Summary Indirect ELISA was used to quantify (+)-abscisic acid (ABA) in developing zygotic embryo and megagameto-phyte tissues from two loblolly pine (Pinus taeda L.) mother trees. On a dry weight basis, embryonic and megagametophytic ABA concentrations were highest during early development and decreased steadily to their lowest values at cone ripening. Embryonic ABA concentration was significantly higher than megagametophytic ABA concentration. The ABA content of embryos was lowest during early to mid-development; it in-creased rapidly during mid-development, subsequently de-clined, and then either remained constant or declined at cone ripening (late development). Total ABA per embryo increased rapidly just before the rapid increase in embryo dry weight. Total ABA per megagametophyte remained constant through-out development, except during mid-development when it tem-porarily declined.
Keywords: ELISA, enzyme-linked immunosorbant assay, lob-lolly pine, seed maturation, somatic embryogenesis.
Introduction
Putative roles for (+)-ABA in seed development include inhi-bition of precocious germination, promotion of storage protein synthesis and desiccation tolerance, suppression of reserve mobilization, and induction of dormancy (reviewed by Black 1991). During somatic embryogenesis in Picea glauca (Moench) Voss (Attree et al. 1992), Picea engelmanni Parry ex Engelm. (Roberts 1991), Picea abies (L.) Karst. (Hakman et al. 1990) and Pinus taeda L. (Becwar and Feirer 1989), exo-genously applied ABA can inhibit precocious germination and stimulate the accumulation of storage nutrients, lipids and proteins. Also, in combination with polyethylene glycol, ABA improves desiccation tolerance in somatic embryos of P. glauca by enhancing lipid biosynthesis (Attree et al. 1992). Very little is known about changes in ABA concentrations during conifer zygotic embryogenesis. It seems likely that ABA plays a similar critical role in the development of zygotic and somatic embryos of conifers as has been observed for such species as wheat and barley (Quarrie et al. 1988), pea (Wang et al. 1987), rice (Grossmann et al. 1986) and bean (Prevost and Le Page-Degivry 1985).
This study was undertaken to gain a better understanding of the role of ABA in regulating morphological events during zygotic embryogenesis in loblolly pine. The specific objective was to quantify (+)-ABA concentrations in embryos and gametophytes during development from fertilization to cone ripening using an indirect enzyme-linked immunosorbant as-say (ELISA). A second objective was to use the data to develop a model to assist in determination of optimal ABA require-ments for a somatic embryogenesis system.
Materials and methods
Reagents
Phosphate-buffered saline plus Tween (PBS/Tween) contained 13.6 g potassium monophosphate, 29.2 g sodium chloride per liter plus 0.05% Tween 20 (pH 7.4). Blocking solution con-tained 5% (w/v) bovine serum albumin (BSA) (Sigma, A-3425) in PBS/Tween. Assay buffer contained 0.1% (v/v) BSA in PBS/Tween.
The ABA-4′-BSA conjugate was prepared according to Quarrie and Galfre (1985) and stored at −20 °C. The conjugate was diluted (1/100,000) in 0.05 M sodium carbonate (pH 9.6) before use. The monoclonal antibody (mAb) raised against free cis-(+)-ABA (Mertens et al. 1983) was from Idetek Inc., (San Bruno, CA). The mAb was dissolved in 2 ml of assay buffer, and 10 µl aliquots were stored at −80 °C. Before use, mAb was further diluted (1/5000) in assay buffer. Goat anti-mouse antibody-biotin conjugate (GAM-biotin conjugate, Sigma, B-7264) was stored as 10 µl aliquots at −20 °C. The conjugate was diluted 1/5000 in assay buffer before use. Strep-tavidin-poly-HRP conjugate (Research Diagnostics Inc., Flan-ders, NJ, No. RDI-pHRP20-SA) was stored at −20 °C in 10 µl aliquots. The conjugate was diluted 1/5000 in assay buffer before use. A 10 mg ml−1 solution of tetramethyl-benzidine (TMB) substrate (Sigma, T-2885) in DMSO was diluted 1/1000 in 100 mM sodium acetate (pH 5.5). A 3% solution of H2O2 was added to yield a final concentration of 0.002%. Abscisic acid (ABA) standards (15.8 ng to 0.25 pg (+)-ABA per 100 µl of assay buffer) were prepared by serial dilution of a 50 mM (+)-cis-ABA stock solution obtained by dissolving (±)-cis,trans-ABA (Sigma, A-1012) in absolute methanol. The
Abscisic acid and zygotic embryogenesis in
Pinus taeda
RENE H. KAPIK,
1,2RONALD J. DINUS
3and JEFFREY F. D. DEAN
41 Kimberly-Clark, Technical Papers, 1400 Holcomb Bridge Road, Roswell, GA 30076, USA
2 Present address: 503 Maple Street, Munising, MI 49826, USA
3 Institute of Paper Science and Technology (IPST), Forest Biology Group, 500 10th St. NW, Atlanta, GA 30318, USA
4 Department of Biochemistry, University of Georgia, Athens, GA 30602, USA
Received May 31, 1994
stock solution was stable for at least 3 months when stored at −20 °C in the dark. The small amount of methanol in the standards did not affect the performance of the antibody (data not presented).
