Summary We investigated changes in photochemical activ-ity and cold hardiness of detached needles of three clones of Picea abies (L.) Karst. by measuring variable chlorophyll fluorescence (Fv/Fm), before and after artificial freezing, from September to June. Photochemical activity varied considerably during the study, but only minor differences in photochemical activity among the clones were observed before freezing. Pho-tochemical activity was high during early fall and then declined from November until April. Photochemical activity was at a minimum in April and then increased quickly to high values in May.
During the period from late September to October, and also during the winter, differences in Fv/Fm ratios after artificial freezing to below −10 °C were observed among clones, indi-cating clonal differences in cold hardiness and hardiness devel-opment. The clone having an average height of 2.3 m after 11 years showed consistently lower cold hardiness than clones that had reached average heights of 4.0 and 5.0 m. There were also differences in the temperature requirement for bud flush-ing among clones.
Keywords: chlorophyll fluorescence, winter stress.
Introduction
Species native to cold regions maintain their physiological integrity during winter as a result of seasonal physiological, biochemical and morphological adaptations (Levitt 1980). However, these species also exhibit short-term acclimation to fluctuations in temperature and other climatic factors (Hän-ninen and Pelkonen 1989), and thus are able to maintain an optimal balance among physiological processes, for instance between growth and frost hardiness (Rehfeldt 1992). Although the various physiological processes involved in acclimation are triggered by environmental factors (Junttila and Kaurin 1990), they are genetically controlled (Engler 1913, Glerum 1976). Regulation of this genetic control is, however, poorly understood.
Chlorophyll a fluorescence has been used as a probe to assess the physiological state of the photosynthetic system (Krause and Weis 1984, Schreiber and Bilger 1987,
Lichten-thaler 1988). Chlorophyll fluorescence can also be used to determine low-temperature tolerance of Norway spruce (Picea abies (L.) Karst.) foliage (Vidaver et al. 1989, Adams and Perkins 1993). Pisek and Winkler (1958) demonstrated that the potential for net photosynthesis varies with season and alti-tude. Frequent frosts in fall decrease the potential for photo-synthesis, which does not recover until temperatures rise in the spring (Bolhàr-Nordenkampf and Lechner 1988, Lundmark et al. 1988).
It has been suggested that the decline in photosynthesis in response to low temperatures is partly due to a cold-induced increase in susceptibility to photoinhibition caused by high irradiances (Öquist 1985). High irradiances combined with chilling temperatures cause photoinhibition in P. abies (Lund-mark and Hällgren 1987, Bolhàr-Nordenkampf and Lechner 1988).
Despite many studies on the photochemical activity and frost hardiness of conifers, several aspects of the ecophysi-ological significance of these studies have not been considered in detail. The objectives of this study were to determine whether clonal differences in cold hardiness and growth are related to photochemical activity. We measured variable chlo-rophyll fluorescence of genetically defined clones of Norway spruce grown in a field test, before and after artificial freezing, from early fall to early summer.
Materials and methods
Study site and material
The study was conducted on three nonjuvenile clones of Nor-way spruce that formed part of a clonal field-test at Sävar (63°54′ N, 20°33′ E, altitude 10 m) in northern Sweden. Before the present study, the clonal material had been subjected to several steps of selection, each with a different selection inten-sity. The ortets in the clone test were originally selected in 1977 from seedlings from two seed orchards, based on 3-year-old height. The two seed orchards were located within 25 km of the study site. Average clonal origins of the two seed or-chards were 63°42′ N, 17°86′ E, altitude 211 m, and 64°05′ N, 16°41′ E, altitude 347 m. One of the clones used in this study,
Seasonal variation in photochemical activity and hardiness in clones of
Norway spruce (
Picea abies
)
JOHAN WESTIN,
1LARS-GÖRAN SUNDBLAD
1and JAN-ERIK HÄLLGREN
21 The Forestry Research Institute of Sweden, P.O. Box 3, S-918 21 Sävar, Sweden
2 Department of Forest Genetics and Plant Physiology, Faculty of Forestry, Swedish University of Agricultural Sciences, S-901 83 Umeå,
Sweden
Received November 23, 1994
Clone 71, was derived from the high altitude seed orchard and Clones 90 and 115 were from the low altitude seed orchard. Cuttings from selected seedlings (the ortets) were rooted in May 1977 and grown in a nursery close to the study site until May 1983. A sample of 22 clones were thereafter selected, classified by height category (above average (+), average (m), and below average (−)) and planted in the field test used in this study. Each clone was represented by 11 ramets planted in rows in a north-east direction and with a quadratic spacing of 2 m. The three clones used in the present study were Clone 71 (height category −), Clone 90 (height category m) and Clone 115 (height category +). All 11 ramets of each clone were well established at the time of the study.
