequence was determined. Nucleotide sequences resembling the auxin-responsive elements (AuxRE) D1 and D4 (the TGTCTC element) in theGH3 gene from soybean, and the auxin-responsive domain (AuxRD) B in PS-IAA4/5 from pea were found in the 5%-flanking region of theCMe-ACS2 gene. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:ACC synthase; Auxin; Ethylene; Melon (Cucumis meloL.); Ripening fruit
www.elsevier.com/locate/plantsci
1. Introduction
Ethylene is a gaseous plant hormone, and is produced in plants during seed germination, leaf abscission, organ senescence and fruit ripening, and its production is also greatly increased by stress and the presence of auxin [1].
1-Aminocyclopropane-1-carboxylate (ACC) synthase (ACS, S -adeno-syl-L-methionine methylethioadenosine-lyase, EC
4.4.1.14), which catalyzes the conversion of S -adenosylmethionine to ACC, is a rate-limiting en-zyme in the ethylene production pathway of higher plants [1,2], and is encoded by a multigene family. The amino acid sequences encoded by allACSgenes contain seven conserved regions [1]. Using appro-priate primers specific for the conserved sequences, several ACS genes have been isolated from Ara -bidopsis[3 – 6], mung bean [7 – 12], rice [13], tomato [14 – 18] and winter squash [19 – 21]. Each member of this gene family is differentially expressed in
The sequences ofpCMe-ACS3andCMe-ACS2reported herein have been deposited in the DNA Data Bank of Japan under the accession numbers D86241 and D86242, respectively.
* Corresponding author. Tel./fax: +81-47-3088863.
E-mail address:[email protected] (T. Sato).
response to different signals, such as wounding, ripening, senescence and the presence of auxin, as well as in different tissue types [1].
Ethylene production was increased in a climac-teric fruit during ripening, and ethylene induced the expression of ripening-related genes in the fruit and accelerated ripening. The low level of ethylene produced in fruit during the preclimacteric stage (the system 1 ethylene production) was suggested to act as a stimulus for the induction of genes for ethylene production at the ripening stage [22]. Melon is a climacteric fruit, in which ethylene production is known to increase markedly during ripening. It was previously found that a wound-re-sponsive ACS gene (CMe-ACS1) was mainly ex-pressed in mesocarp tissues in the ripening fruit, which played a key role in the marked increased in the production of ethylene at the ripening stage [23]. The mRNA of CMe-ACS2 was also detected in a ripening melon fruit at a preclimacteric stage [23]. CMe-ACS2 was isolated from auxin-treated etiolated seedlings, but the expression patterns and the stimuli for gene induction were not clarified. The mRNA levels ofACSgenes were usually very low in ripening melon fruit, and the nucleotide sequences of someACSgenes were similar to each other. It is not easy to detect the mRNA levels of each ACSgene by Northern hybridization. If one is to understand the expression patterns of ACS genes in plant tissues, RT-PCR analysis could be employed as a useful tool to identify low levels of mRNA in the tissues.
In this experiment, two cDNA fragments encod-ing the auxin-responsive ACS gene (CMe-ACS2 and 3) were isolated from etiolated melon seedlings, and their expression patterns in the etio-lated seedlings and developing melon fruit were studied. A genomic DNA encoding CMe-ACS2 was isolated and its structure determined.
2. Material and methods
2.1. Plant materials
Melon seedlings (Cucumis melo L. cv. AMS) were grown for 6 days at 28°C in the dark. The tissues (about 0.5 g) were incubated in petri dishes (90 mm in diameter) with 2 ml of 20 mM phos-phate buffer (pH 6.8) containing various chemicals for 2 h at 28°C. The tissue in the petri dish was
placed in a gas-tight box (3 l) for treatment with ethylene or 2,5-norbornadiene (NBD). The tissue was frozen by liquid nitrogen, and stored at −80°C. Melon plants (cv. Takami) were grown in a greenhouse. Fruits were harvested on day 55 after pollination and incubated at 25°C for prede-termined durations.