Plant material
Loblolly pine cones from two seed orchards were shipped overnight, on ice packs, on a weekly basis from June to Octo-ber 1992 (i.e., the period from fertilization to cone ripening). The two mother trees were designated as UC (Bellville, GA) and WV (Summerville, SC). On delivery, seeds were immedi-ately extracted from the cones, and embryos, suspensors and megagametophytes were separated under a stereomicroscope at 4 °C. The embryos were scored for morphological develop-ment and briefly rinsed in distilled water to remove any hor-mones that may have leaked from the megagametophyte during dissection. The embryo, suspensor and megagameto-phyte tissues were frozen separately in liquid nitrogen, lyo-philized and stored at −80 °C until analyzed.
Morphological stages of embryo development
The morphological development of the embryos was scored according to a method developed at the Institute of Paper Science and Technology (Pullman and Webb 1994). Stage 1: proembryos up to the 12-cell stage; found mainly in the arche-gonium; approximately 3 days after fertilization (DAF). Stage 2: embryo-proper (polyembryony); embryos are distinct and translucent, and still found at the micropylar end of the mega-gametophyte; approximately 6 DAF. Stage 3: dominant em-bryo-proper present; it is white, opaque, and located at the chalazal end of the megagametophyte; approximately 39 DAF. Stage 4: embryo-proper is similar to Stage 3, but is larger longitudinally and radially, and is still dome-shaped and opaque; approximately 43 DAF. Stage 5: similar to Stage 4, except the apical meristem is visible; approximately 45 DAF. Stage 6: similar to Stage 5, except the cotyledon primordia are barely visible below the apical meristem; approximately 47 DAF. Stage 7: similar to Stage 6, except the cotyledons are elongated, but do not overtop the apical meristem; approxi-mately 49 DAF. Stage 8A: similar to Stage 7, except the cotyledons overtop the apical meristem; apical meristem is still visible; approximately 52 DAF. Stage 8B: similar to Stage 8A, except the embryo and cotyledon lengths are longer; approxi-mately 57 DAF. Stage 9: cotyledons are curved and joined at their tips; apical meristem is not visible from any angle; tissue was collected weekly and subdivided by percent water content of tissue because growth continued without obvious morpho-logical change (Stages 9A--9H).
Determination of ABA
Isolated tissues were ground with a cold glass rod, weighed, then placed in screw-cap Teflon tubes and extracted in 10 ml of 80% methanol (adjusted to pH 7) containing 25 mg l−1 butylated hydroxytoluene as an antioxidant (Milborrow and Mallaby 1975, Neill et al. 1983, Neill and Horgan 1987). Approximately 5 × 105 Bq of [3H]-ABA (TRK.644, Amer-sham, Arlington Heights, IL) was added as an internal
stand-ard. The tubes were purged with nitrogen, and the tissues were extracted overnight with stirring at 4 °C in the dark.