Measurements of tree height and bud flushing
The height of each ramet, defined as the height from ground level up to the uppermost bud of the leading shoot, was meas-ured in May 1994 with a measuring pole. In 1992, the actual date of flushing for each clone was not recorded, but was estimated from the accumulated degree days (> 5 °C) that were required for the clones to flush in the spring of 1994. The clones were considered to be flushing when new needles were clearly visible through the bud scales of terminal buds and new needles were elongated to approximately twice the length of the original bud.
Collection of needles
From each ramet, 10 to 15 needles were collected from the uppermost 1991 whorl and placed in plastic bags. Current-year needles were collected from the midsections of the whorl-branches in all directions. If no clear whorl existed, needles were collected from terminal shoots below the potential lead-ing shoot. Needles were kept in the bags throughout the pretreatment, measurement and freezing procedures. After col-lection, the bags were randomly divided into five groups, corresponding to five freezing temperatures. Between collec-tion and measurements, the bags were stored at 5 °C for 1--16 h. Duration of storage did not significantly alter the results of the fluorescence measurements.
Measurement of chlorophyll fluorescence
Chlorophyll fluorescence was measured in darkness (weak, green guiding light provided by a Philips PF710E light bulb) with a plant stress fluorometer (BioMonitor, Malmö, Sweden; described by Öquist and Wass 1988) after needles had been allowed to adapt to darkness for at least 1 h at 5 °C. Actinic light intensity was 200 µmol m−2 s−1 and the duration of excitation was 5 s. The irradiance was considered to be satu-rating because higher intensities did not result in higher Fv/Fm ratios. During measurements, needles were aligned within the bags and the end of the optical fiber of the fluorometer was placed on top of the needles in direct contact with the plastic bag. When no variable chlorophyll fluorescence could be de-tected, the sample was given a value of zero.
Freezing of needles
Needles were frozen in the bags by immersion in ethanol in a
programmable low-temperature thermostat bath (Lauda RKP 20-D, Lauda-Königshofen, Germany) equipped with an exter-nal freezing bath. Before freezing, the initial temperature of the freezing bath was between 15 and 20 °C. The temperature was first lowered to 5 °C in 2 h and then at a rate of 5 °C h−1 to
−25 °C. Sets of samples were withdrawn from the freezing bath at 5 °C intervals and directly (within approximately 1--2 min) stored at 5 °C in darkness. Chlorophyll fluorescence measurements of samples after freezing were made by the same procedure as for measurements before freezing and were performed within 2--16 h after the freezing treatments. Dura-tion of storage did not significantly alter the results of the fluorescence measurements.
Statistical analysis
Data were subjected to analysis of variance and estimation of least-square means according to the GLM procedures of the SAS software package (SAS Institute Inc., Cary, NC). To estimate mean Fv/Fm ratios before and after freezing, two separate models were used with individual Fv/Fm ratios as dependent variables (Equation 1):
γik = ci + eik, (1)
where γik are individual observations of Fv/Fm ratios before or after freezing, ci is the fixed clone effect, and eikare random residual deviations assumed to be normally independently distributed.