2.2. Estimation of ethylene concentration
A melon fruit was placed in a gas-tight box (12 l) at 25°C for 1 h. The ethylene concentration in the box was measured by gas chromatography (Hitachi 3000) using an alumina column (f2.5 mm×1 m) [23].
2.3. Cloning of auxin-responsi6e ACS genes from
melon
Total RNA was isolated from auxin-treated (100 mM) etiolated melon seedlings and a cDNA library from the RNA was constructed in pBlue-script SK (−) using a vector primer [24]. Two ACC synthase-related gene fragments (about 1.1 kbp) were obtained by PCR amplification of the cDNA.
A genomic library of melon was constructed into the lGEM11 vector (Promega, Madison, USA) and screened with a 32P-labeled cDNA
probe of pCMe-ACS2 [25]. The inserted DNA in the lGEM11 vector was subcloned in pBluescript SK (−).
2.4. Analysis of RNA
RT-PCR analysis was performed to determine the levels of mRNA of the ACS genes in the tissues. The first strand of cDNA was synthesized with 10 mg of the total RNA and the Rv primer which was specific for a conserved sequence of the ACSgenes (box 7) [1]. A cDNA fragment ofACS mRNA was amplified up to 20 cycles with the Rv primer and one of the Fw primers (Fw1, 2 and 3) specific for each ACS gene, and was detected by Southern hybridization with a 32P-labeled probe
specific for each ACS mRNA.
Fw primers:
Fw1 (CMe- GACCATTGCAAGAAGGAACTC
CGAAGAGGAGGAAGTTTGGA Fw3 (CMe
-AGAGA, ACS3)
Rv primer AARCAAACYCGRAACCAWCC
TGGYTC.
To determine the levels of mRNA in the total RNA fraction, the level of mRNA from the actin gene was measured by RT-PCR analysis with actin-specific primers (a forward primer
CAY-ACWGTYCCNATHTAYGARGGNTA and a
reverse primer CTGATRTCNACRTCRCAYT-TCAT).
2.5. General methods
The general methods adopted for the manipula-tions of DNA and RNA have been described by Sambrook et al. [26].
3. Results
3.1. Cloning of two cDNA fragments of ACS genes from etiolated melon seedlings and expression of the genes in the seedlings
Two cDNA fragments (about 1.1 kbp) from a cDNA library of indole acetic acid (IAA)-treated etiolated seedlings were isolated by PCR amplifi-cation and their nucleotide sequences determined. The deduced amino acid sequences of both cDNA fragments contained seven conserved regions
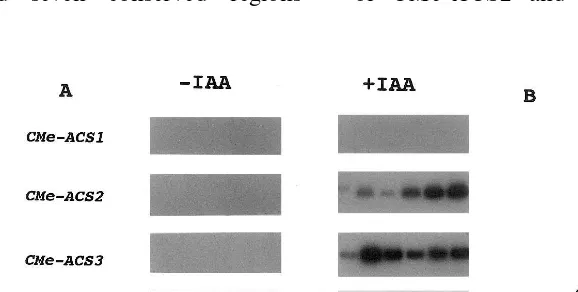
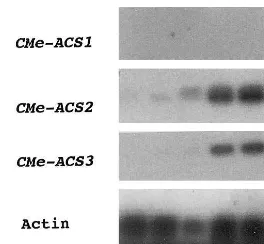
found in other ACS isoenzymes from various plant species (data not shown) [1], indicating that the genes isolated from melon were CMe-ACS2 and 3. RT-PCR analyses were performed to study the expression patterns of CMe-ACS2 and 3 in etiolated melon seedlings. The seedlings were cut into six pieces, and the mRNA levels of the ACS genes in each piece were determined (Fig. 1). The mRNA levels of CMe-ACS1, 2 and 3 were very low in the tissues not subjected to IAA treatment. CMe-ACS2 was strongly expressed in the roots and lower parts of hypocotyls following treatment with IAA (100 mM) for 2 h. The mRNA levels of CMe-ACS3 were also markedly increased in the hypocotyls and roots following IAA treatment (100 mM). The expressions of CMe-ACS2 and 3 were low in the cotyledons even following the treatment of the tissues with IAA (100 mM). Ex-pressions of CMe-ACS1 could not be detected in etiolated seedlings under the above conditions. A dose-response experiment of IAA showed that treatment with a low concentration of IAA (1mM) was sufficient to increase the mRNA levels of CMe-ACS2 and 3 in the hypocotyls (Fig. 2). The expression of CMe-ACS2 was more sensitive to low levels of IAA than that of CMe-ACS3. The effects of plant hormones on the mRNA levels of the ACS genes in etiolated hypocotyls are shown in Fig. 3. Treatment with naphthalene acetic acid (NAA, 100 mM) and 2,4-dichlorophenoxy acetic acid (2.4-D, 100 mM) increased the mRNA levels of CMe-ACS2 and 3 in etiolated hypocotyls.