The homogenate was centrifuged at 2000 g for 15 min, and the supernatant removed. The pellet was re-extracted in 1 ml of solvent. The supernatants were pooled, passed through a 0.45 µm nylon filter, and reduced to near dryness in a rotoeva-porator at 35 °C. The remaining aqueous phase was diluted to 1000 µl with assay buffer for analysis by indirect ELISA. The procedure averaged an [3H]-ABA recovery of 81% (95% CI of 3%).
The ELISA procedure was similar to the method described by Walker-Simmons (1987) except for the substitution of a biotin-streptavidin-multiple horseradish peroxidase (HRP) system for added amplification. We used Immulon-2, flat-bot-tom, 96-well microtitration plates (Dynatech Laboratories, Chantilly, VA) (Walker-Simmons 1987). An aliquot of 200 µl of diluted ABA-4′-BSA conjugate was added to each well of the microtiter plate except those in the outside rows, which were previously shown to produce inconsistent results (Ross et al. 1987). The plates were sealed with Parafilm and incubated overnight at 4 °C in the dark. The plates were then aspirated and washed with PBS/Tween. Approximately 300 µl of 5% BSA solution was then added to each well to block the remain-ing protein adsorption sites, and the plates were incubated for 45 min at 37 °C in the dark. The wells were aspirated and washed with PBS/Tween. The ABA standards and samples were diluted 1/1 with diluted mAb and incubated overnight at 4 °C in the dark. Aliquots (200 µl) of the ABA standards and samples that had been incubated with mAb were added to the plate and then incubated for 90 min at 37 oC in the dark. The wells were aspirated and washed with PBS/Tween. Diluted GAM-biotin conjugate (200 µl) was added to each well, and the plate was incubated for 90 min at 37 °C in the dark. The wells were aspirated and washed with PBS/Tween. Diluted streptavidin-poly-HRP conjugate (200 µl) was added to each well, and the plate was incubated for 90 min at 37 °C in the dark. The wells were aspirated, washed with PBS/Tween, and once with 300 µl of 100 mM sodium acetate per well to remove chloride ions, which are inhibitory to peroxidase activity. The TMB solution (200 µl) was then added to each well, and the blue color was allowed to develop at room temperature in the dark. Reactions were stopped with 40 µl of 1.5 NH2SO4, and the absorbance was determined at 450 nm. The concentration of (+)-ABA in the samples was calculated by performing a log transformation and comparing the results to the calibration curve of (+)-ABA for each plate (Weiler et al. 1986).
The GC/MS-SIM validation of the indirect ELISA
a DB-1 (J&W Scientific Ltd.) 15 m × 0.25 mm × 0.25 µm film. The injection and interface temperatures were 300 °C. Data were collected using the SIM program, monitoring four ions: m/e 190 (endogenous ABA), m/e 194 (2H6-ABA), and m/e 162 and 166 to monitor impurities. Validation of the ELISA by GC/MS-SIM demonstrated good agreement between the two methods and showed no cross-reactants or interferants to the ELISA (data not presented).
Statistical analysis
Statistical significance of the multiple pairwise comparisons for ABA in the tissues was based on Bonferroni-Welch which assumes normal distributions but unequal variances between samples. Analyses were carried out at 95% confidence inter-vals (α = 0.05).