Means for each temperature regime on each measurement date were estimated separately. Tukey’s studentized range (HSD) test was used to test whether differences in mean Fv/Fm ratios between clones on a single measurement date were statistically significant at P = 0.05.
Results
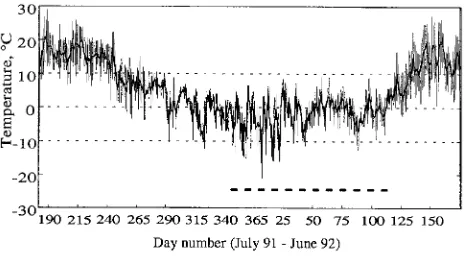
Mean maximum and minimum air temperatures (recorded at a site 20 km from the study site), before and during the study period, are shown in Figure 1. It should be noted that the winter, from November until the beginning of April, was
usually mild for this latitude.
In May 1994, average tree heights for Clones 71, 90 and 115 were 2.3, 4.0 and 5.0 m, respectively. Clones 71 and 115 flushed on June 3, 1994, whereas Clone 90 flushed 1 week later on June 10, 1994 (Table 1).
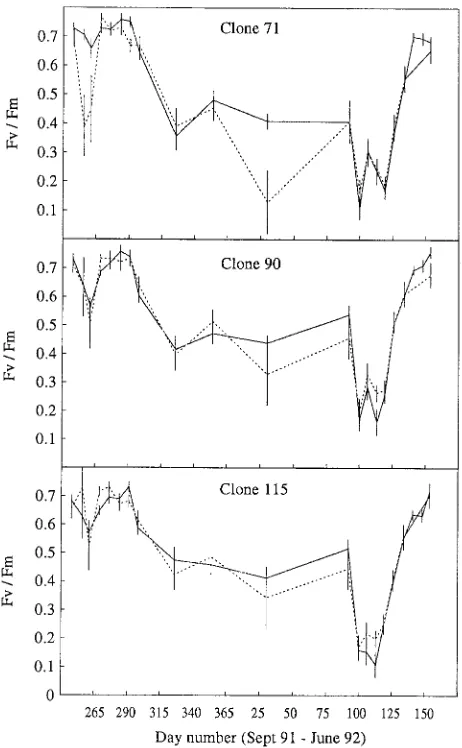
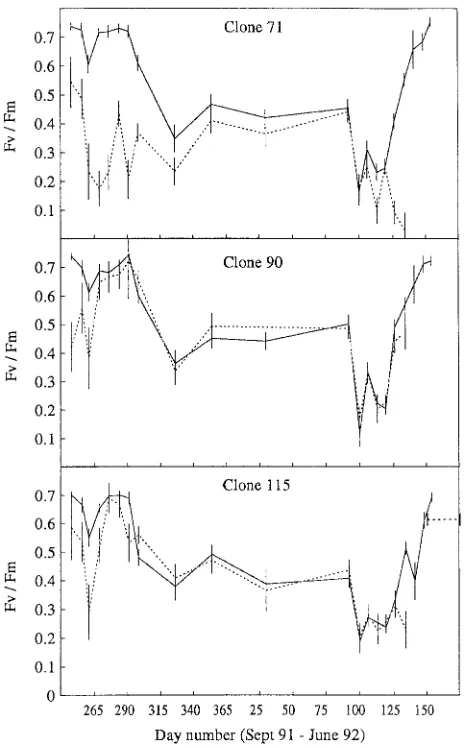
The effects of freezing temperatures on the Fv/Fm ratios of needles of each clone over time are shown in Figures 2--4. When Fv/Fm ratios before freezing were used as a dependent variable in the analyses of variance, there were no statistically significant differences among clones. However, on several occasions, Clone 71 had slightly higher Fv/Fm ratios than Clones 90 and 115. All clones showed similar patterns of development of photochemical activity during the study period from September 1991 to June 1992.
