T
he combination of biochemical, physiological and genetic studies in genetically engineered transgenic plants has pro-ven to be extremely useful for analysing biochemical and developmental processes. Examples include the enhanced or ec-topic expression of enzymes and regulatory proteins, and the ex-pression of antisense RNA or dominant negative proteins that reduce the amount of a gene product. The availability of a broad spectrum of promoters that differ in their ability to regulate the temporal and spatial expression patterns, dramatically increases the application of transgenic technology.In this context, promoters that are only activated in response to a specific chemical are particularly valuable. In E. coli, an exam-ple of a promoter that provides such fidelity is the IPTG inducible
lacpromoter, which is routinely used whenever expression of the recombinant protein is going to interfere with growth. Similarly, if a foreign gene product expressed in plants is going to interfere with regeneration, growth or reproduction, an inducible promoter is required. With such a tool, plants can be regenerated while the promoter is inactive. Further analysis can then be performed after activating expression of the transgene. When working with higher plants, arguments other than the lethality of the gene product may
favour the use of an inducible promoter over a constitutive one (Box 1). Moreover, in addition to being a valuable means of eluci-dating gene function, chemically inducible promoters are increas-ingly relevant to the improvement of crops by genetic engineering. Examples of biotechnological applications are listed in Box 1.
If only very low amounts of a gene product are required for a cellular process, then the expression level of the promoter should be close to zero in the absence of the inducer. In this case, high expression levels in the presence of the inducer are not an essen-tial requirement. In contrast, if only high amounts of a gene prod-uct are effective, residual activity in the absence of the inducer can be tolerated, but it should be inducible to high levels in the pres-ence of the inducer. Ideally, both features – very low expression levels in the absence of the inducer, and high expression levels in the presence of the inducer – should be combined in one system as it is more broadly applicable. A further advance in use of a chemi-cally inducible promoter is its use in combination with tissue spe-cific promoters, so that gene expression can be restricted to a given tissue at a specific time.
It is important that the chemical used is highly specific for the target promoter: it should neither influence the expression of other genes nor affect other cell functions. Uptake is another important issue: once it is applied, either by foliar spray or root drenching, the chemical should enter every cell. Additional requirements that an ideal inducer should meet are given in Box 2.
To provide such tools, two different strategies are being adopted1 . First, plant promoters that respond to a given chemical have been isolated: obviously, this concept will provide expression systems that are not specific to the transgene alone. The second strategy involves using regulatory elements from other organisms that respond to chemicals that are not usually encountered by plants.
Promoters that respond to chemical
inducers
Christiane Gatz and Ingo Lenk
The introduction of foreign genes constitutes a powerful tool with which to study and improve the genetic resources of plants. Transgene expression levels and expression pat-terns can be adjusted by combining the protein coding region with a suitable promoter. There is a diverse spectrum of endogenous plant promoters and these are currently being broad-ened by the development of chimeric promoters that respond to otherwise inactive chemicals. This range includes promoters that respond to inducers such as the antibiotic tetracycline, the steroid dexamethasone, the copper ion, ethanol or the agrochemical RH-5992. These chimeric promoters offer a range of options for transgene design for experimental and field use.
Box 1. Applications of chemically inducible promoters
Basic research
• Expression of gene products that interfere with regeneration, growth or reproduction.
• Expression of gene products at different stages of development. • Differentiation between primary and secondary effects. • Analysis of primary effects before homeostatic mechanisms
start to counteract.
• Clear correlation between induction of the transgene and occur-rence of an altered phenotype.
Biotechnological applications
• Construction of a conditional male sterility system.
• Expression of transgenes that interfere with regeneration, growth or reproduction (biofarming).
• Conditional expression of resistance genes as a means of pest management to delay adaptive processes of the pathogen. • Simultaneous induction of processes such as flowering and leaf
abscission.
Box 2. Properties required for an ideal inducer
• High specificity for the transgene, no toxicity. • High environmental compatibility.
• Convenient application by foliar spraying or root drenching. • High efficiency at low concentrations and low use rates. • Low costs.
Both strategies have yielded useful tools that respond to a variety of unrelated chemicals (Fig. 1).
Endogenous plant promoters
A prominent example of a plant promoter that responds to chemi-cal treatment is the PR-1apromoter1
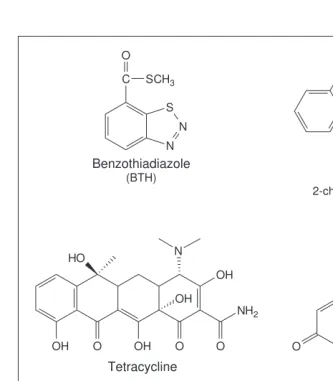
. The PR-1apromoter, which drives expression of a defence gene, is normally induced upon pathogen attack, forms of oxidative stress and leaf senescence, but it also responds to benzothiadiazole2
(BTH, Fig. 1). BTH can be applied by foliar spraying and is available under the trade name BION (Novartis). The PR-1a promoter has been used to drive expression of the Bacillus thuringiensisd-endotoxin in transgenic plants. Insect feeding damage was only inhibited in the chemically treated plants. In those experiments, the alternative inducer iso-nicotinic acid (INA) was used instead of BTH (Ref. 2). The PR-1a
promoter can thus be used conditionally to express resistance genes. As permanent expression of a resistance protein favours the adaptive processes of a pathogen, the PR-1a/BTH system might constitute a means of reducing these selection pressures to ensure improved pest management. In this context, the fact that the chemi-cal also induces a whole battery of other defence genes may thus be tolerated.