Results and discussion
Concentrations and contents of ABA in developing tissues
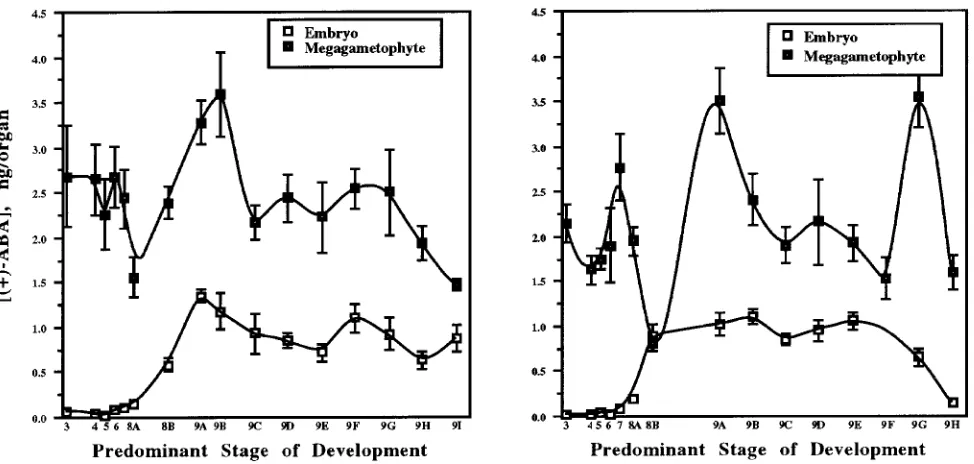
Changes in ABA contents and concentrations during develop-ment of embryos and megagametophytes are illustrated for UC and WV trees in Figures 1 and 2, respectively. Chronological spacing of the data points along the abscissa represents the stages of embryo development, i.e., rapid morphological de-velopment in Stages 4--8A was followed by slower develop-ment during maturation. Stage 9 tissue was collected on a weekly basis. Abscisic acid was detected in all tissues tested.
Megagametophytic ABA contents at Stages 8A and 9A were significantly lower and higher, respectively, than the ABA contents at all other stages. No other significant change in megagametophytic ABA content was detected. The ABA
con-tents of megagametophytes were significantly higher than the ABA contents of the corresponding embryo tissues, a trend that appears to be species dependent (Jones and Brenner 1987, Piaggesi et al. 1991).
We compared the changes in ABA concentrations and con-tents during zygotic embryogenesis in loblolly pine with the changes observed in herbaceous species, because no compara-ble study has been reported for gymnosperms. In many herba-ceous species, ABA is low or undetectable during early embryo development. Abscisic acid concentrations and con-tents tend to increase to maximum values by the midpoint of the maturation process, and then decrease to low values at or near full maturity (Quarrie et al. 1988, Napier et al. 1989, Xu et al. 1990, Groot et al. 1991). We did not observe this trend in either embryo or megagametophyte tissues of loblolly pine (Figure 1). In loblolly pine embryos, there was a significant increase in ABA content from Stages 8A to 9A, followed by either a decline and a subsequent increase in ABA content in embryo tissue from the UC mother tree or a rapid drop in ABA content in embryo tissue from the WV mother tree. This trend was similar to that found for ABA contents in embryos from Acer platanoides L. (Pinfield and Gwarazimba 1992), Prunus persica L. cv Springcrest (Piaggesi et al. 1991), Phaseolus coccineus L. (Perata et al. 1990) and Pisum sativum L. (Wang et al. 1987).
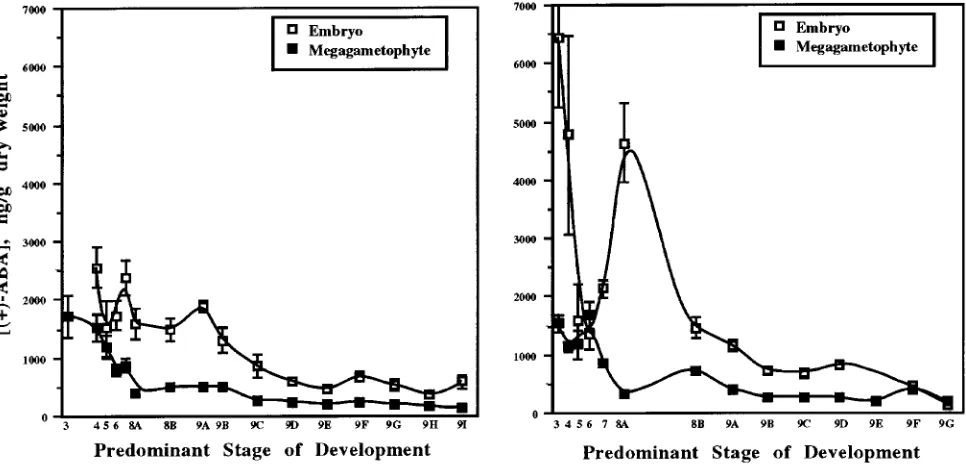
Generalizations about trends in ABA concentrations during embryo development in herbaceous plants were not applicable to loblolly pine embryos (Figure 2). Overall, the highest ABA concentrations in embryos were found during early embryo development. Subsequently, the ABA concentrations declined gradually to reach minimum values at cone ripening. A similar trend has been observed in embryos of Zea mays L. (Jones and
Brenner 1987) and Triticum aestivum L. (Walker-Simmons 1987). We observed two significant ABA peaks in loblolly pine embryos: Stages 7 and 9A for the UC mother tree and Stages 3 and 8A for the WV mother tree. Two ABA peaks were also observed in developing embryos of Phaseolus coccineus (Perata et al. 1990) and Phaseolus vulgaris L. (Hsu 1979), and three to four peaks were observed in Brassica napus L. (Finkelstein et al. 1985). The ABA peaks in these plants did not necessarily occur at the same stage of embryo development. The concentration of ABA in loblolly pine embryos was sig-nificantly higher than in megagametophyte tissue. This trend appears to be species dependent (Jones and Brenner 1987, Quarrie et al. 1988, Xu et al. 1990).