Fig. 1. Expression of ACS genes in the etiolated melon seedlings following treatment with indole acetic acid (IAA). Melon seedlings were grown for 6 days at 28°C in the dark. Seedlings were cut into six pieces as shown in panel B. The tissue pieces were incubated in 20 mM phosphate buffer (pH 6.8) containing IAA (100mM) for 2 h. To determine the mRNA levels of theACS
Fig. 2. Effect of indole acetic acid (IAA) concentration on the mRNA levels of the ACSgenes in etiolated hypocotyls. The hypocotyl tissues were incubated in media with various con-centrations of IAA for 2 h. Lane 1, no IAA; lane 2, IAA (0.1
mM); lane 3, IAA (0.5mM); lane 4, IAA (1mM); lane 5, IAA (10mM); lane 6, IAA (50 mM).
4A). The mRNA level of CMe-ACS2 was markedly increased in melon fruit at 3 days after pollination (Fig. 4A). The expression of CMe -ACS3 was only slightly increased after pollination. Ethylene production in the ripening melon fruit was increased, and peaked on day 7 after harvest (Fig. 4B). The mRNA levels of CMe-ACS1, 2 and 3 were very low in the mesocarp tissues at harvest. Those of CMe-ACS2 and 3 were temporally in-creased in the mesocarp tissues on approximately the 3rd and 5th days after harvest, although the mRNA level of CMe-ACS3 was lower than that ofCMe-ACS2. The increase in the mRNA level of CMe-ACS1 was noted first in mesocarp tissues at day 5 after harvest (Fig. 4B).
3.3. Characterization of a genomic DNA encoding CMe-ACS2
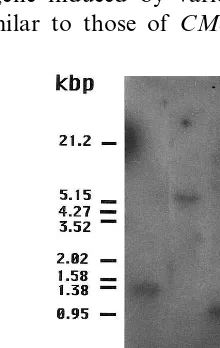
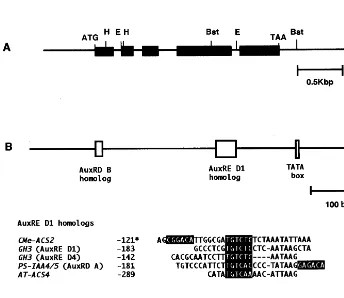
A genomic DNA encodingCMe-ACS2 was iso-lated and sequenced (accession number D86242). Southern blot analysis showed that each restric-tion digesrestric-tion fragment of CMe-ACS2 byEcoR I, Bgl II, BstX I and Hind III gave a single band, and the sizes of the fragments were in good agree-ment with those calculated from the nucleotide sequence (Fig. 5). CMe-ACS2 consisted of five exons and four introns. The number and size of the exons and the location of introns were identi-cal to those in CMe-ACS1 of melon [25] and CP-ACS1A/1B of zucchini [27]. The deduced amino acid sequence of CMe-ACS2 consisted of 490 amino acids (54.09 kDa). The homology of the deduced amino acid sequence of CMe-ACS2 to those of other ACS genes is shown in Table 1. The amino acid sequence of CMe-ACS2 was ho-mologous to those of VR-ACS1 of mung bean (71% similarity)[7], PS-ACS2 of pea (71% similar-ity) [28,29], LE-ACS6 of tomato (71% similarity) [15] and AT-ACS6 of Arabidopsis(69% similarity) [4]. A putative TATA box (tatatatat) and a poly (A)-signal (aataa and aattaa) were found at 5%- and 3%-flanking regions, respectively. Nucleotide se-quences resembling auxin-responsive elements (AuxREs) were found in the 5%-flanking region of
the CMe-ACS2 gene (Fig. 6).