For biotechnological applications, commercially certified chemi-cals are an attractive option for chemical regulation. Safeners are agrochemicals that increase the tolerance of plants to herbicides1
.
They appear to induce a smaller set of genes as compared with BTH, most likely those genes that encode enzymes responsible for detoxifying xenobiotics. Several safener-inducible promoters have been cloned from maize1
. One of these, the In2-2promoter, has been fused to a reporter gene and tested in transgenic Arabidopsis3
. The promoter was inactive in the absence of the safener benzenesulfonamide (Fig. 1), and induction occurred only in roots, apical meristems and hydathodes. Induction was coupled to retarded growth. After transfer of the plants to safener-free medium, the promoter was again inactivated and normal plant devel-opment was restored. This feature limits its application to experiments that require only transient expression in the above-mentioned tissues. The In2-2promoter might still be valuable for controlling gene expression in maize, as the expression pattern is more favourable1
. Also, the concentrations of safeners required for induction do not af-fect monocots as severely as dicots.
Gene regulation by regulatory elements from non-plant organisms
Organisms that are distant from plants in evolutionary terms have developed mecha-nisms of gene regulation that respond to external signals that are not usually en-countered by higher plants. Antibiotics, for example, predominantly affect prokaryotes, which in turn have evolved antibiotic-inducible resistance genes4
. Lactose (or ana-logues such as IPTG) induces metabolic genes in enteric bacteria5
, but such pro-cesses are unlikely to affect plant genes. Specific steroid hormones control animal development by altering gene expression patterns6
, but there is no equivalent target molecule in plants. If the underlying regulatory mechanisms involve the binding of only one regulatory protein to specific cissequences, they can be engineered to work in plants, thus yielding expression systems that respond to highly specific chemicals.
This strategy requires the expression of two genes in transgenic plants: the gene encoding the protein responsible for the regu-lation (transcriptional repressor or activator) and the gene of inter-est, under the control of a suitable target promoter. This strategy is more complex than the use of endogenous plant promoters, which only require the transfer of the gene of interest under the control of the inducible promoter. The use of heterologous regu-latory elements includes systems that respond to the antibiotic tetracycline (tc, Figs 2 and 3)7,8
, the steroid dexamethasone (dx, Fig. 4)9
, the copper ion (Fig. 5)10
or IPTG (Ref. 11). Here, we have focused on their most recent applications, and the use of two novel systems that respond to ethanoll2
and RH-5992 (I. Jepson, pers. commun.).
The tetracycline-inducible promoter
The concept of using a regulatory protein from a prokaryote to control plant gene expression was first realized through the use of the bacterial repressor protein TetR, which binds to tetoperator DNA only in the absence of its inducer tc (Ref. 1). The TetR–tet Fig. 1.Molecules used for the chemical induction of transgene expression in plants.
operator–tc interaction has now been engineered to regulate plant gene expression (Fig. 2). When using b-glucuronidase as a re-porter system, 500- to 800-fold induction is routinely observed, with induced levels reaching the levels of the CaMV 35S promoter.
The benefits of the tc-inducible promoter were exploited recently for the conditional overexpression of isopentenyl transferase (ipt), an enzyme that catalyzes a rate-limiting step in cytokinin
biosyn-thesis13,14
. Even small amounts of the ipt protein lead to cytokinin-induced morphological changes. This makes the iptgene an ideal marker for determining how leaky a regulatory system is in its un-induced state. Untreated transgenic plants appear to be very simi-lar to the wild type, with residual ipt activity being only visible by the somewhat delayed senescence. Application of tc leads to inac-tivation of TetR, allowing the synthesis of ipt transcripts at far
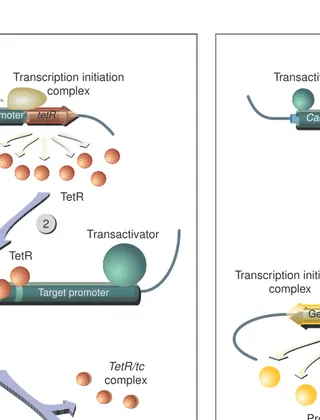
Fig. 2. The tetracycline-inducible expression system. (1) The Tn10 encoded Tet repressor (TetR, Mr24 kDa) is synthesized under the
control of a strong constitutive promoter (e.g. CaMV 35S promoter). (2) The target promoter (e.g. a modified CaMV 35S promoter) contains three 19 bptetoperator sites that flank the TATA box. By binding to these sequences, three TetR dimers interfere with the assembly of the transcription initiation complex. As the transcription initiation complex is stabilized by multiple pro-tein–protein interactions, stringent repression requires high levels of TetR. (3) The antibiotic tc binds to TetR with a high affinity, abolishing its DNA-binding ability. (4) Under these conditions, repression is relieved, leading to the synthesis of the gene product. This system has been shown to work in tobacco, potato and tomato plants, but not in Arabidopsis, which might not tolerate the amounts of TetR needed for efficient repression.