It has been suggested that embryos synthesize ABA (Bray and Beachy 1985). In loblolly pine embryos, however, the initial increase in ABA occurred concomitantly with an appar-ent decrease in ABA contappar-ent in the megagametophyte (Fig-ure 1), suggesting that loblolly pine embryos may import ABA from the megagametophyte. In loblolly pine, there are no direct vascular connections between the developing embryo and the mother plant, and so it is possible that the embryo obtains all of its water and nutrients from the megagameto-phyte tissues (Morris et al. 1991). Rates of transfer of water and nutrients are likely to be greatest during early tissue development when water content is high and the tissues are in intimate contact with each other. During later stages of devel-opment, when desiccation is rapid, the outer cell layers of the embryo and megagametophyte dry out and break close con-tact, thus restricting the movement of water and nutrients. This developmental change might explain the increase in mega-gametophtyic ABA without a corresponding change in embry-onic ABA in samples from the WV mother tree at Stage 9F.
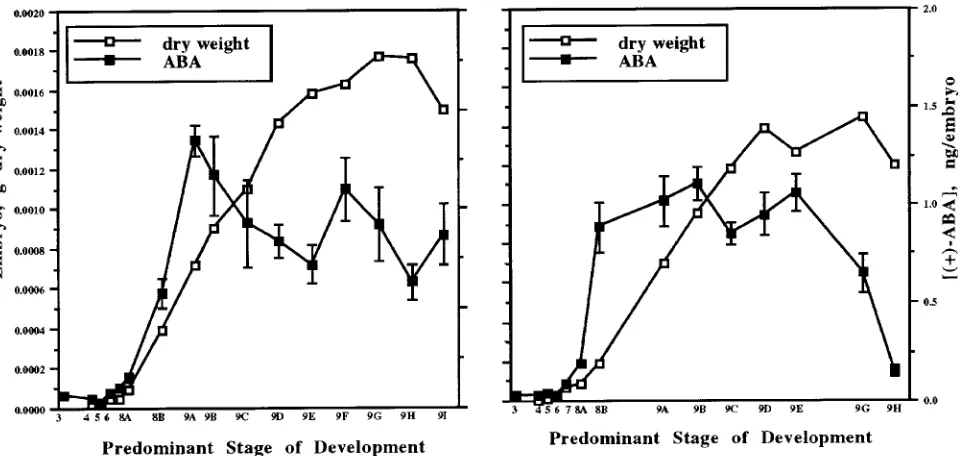
In embryos, there was a rapid increase in ABA just before an
increase in dry weight (Figure 3). This trend has been observed in many species, including Zea mays (Jones and Brenner 1987), Glycine max (L.) Merrill (Ackerson 1984) and Triticum aestivum (King 1976). Although increases in ABA may trigger increases in embryo dry mass, further evidence is necessary to substantiate any causal relationship between ABA and either storage product accumulation or desiccation. In Gossypium, high concentrations of specific late-embryogenesis-abundant (LEA) proteins coincide with an increase in endogenous seed ABA concentrations, and these proteins can also be prema-turely induced by incubation of immature seeds in ABA (Galau et al. 1986, Skriver and Mundy 1990). It is not known whether the production of similar proteins in loblolly pine embryos is correlated with the increase in ABA content.