4. Discussion
Auxin affects the production of ethylene in higher plants, and ethylene produced in the tissues Fig. 3. Effects of plant hormones and some chemicals on the
mRNA levels of theACS genes in etiolated hypocotyls. The tissues were incubated in 2 ml of 20 mM phosphate buffer containing various chemicals for 2 h. For treatment with ethylene, petri dishes containing the tissues were placed in a gas-tight box for 2 h at 28°C. Panel A: lane 1, no chemical; lane 2, indole acetic acid (IAA) (100mM); lane 3, naphthalene acetic acid (NAA) (100 mM); lane 4, 2,4-dichlorophenoxy acetic acid (2,4-D) (100 mM); lane 5, methyl jasmonate (50
mM); lane 6, abscisic acid (ABA) (50 mM); lane 7, BA (10
mM); lane 8, salicylic acid (50 mM). Panel B: lane 1, no chemical; lane 2, IAA (100mM); lane 3, NBD (3500 ppm); lane 4, IAA (100 mM)+NBD (3500 ppm); lane 5, ethylene (50 ppm); lane 6, ethylene (50 ppm)+IAA (100mM).
Other plant hormones [abscisic acid (ABA) (50
mM), BA (10 mM), ethylene (50 ppm), methyl jasmonate (50mM)] and salicylic acid (50mM) did not affect the mRNA levels of CMe-ACS2 and 3 (Fig. 3A). Treatment with NBD, an inhibitor of ethylene activity, increased the mRNA levels of CMe-ACS2 in etiolated hypocotyls with or with-out IAA treatment (Fig. 3B).
3.2. Changes in the mRNA le6els of ACS genes in
melon fruit
Fig. 4. Changes in the mRNA levels of theACSgenes in melon fruit after pollination (panel A) and during ripening (panel B). Total RNA was isolated from whole fruit (panel A) and the mesocarp tissues (panel B). The ethylene production in the fruit was measured as described in Section 2.
controls the development of plants [1,22]. Two or three auxin-responsive ACS genes have been isolated from various plants [3,4,7,10 – 13,17,29]. They were classified into at least two subfamilies by means of a phylogenetic analysis of amino acid sequences [13]. The physiological roles of the genes during the development of plants and the molecular mechanisms of their expression in the presence of auxin, however, are not yet understood. Two cDNA fragments of the melon ACS gene family (pCMe-ACS2 and 3) were isolated from auxin-treated etiolated seedlings by PCR amplification, and studied their expression patterns in the etiolated melon seedlings and the melon fruit.
CMe-ACS3 was highly homologous at the amino acid level to CM-ACS2, LE-ACS3, AT-ACS4, VR-ACS6 and VR-ACS7 (Table 1). Since the expressions of most of these ACSgenes were induced by auxin without protein synthesis and 5%-flanking sequences of the genes contained auxin-responsivecis-elements, they were proposed to be the primary auxin-responsive ACS genes in theACSgene family [3,10,19]. The mRNA level of CMe-ACS3 increased in etiolated melon seedlings following treatment with auxins (IAA, NAA and 2,4-D) (Fig. 3). Other plant hormones (ABA, BA, ethylene and methyl jasmonate) did not affect the mRNA level of CMe-ACS3. The expression patterns of CMe-ACS3 were in good agreement with those of the primary auxin-responsive genes in the ACS gene family [10].