When Fv/Fm ratios after freezing were used as a dependent variable in the analyses of variance, statistically significant differences among clones were observed on several occasions for freezing temperatures below −10 °C. During the period from late September to late October, at all freezing treatments below −10 °C, the Fv/Fm ratio of Clone 71 was significantly more affected by freezing than the Fv/Fm ratios of the other clones. Similarly, in May, at all freezing temperatures below
−10 °C, in most cases, the Fv/Fm ratios of Clones 71 and 115 were more affected by freezing than that of Clone 90.
Discussion
Night frosts in early September resulted in minor and tempo-rary declines in variable chlorophyll fluorescence measured before artificial freezing. By the end of October, however, a permanent decrease in variable chlorophyll fluorescence had occurred. The decrease coincided with day and night average temperatures below freezing and occasional night tempera-tures down to −10 °C. This pattern was consistent for all three clones and is similar to the patterns previously observed in Norway spruce (Bolhàr-Nordenkampf and Lechner 1988, Lundmark et al. 1988).
In parallel with the decrease in variable chlorophyll fluores-cence before freezing, frost hardiness increased. The increase in frost hardiness was evident as a decrease in the difference in variable chlorophyll fluorescence before and after freezing. The rate of hardening was most rapid when the daily mean temperature fell to near or below 5 °C. Similar results have been reported by Heide (1974) and Aronsson (1975) who showed that both short days and chilling are needed to induce cold hardiness in Norway spruce. Repo (1992) found that, for both Scots pine (Pinus sylvestris L.) and Norway spruce,
temperatures between 10 and 0 °C represent the most efficient interval for induction of cold hardening.
In contrast to photochemical activity, frost hardiness and responses to artificial freezing differed among clones from late September to late October. Clone 71 appeared less cold hardy than Clones 90 and 115; it also showed a 1--2 weeks later induction of cold hardening and was severely affected by freezing temperatures below −15 °C in mid-October.
Table 1. Date for flushing in 1994 for each clone and the estimated dates for flushing in 1992 based on accumulated degree days required in 1994. Clone Date for flushing Accumulated degree days (5 °C) Date in 1992 when Day number in 1992 when in 1994 required for flushing in 1994 accumulated degree days accumulated degree days
(1994) achieved (1994) achieved
71 June 3 73 May 24 145
90 June 10 111 May 28 149
115 June 3 73 May 24 145
Figure 2. Mean values of the variable chlorophyll fluorescence ratio,
Fv/Fm, for Clones 71, 90 and 115 from September 1991 to June 1992,
Throughout the winter, from November until the beginning of April, photochemical activity was low and stable with Fv/Fm ratios ranging between 0.35 and 0.5 for all three clones. The fluctuations in Fv/Fm ratios that occurred seemed to coincide with fluctuations in temperature. Although the weather during this period was unusually mild with day temperatures occa-sionally above 5 °C, photochemical activity did not recover to high values in any of the clones. Because of the long intervals between measurements during winter, it is possible that the actual variation in fluorescence ratios was larger than is evi-dent from Figures 2--4.
During the winter, Clones 90 and 115, but not Clone 71, were frost hardy and did not exhibit further decreases in vari-able chlorophyll fluorescence in response to artificial freezing to −25 °C. In April, the Fv/Fm ratio in all three clones decreased to a minimum, although air temperatures in April were not significantly different from those in previous months. Thus,
temperature was not the only factor that affected photochemi-cal activity. There are several studies in which low tempera-tures were found to increase the susceptibility to photoinhibition caused by high light intensities. Strand and Öquist (1985a, 1985b) concluded that, in Scots pine, both low temperatures and freezing temperatures resulted in increased photodamage during periods of high irradiance. The global irradiation in April 1992 was 85.6 kWh m−2 (SMHI 1992b), which is less than the average (since 1959) of 110.8 kWh m−2, but is still considerably higher than in March (49.1 kWh m−2) (SMHI 1992a). Incident light intensities on nonshaded needles could have been further increased as a result of reflected light from late snow cover. We conclude, therefore, that the decrease in Fv/Fm in April in Norway spruce needles in the field was caused by photoinhibition (cf. Strand and Öquist 1985b).