Conclusions
Trends in (+)-ABA concentrations and contents were similar in seed tissues from two loblolly pine mother trees grown in different locales. The ABA peaks did not correspond to any observable morphogenetic changes in the embryos. However, a rapid increase in ABA content in embryos occurred just before the rapid increase in embryo dry weight. Although a causal relationship is suggested, it is not known if the increase in ABA content triggered the increase in embryo dry mass. In Norway spruce, an increase in ABA appears to stimulate late-stage synthesis of triacylglycerides and storage lipids (Feirer et al. 1989).
The ABA content of megagametophyte tissue did not change significantly during development (Figure 1). We attrib-ute the apparent decline in ABA concentration during develop-ment (Figure 2) to an increase in dry weight, and not to a decrease in ABA. Furthermore, we suggest that the ABA
sent in embryonic tissue is imported from the megagameto-phyte, especially during early development when water con-tent is high and the tissues are in close contact with each other.
Acknowledgments
We thank Union Camp (UC) and Westvaco (WV) Corporations for the supplies of cones throughout 1991 and 1992. Financial support from the Institute of Paper Science and Technology and its member compa-nies is also gratefully acknowledged. The 2H6-ABA was synthesized by Dr. Laurent Rivier, and was a gift from Dr. R.P. Pharis, University of Calgary, Calgary, Alberta, Canada.
References
Ackerson, R.C. 1984. Regulation of soybean embryogenesis by ab-scisic acid. J. Exp. Bot. 35:403--413.
Attree, S.M., M.K. Pomeroy and L.C. Fowke. 1992. Manipulation of conditions for the culture of somatic embryos of white spruce for improved triacylglycerol biosynthesis and desiccation tolerance. Planta 187:395--404.
Becwar, M.R. and R.P. Feirer. 1989. Factors regulating loblolly pine (Pinus taeda L.) somatic embryo development. In Proc. 20th South. For. Tree Improv. Conf., Charleston, SC, pp 178--185.
Black, M. 1991. Involvement of ABA in the physiology of developing and mature seeds. In Abscisic Acid: Physiology and Biochemistry. Eds. W.J. Davies and H.G. Jones. Bios Scientific Publishers, Ox-ford, U.K., 89--101.
Bray, E.A. and R.R. Beachy. 1985. Regulation by ABA of β -congly-cinin expression in cultured developing soybean cotyledons. Plant Physiol. 79:746--750.
Finkelstein, R.R., K.M. Tenbarge, J.E. Shumway and M.L. Crouch. 1985. Role of ABA in maturation of rapeseed embryos. Plant Physiol. 78:630--636.
Feirer, R.P., J.H. Conkey and S.A. Verhagen. 1989. Triglycerides in embryogenic conifer calli: a comparison with zygotic embryos. Plant Cell Rep. 8:207--209.
Galau, G.A., D.W. Hughes and L. Dure III. 1986. Abscisic acid induction of cloned cotton late embryogenesis-abundant (LEA) mRNAs. Plant Mol. Biol. 7:155--170.
Groot, S.P.C., I.I. van Yperen and C.M. Karssen. 1991. Strongly reduced levels of endogenous abscisic acid in developing seeds of tomato mutant sitiens do not influence in vitro accumulation of dry matter and storage proteins. Physiol. Plant. 81:73--78.
Grossmann, C., H.O. Schmidt and J. Jung. 1986. Changes in mem-brane permeability and mineral, phytohormone, and polypeptide composition in rice suspension cells during growth and under the influence of the growth retardant tetcyclacis. Plant Cell Rep. 5:315--318.
Hakman, I., P. Stabel, P. Engstrom and T. Eriksson. 1990. Storage protein accumulation during zygotic and somatic embryo develop-ment in Picea abies (Norway spruce). Physiol. Plant. 80:441--445. Hsu, F.C. 1979. Abscisic acid accumulation in developing seeds of
Phaseolus vulgaris L. Plant Physiol. 63:552--556.