The amino acid sequence of CMe-ACS2 was homologous to those ofVR-ACS1, PS-ACS2 and AT-ACS6 (Table 1). The latter were members of the multi-responsive ACS gene subfamily, and their mRNA levels were increased in tissues following not only treatment with auxin but also touching and wounding [4,29,30]. The effects of plant hormones on the expression of CMe-ACS2 in etiolated melon seedlings were studied. The results indicated that CMe-ACS2 was an auxin-responsive ACS gene and the expression patterns of the gene induced by various plant hormones were similar to those of CMe-ACS3 in etiolated
Table 1
Predicted amino acid sequence identity among various 1-Aminocyclopropane-1-carboxylate (ACC) synthasesa
CMe-ACS2 (%)
Gene product CMe-ACS3 (%)
CMe-ACS1 62 53
CMe-ACS2 100 55
PS-ACS2 71 52
55 71
VR-ACS1
51
AT-ACS6 69
55 71
LE-ACS6
56
CMe-ACS3 100
90 53
CM-ACS2
57
VR-ACS6 75
VR-ACS7 57 77
75 54
LE-ACS3
70
AT-ACS4 57
aAT-ACS4 and 6,Arabidopsis thaliana[3,4];CM,Cucubita
maxima (winter squash); CMe, Cucumis melo (melon) ([24]; this work);LE,Lycopersicon esculentum(tomato) [15,17];PS,
Pisum sati6um(pea) [28,29];VR,Virgina radiata(mung bean) [7,10–12]. Underline indicates auxin-responsive genes.
hypocotyls, except for the response to NBD (Fig. 3). The mRNA level of CMe-ACS2 increased in etiolated hypocotyls following treatment with NBD with or without IAA in the medium. These results indicated that the low level of ethylene produced in tissues might function to reduce the mRNA level of CMe-ACS2 in the tissues. Similar results were obtained in the experiment in which NBD treatment increased the mRNA level ofVR -ACS1 in etiolated mung bean roots [10]. The basal mRNA level of CMe-ACS2 in etiolated seedlings was sometimes higher than that of CMe-ACS3. These results indicated that not only auxin but also other signals, such as touch signal, might induce the expression ofCMe-ACS2, becauseVR -ACS1 and AT-ACS6 were reported as being touch-induced ACS genes, and were sometimes strongly expressed in control tissues [4,10,30]. Even if a detectable level of mRNA inCMe-ACS2 was present in intact etiolated hypocotyls,
treat-Fig. 6. Structure of the 1-aminocyclopropane-1-carboxylate synthase gene (CMe-ACS2) gene. Panel A: Gene organization of
ment with IAA increased the mRNA level of CMe-ACS2 in the tissues (Fig. 3).
The expression patterns of ACS genes in devel-oping and ripening melon fruit were studied (Fig. 4). Enlargement of the melon fruit began within 5 days after pollination, and continued until day 35 [31]. The mRNA levels of CMe-ACS2 increased within 3 days after pollination and high levels were maintained for at least 12 days during the period when the melon fruit was rapidly developing (Fig. 4). The increase in the level ofCMe-ACS2 mRNA should reflect that of auxin in the developing fruit. The mRNA levels of Cm-ERS1 and Cm-ETR1, which were ethylene receptor genes of melon, were also increased in the developing melon fruit [32]. Although the level of ethylene in young fruit was very low, the low level of ethylene in the fruit might play a role in the control of its development. The mRNA level of CMe-ACS2 decreased at the time of harvest (55 days after pollination), but was transiently increased in mesocarp tissues at the preclimacteric stage (day 3 to day 5 after harvest). The expression pattern of CMe-ACS3 was very similar to that ofCMe-ACS2 in the melon fruit at the preclimacteric stage, although the mRNA level of CMe-ACS3 was lower than that of CMe -ACS2. Nakatsuka et al. [15] reported that the expression of three ACS genes (LE-ACS1b, LE -ACS3 and LE-ACS6) was detectable in the tomato fruit at the preclimacteric stage, and that LE-ACS6 played an important role in ethylene production at the preclimacteric stage (in the sys-tem 1 ethylene production) [22]. LE-ACS6 was homologous at the amino acid level to CMe -ACS2, and was a touch-induced ACSgene [33]. It might be possible that a certain touch signal in mesocarp tissues increased the mRNA level of CMe-ACS2 at the preclimacteric stage. However, the increase in the mRNA level of CMe-ACS3 indicated that the level of endogenous auxin might be increased in mesocarp tissues at the preclimac-teric stage. The increased level of auxin in the tissues might stimulate the expression of CMe -ACS2 in the melon fruit.