Because fluorescence ratios in April were low, additional depression in response to artificial freezing was less reliable as
Figure 3. Mean values of the variable chlorophyll fluorescence ratio,
Fv/Fm, for Clones 71, 90 and 115 from September 1991 to June 1992,
before freezing (solid line) and after freezing to −15 °C (dotted line). Standard error of the means are indicated by vertical bars. For each clone, the estimated starting date of flushing plus 25 days is indicated by a horizontal dotted line; n = 1--5.
Figure 4. Mean values of the variable chlorophyll fluorescence ratio,
Fv/Fm, for Clones 71, 90 and 115 from September 1991 to June 1992,
a frost hardiness indicator than if initial Fv/Fm ratios had been high. However, even in April, Clone 71 was more sensitive to low temperatures than Clones 90 and 115. The spring recovery in variable chlorophyll fluorescence was rapid in all three clones and occurred when air temperatures increased in May, which is in accordance with recovery studies in Scots pine (Ottander and Öquist 1991). A similar but slower response was observed in Scots pine and Norway spruce by Lundmark et al. (1988) and in Norway spruce by Bolhàr-Nordenkampf and Lechner (1988).
A tendency toward dehardening was observed in mid-May in Clones 71 and 115. Based on fluorescence ratios after freezing, Clone 90 was the hardiest clone in late spring--early summer and, in 1994, it also flushed 1 week later than Clones 71 and 115 (Table 1).
Because the fluorescence ratios before freezing were similar for all three clones on all occasions, we conclude that the differences in cold hardiness observed among the clones were not related to photochemical activity. Also, photochemical activity did not explain the large differences in growth of the clones. The low hardiness of Clone 71 could, however, provide a possible explanation for its stunted growth. The difference in growth between Clones 90 and 115 is more difficult to explain. Perhaps the late flushing and dehardening of Clone 90 resulted in a shorter growth period, whereas the early flushing and dehardening of Clone 115, if it did not result in significant frost damage, provided a longer growth period.
Acknowledgments
The authors thank staff members at Sävar for their help in collecting, freezing and measuring samples. We also thank Lecturer Sören Holm at the Swedish University of Agricultural Sciences, Faculty of For-estry, for valuable advice on the statistical analysis of data.
References
Adams, G.T. and T.D. Perkins. 1993. Assessing cold tolerance in Picea
using chlorophyll fluorescence. Environ. Exp. Bot. 33:377--382. Aronsson, A. 1975. Influence of photo- and thermoperiod on the initial
stages of frost hardening and dehardening of phytotron-grown seed-lings of Scots pine (Pinus sylvestris) and Norway spruce (Picea abies (L.) Karst.). Stud. For. Suec. 128:1--20.
Bolhàr-Nordenkampf, H.R. and E.G. Lechner. 1988. Temperature and light dependent modifications of chlorophyll fluorescence kinetics in spruce needles during winter. Photosynth. Res. 18:287--298. Engler, A. 1913. Einfluss der Provenienz des Samens auf die
Eigen-schaften der forstlichen Holzgewächse. Mitt. Schweiz. Central. Forstl. Versuchsw. 10:189--386.
Glerum, C. 1976. Frost hardiness of forest trees. In Tree Physiology and Yield Improvement. Eds. M.G.R. Cannell and F.T. Last. Proc. Conf. Physiol. Genet. For. Tree Yield, Gorebridge, Scotland, pp 403--420.
Heide, O.M. 1974. Growth and dormancy in Norway spruce ecotypes. I. Interaction of photoperiod and temperature. Physiol. Plant. 30:1--12.