Jones, R.L. and M.L. Brenner. 1987. Distribution of abscisic acid in maize kernel during grain filling. Plant Physiol. 83:905--909. King, R.W. 1976. Abscisic acid in developing wheat grains and its
relationship to grain growth and maturation. Planta 132:43--51. Mertens, R., B. Deus-Neumann and E.W. Weiler. 1983. Monoclonal
antibodies for the detection and quantitation of the endogenous plant growth regulator, abscisic acid. FEBS Lett. 160:269--272. Millborrow, B.V. and R. Mallaby. 1975. Occurrence of methyl
(+)-ab-scisate as an atrifact of extraction. J. Exp. Bot. 26:741--748. Morris, P.C., P.C. Jewer and P.J. Bowles. 1991. Changes in water
relations and endogenous abscisic acid content of wheat and barley grains and embryos during development. Plant Cell Environ. 14:443--446.
Napier, J.A., J.M. Chapman and M. Black. 1989. Calcium-dependent induction of novel proteins by abscisic acid in wheat aleurone tissue of different developmental stages. Planta 179:156--164.
Neill, S.J. and R. Horgan. 1987. Abscisic acid and related compounds.
In Principles and Practice of Plant Hormone Analysis, Vol. 1. Academic Press, New York, NY, pp 111--167.
Neill, S.J., R. Horgan and J.K. Heald. 1983. Determination of the levels of abscisic acid-glucose ester in plants. Planta 157:371--375. Perata, P., P. Picciarelli and A. Alpi. 1990. Pattern of variations in abscisic acid content in suspensors, embryos, and integuments of developing Phaseolus coccineus seeds. Plant Physiol. 94:1776--1780.
Piaggesi, A., P. Perata, C. Vitagliano and A. Alpi. 1991. Level of abscisic acid in integuments, nucellus, endosperm, and embryo of peach seeds (Prunus persica L. cv Springcrest) during develop-ment. Plant Physiol. 97:793--797.
Pinfield, N.J. and V.E.E. Gwarazimba. 1992. Seed dormancy in Acer: the role of abscisic acid in the regulation of seed development in
Acer platanoides L. Plant Growth Regul. 11:293--299.
Prevost, I. and M.Th. Le Page-Degivry. 1985. Changes in abscisic acid content in axis and cotyledons of developing Phaseolus vulgaris
embryos and their physiological consequences. J. Exp. Bot. 36:1900--1905.
Pullman, G. and D. Webb. 1994. An embryo staging system for comparison of zygotic and somatic embryo development. TAPPI R&D Division Biological Sciences Symposium, Minneapolis, MN, pp 31--34.
Quarrie, S.A. and G. Galfre. 1985. Use of different hapten-protein conjugates immobilized on nitrocellulose to screen monoclonal antibodies to abscisic acid. Anal. Biochem. 151:389--99.
Quarrie, S.A., R. Tuberosa and P.G. Lister. 1988. Abscisic acid in developing grains of wheat and barley genotypes differing in grain weight. Plant Growth Regul. 7:3--17.
Roberts, D.R. 1991. Abscisic acid and mannitol promote early devel-opment, maturation and storage protein accumulation in somatic embryos of interior spruce. Physiol. Plant. 83:247--254.
Ross, G.S., P.A. Elder, J.A. McWha, D. Pearce and R.P. Pharis. 1987. The development of an indirect enzyme linked immunoassay for abscisic acid. Plant Physiol. 85:46--50.
Skriver, K. and J. Mundy. 1990. Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2:503--512.
Walker-Simmons, M. 1987. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol. 84:61--66.
Wang, T.L., S.K. Cook, R.J. Francis, M.J. Ambrose and C.L. Hedley. 1987. An analysis of seed development in Pisum sativum. IV. Abscisic acid accumulation. J. Exp. Bot. 38:1921--1932.
Weiler, E.W., J. Eberle and R. Mertens. 1986. Plant growth substances. Berlin, Heidelberg, Germany, pp 22--25.