CMe-ACS2 and 3 were auxin-responsive ACS genes in melon; however, their expression levels in the etiolated seedlings and the developing fruit differed from each other. To understand the regu-latory mechanisms of ACS gene expression by auxin, it is necessary to isolate the genomic DNA encoding them. As the first step toward achieving
this, the genomic DNA encoding CMe-ACS2 was isolated and its nucleotide sequence determined. Southern blot analysis showed that the restriction digestion fragments of CMe-ACS2 gave a single band, and suggested thatCMe-ACS2 was a single-copy gene in each haploid genome (Fig. 5). The 5%-flanking region of CMe-ACS2 contained a palindromic repeat of TGTCTC-like elements [39] and a putative auxin-responsivecis-element resem-bling the auxin-responsive domain (AuxRD) B of PS-IAA4/5 from pea [34,35], which were located about 100 and 300 bp upstream from TATA box, respectively (Fig. 6B). The TGTCTC elements were found in the 5%-flanking region of the GH3
gene from soybean (AuxRE D1 and D4) [36,37], PS-IAA4/5 (AuxRD A) [34,35] and AT-ACS4 from Arabidopsis [3,38]. Ulmasov et al. [39] showed that the palindromic repeats of the TGTCTC elements functioned as AuxREs and the auxin response factor (ARF) 1 bound to TGTCTC AuxREs. These results indicated that the expression of CMe-ACS2 by auxin might be mediated, in part, by members of the TGTCTC ARF family.
Acknowledgements
We thank T. Kote of the Japan Horticulture Productivity Institute for the supply of melon fruit used in this study and Dr H. Mori of Nagoya University for the gift of the vector primer. This work was supported in part by a grant from the Ministry of Education, Science, Sports and Cul-ture of Japan (no. 05276102).
References
[1] H. Kende, Ethylene biosynthesis, Annu. Rev. Plant Physiol. Plant Mol. Biol. 44 (1993) 283 – 307.
[2] S.F. Yang, N.E. Hoffman, Ethylene biosynthesis and its regulation in higher plants, Annu. Rev. Plant Physiol. 35 (1984) 155 – 189.
[3] S. Abel, M.D. Nguyen, W. Chow, A. Theologis,ACS4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Ara
-bidopsis thaliana, J. Biol. Chem. 270 (1995) 19093 – 19099.
[5] X. Liang, S. Abel, J.A. Keller, N.F. Shen, A. Theologis, The 1-aminocyclo-propane-1-carboxylate synthase gene family of Arabidopsis thaliana, Proc. Natl. Acad. Sci. USA 89 (1992) 11046 – 11050.
[6] D. Van Der Straeten, R.A. Rodrigues-Pousada, R. Vil-larroel, S. Hanley, H.M. Goodman, M.V. Montague, Cloning, genetic mapping, and expression analysis of an
Arabidopsis thaliana gene that encodes 1-aminocyclo-propane-1-carboxylate synthase, Proc. Natl. Acad. Sci. USA 89 (1992) 9969 – 9973.
[7] J.R. Botella, J.M. Arteca, C.D. Schlangnhaufer, R.N. Arteca, A.T. Phillips, Identification and characterization of a full-length cDNA encoding for an auxin-induced 1-aminocyclopropane-1-carboxylate synthase from etio-lated mungbean hypocotyl segments and expression of its mRNA in response to indole-3-acetic acid, Plant Mol. Biol. 20 (1992) 425 – 436.
[8] J.R. Botella, C.D. Schlangnhaufer, J.M. Arteca, R.N. Arteca, A.T. Phillips, Identification of two new members of the 1-aminocyclopropane-1-carboxylate synthase multigene family in mungbean, Gene 123 (1993) 249 – 253.
[9] J.R. Botella, C.D. Schlangnhaufer, R.N. Arteca, A.T. Phillips, Identification and characterization of three pu-tative genes for 1-aminocyclopropane-1-carboxylate syn-thase from etiolated mungbean hypocotyl segments, Plant Mol. Biol. 18 (1992) 793 – 797.