Hänninen, H. and P. Pelkonen. 1989. Dormancy release in Pinus sylvestris L. and Picea abies (L.) Karst. seedlings: effects of inter-mittent warm periods during chilling. Trees 3:179--184.
Junttila, O. and Å. Kaurin. 1990. Environmental control of cold accli-mation in Salix pentandra. Scand. J. For. Res. 5:195--204. Krause, G.H. and E. Weis. 1984. Chlorophyll fluorescence as a tool in
plant physiology. II. Interpretation of fluorescence signals. Photo-synth. Res. 5:139--157.
Levitt, J. 1980. Responses of plants to environmental stresses. Vol. 1. Academic Press, New York, pp 139--149.
Lichtenthaler, H.K. 1988. In vivo chlorophyll fluorescence as a tool for
stress detection in plants. In Applications of Chlorophyll
Fluores-cence. Ed. H.K. Lichtenthaler. Kluwer Academic Publishers, Dor-drecht, The Netherlands, pp 129--142.
Lundmark, T. and J.-E. Hällgren. 1987. Effects of frost on shaded and exposed spruce and pine seedlings planted in the field. Can. J. For. Res. 17:1197--1201.
Lundmark, T., J.-E. Hällgren and J. Hedén. 1988. Recovery from winter depression of photosynthesis in pine and spruce. Trees 2:110--114.
Ottander, C and G. Öquist. 1991. Recovery of photosynthesis in winter-stressed Scots pine. Plant Cell Environ. 14:345--349. Öquist, G. 1985. Effects of winter stress on chlorophyll organization
and function in Scots pine. J. Plant Physiol. 122:169--179. Öquist, G. and R. Wass. 1988. A portable, microprocessor operated
instrument for measuring chlorophyll fluorescence kinetics in stress physiology. Physiol. Plant. 73:211--217.
Pisek, A. and E. Winkler. 1958. Assimilationsvermögen und Respira-tionen der Fichte (Picea excelsia Link.) in verschiedener Höhenlage und der Zirbe (Pinus sembra L.) an der alpinen Waldgrenze. Planta
51:518--543.
Rehfeldt, G.E. 1992. Early selection in Pinus ponderosa:
compro-mises between growth potential and growth rhythm in developing breeding strategies. For. Sci. 3:661--677.
Repo, T. 1992. Seasonal changes of frost hardiness in Picea abies and Pinus sylvestris in Finland. Can. J. For. Res. 22:1949--1957.
Schreiber, U. and W. Bilger. 1987. Rapid assessment of stress effects on plant leaves by chlorophyll fluorescence measurements. In Plant
Response to Stress. Functional Analysis in Mediterranean Ecosys-tems. Eds. J.D. Tenhunen, F.M. Catarino, O.L. Lange and W.C. Oechel. Springer-Verlag, Berlin, Germany, pp 27--53.
SMHI (The Swedish Meteorological and Hydrological Institute). 1992a. Global strålning. Väder och Vatten, April 1992, 16 p.
SMHI (The Swedish Meteorological and Hydrological Institute). 1992b. Global strålning. Väder och Vatten, May 1992, 16 p. Strand, M. and G. Öquist. 1985a. Inhibition of photosynthesis by
freezing temperatures and high light levels in cold-acclimated seed-lings of Scots pine (Pinus sylvestris). I. Effects on the light-limited
and light-saturated rates of CO2 assimilation. Physiol. Plant.
64:425--430.
Strand, M. and G. Öquist. 1985b. Inhibition of photosynthesis by
freezing temperatures and high light levels in cold-acclimated seed-lings of Scots pine (Pinus sylvestris). II. Effects on chlorophyll fluorescence at room temperature and 77 K. Physiol. Plant. 65:117--123.
Vidaver, W., W. Binder, R.C. Brooke, G.R. Lister and P.M.A. Toivonen. 1989. Assessment of photosynthetic activity of nursery-grown Picea glauca seedlings using an integrating fluorometer to