[10] I.S. Yoon, H. Mori, J.H. Kim, B.G. Kang, H. Imaseki,
VR-ACS6 is an auxin-inducible 1-aminocyclopropane-1-carboxylate synthase gene in mungbean (Vigna radiata), Plant Cell Physiol. 38 (1997) 217 – 224.
[11] W.T. Kim, A. Campbell, T. Moriguchi, H.C. Yi, S.F. Yang, Auxin induces three genes encoding 1-aminocy-clopropane-1-carboxylate synthase in mung bean hypocotyls, J. Plant Physiol. 150 (1997) 77 – 84. [12] H.C. Yi, S. Joo, K.H. Nam, J.S. Lee, B.G. Kan, W.T.
Kim, Auxin and brassinosteroid differentially regulate the expression of three members of 1-aminocyclo-propane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.), Plant Mol Biol. 41 (1999) 443 – 454.
[13] T.I. Zarembinski, A. Theologis, Anaerobiosis and plant growth hormones induce two genes encoding 1-aminocy-clopropane-1-carboxylate synthase in rice (Oryza sati6a L.), Mol. Biol. Cell 4 (1993) 363 – 373.
[14] J.E. Lincoln, A.D. Campbell, J. Oetiker, W.H. Rottmann, P.W. Oeller, N.F. Shen, A. Theologis, LE
-ACS4, a fruit ripening and wound-induced 1-aminocy-clopropane-1-carboxylate synthase gene of tomato (Lycopersicon esculentum), J. Biol. Chem. 268 (1993) 19422 – 19430.
[15] A. Nakatsuka, S. Murachi, H. Okunishi, S. Shiomi, R. Nakano, Y. Kubo, A. Inaba, Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxy-late oxidase, and ethylene receptor genes in tomato fruit during development and ripening, Plant Physiol. 118 (1998) 1295 – 1305.
[16] D.C. Olson, J.H. Oetiker, S.F. Yang, Analysis of LE
-ACS3, a 1-aminocyclopropane-1-carboxylic acid syn-thase gene expressed during flooding in the roots of tomato plants, J. Biol. Chem. 270 (1995) 14056 – 14061.
[17] W.H. Rottmann, G.F. Peter, P.W. Oeller, J.A. Keller, N.F. Shen, B.P. Nagy, L.P. Taylor, A.D. Campbell, A. Theologis, 1-Aminocyclopropane-1-carboxylate synthase in tomato is encoded by a multigene family whose transcription is induced during fruit and floral senes-cence, J. Mol. Biol. 222 (1991) 937 – 961.
[18] D. Van Der Straeten, L. Van Wiemeersch, H.M. Good-man, M.V. Montague, Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-car-boxylate synthase in tomato, Proc. Natl. Acad. Sci. USA 87 (1990) 4859 – 4863.
[19] N. Nakagawa, H. Mori, K. Yamazaki, H. Imaseki, Cloning of a complementary DNA for auxin-induced 1-aminocyclopropane-1-carboxylate synthase and differ-ential expression of the gene by auxin and wounding, Plant Cell Physiol. 32 (1991) 1153 – 1163.
[20] N. Nakagawa, Y. Kamiya, H. Imaseki, Nucleotide se-quence of an auxin-regulated 1-aminocyclopropane-1-carboxylic acid synthase gene (Accession No. U37774) fromCucurbita maximaDuch, Plant Physiol. 109 (1995) 1499.
[21] N. Nakajima, H. Mori, K. Yamazaki, H. Imaseki, Molecular cloning and sequence of a complementary DNA encoding 1-aminocyclopropane-1-carboxylate syn-thase induced by tissue wounding, Plant Cell Physiol. 31 (1990) 1021 – 1029.
[22] S.F. Yang, The role of ethylene and ethylene synthesis in fruit ripening, in: W.W. Thomson, E.A. Nothnagel, R.C. Huffaker (Eds.), Plant Senescence: Its Biochemistry and Physiology, The American Society of Plant Physiolo-gists, USA, 1987, pp. 156 – 166.
[23] M. Yamamoto, T. Miki, Y. Ishiki, K. Fujinami, Y. Yanagisawa, H. Nakagawa, N. Ogura, T. Hirabayashi, T. Sato, The synthesis of ethylene in melon fruit during the early stage of ripening, Plant Cell Physiol. 36 (1995) 591 – 596.
[24] T. Miki, M. Yamamoto, N. Nakagawa, O. Ogura, H. Mori, H. Imaseki, T. Sato, Nucleotide sequence of a cDNA for 1-aminocyclopropane-1-carboxylate synthase from melon fruits, Plant Physiol. 107 (1995) 297 – 298. [25] M. Yamamoto, H. Asama, H. Nakagawa, T.
Hira-bayashi, T. Sato, Nucleotide sequence of a wound- and ripening-related 1-aminocyclopropane-1-carboxylate synthase gene (CMe-ACS1, Accession No. AB025906) in melon (Cucumis melo L. cv. AMS), Plant Physiol. 121 (1999) 311.
[26] J. Sambrook, E.F. Fritsch, T. Maniatis, Molecular Cloning: A Laboratory Manual, 2nd Edition, Cold Spring Harbor Laboratory Press, New York, 1989. [27] P.-L. Huang, J.E. Parks, W.H. Rottmann, A. Theologis,
Two genes encoding 1-aminocyclopropane-1-carboxylate synthase in zucchini (Cucurbita pepo) are clustered and similar but differentially regulated, Proc. Natl. Acad. Sci. USA 88 (1991) 7021 – 7025.
[28] S.C. Peck, H. Kende, Sequential induction of the ethyl-ene biosynthetic enzymes by indole-3-acetic acid in etio-lated peas, Plant Mol. Biol. 28 (1995) 293 – 301. [29] S.C. Peck, H. Kende, Differential regulation of genes
[30] J.R. Botella, R.N. Arteca, J.A. Frangos, A mechanical strain-induced 1-aminocyclopropane-1-carboxylic acid synthase gene, Proc. Natl. Acad. Sci. USA 92 (1995) 1595 – 1598.
[31] T. Iwatsubo, H. Nakagawa, N. Ogura, T. Hirabayashi, T. Sato, Acid invertase of melon fruits: immunochemical detection of acid invertase, Plant Cell Physiol. 33 (1992) 1127 – 1133.
[32] K. Sato-Nara, K. Yuhashi, K. Higashi, K. Hosoya, M. Kubota, H. Ezura, Stage- and tissue-specific expression of ethylene receptor homolog genes during fruit develop-ment in musk melon, Plant Physiol. 120 (1999) 321 – 329. [33] M. Tatsuki, H. Mori, Rapid and transient expression of 1-aminocyclopropane-1-carboxylate synthase isogenes by touch and wound stimuli in tomato, Plant Cell Physiol. 40 (1999) 709 – 715.
[34] N. Ballas, L.-M. Wong, A. Theologis, Identification of the auxin-responsive element, AuxRE, in the primary indoleacetic acid-inducible gene, PS-IAA4/5, of pea (Pisum sati6um), J Mol. Biol. 233 (1993) 580 – 596.
[35] N. Ballas, L.-M. Wong, M. Ke, A. Theologis, Two auxin-responsive domains interact positively to induce expression of the early indoleacetic acid-inducible gene
PS-IAA4/5, Proc. Natl. Acad. Sci. USA 92 (1995) 3483 – 3487.
[36] G. Hagen, G. Martin, Y. Li, T.J. Guilfoyle, Auxin-in-duced expression of the soybean GH3 promoter in transgenic tobacco plants, Plant Mol. Biol. 17 (1991) 567 – 579.
[37] Z.-B. Liu, T. Ulmasov, X. Shi, G. Hagen, T.J. Guilfoyle, Soybean GH3 promoter contains mul-tiple auxin-inducible elements, Plant Cell 6 (1994) 645 – 657.
[38] S. Abel, N. Ballas, L.-M. Wong, A. Theologis, DNA elements responsive to auxin, BioEssays 18 (1997) 647 – 654.
[39] T. Ulmasov, G. Hagen, T.J. Guilfoyle, ARF1, a tran-scription facto that binds to auxin response elements, Science 276 (1997) 1865 – 1868.