CaMV35Spromoter tetRtetR
+ tc
CaMV 35S promoter
TetR
TetR Transactivator
Transactivator
Transactivator
Product X No gene expression
Transcription initiation complex
Transcription initiation complex
TetR/tc complex
Target promoter
1
2
4 3
Gene X
Gene X
Target promoter
Fig. 3. The tetracycline-inactivatable expression system. (1) A fusion protein (tTA, Mr 38 kDa), consisting of TetR and the
acid activation domain of Herpes simplexprotein 16 (VP16), is expressed under the control of a strong promoter (e.g. CaMV 35S promoter). (2) The target promoter contains several tetoperators upstream of a short DNA fragment encoding the TATA box. Multiple binding sites guarantee strong activation owing to the synergistic effects of multiple tTA proteins. In contrast to TetR (Fig. 2), tTA does not compete with endogenous transcription factors for access to the binding sites. Thus considerably less amounts of tTA are needed compared with TetR. (3) The antibiotic tc binds to the TetR moiety with high affinity, abolishing its DNA-binding ability. (4) Under these conditions, expression is efficiently turned off. The system has been shown to work in tobacco and
Arabidopsis.
CaMV35Spromoter tetR
tTA
tTA
No gene expression
1
2
Gene X Target promoter
Gene X Target promoter tetVP16 CaMV 35S promoter
Transactivator
Product X
Transcription initiation complex
Transcription initiation complex
tTA/tc complex
4
higher levels. Constitutive expression triggers a highly aberrant phenotype, and induction with saturating amounts of tetracycline (1 mg ml21
) is lethal. By using lower amounts of tc (0.1 mg l21 ), it proved possible to study the metabolic fate of de novo-synthesized
cytokinin in different parts of the plant14
. In combination with grafting experiments this system allowed an assessment of the role of cytokinins as long-range root–shoot signals in the corre-lative control of apical dominance and sequential leaf senes-cence in tobacco. The experiments supported the hypothesis that cytokinin is involved in paracrine signalling14
. The system was also used to obtain transgenic tobacco plants that express high levels of oat arginine decarboxylase in their leaves15
. These experiments were carried out to investigate the action of specific polyamines in different developmental processes. A study with transgenic potato plants has demonstrated that successful antisense RNA experi-ments can also be performed using this promoter16
. The tc-inducible gene expression is also effective in both the leaves and fruits of tomato17
. Whether the plants expressing TetR still exhibit an abnormal phenotype, as described before18
, was unclear.
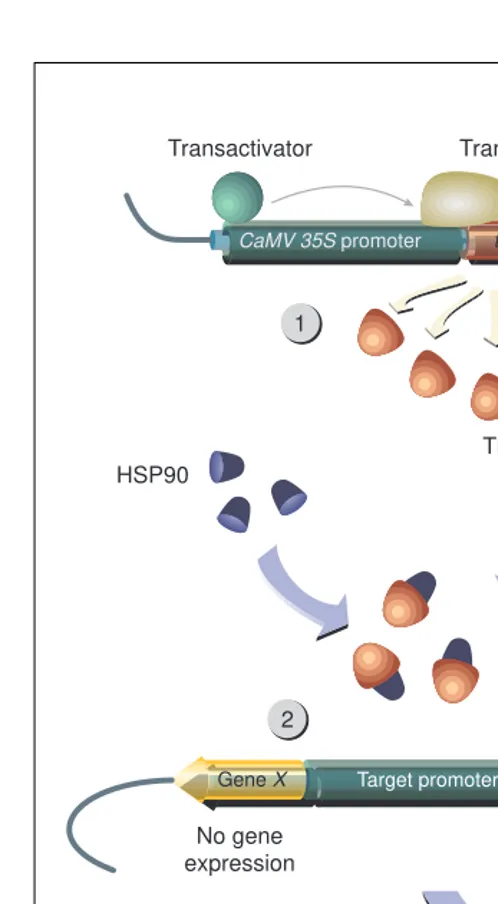
Fig. 4. The dexamethasone-inducible expression system. (1) The fusion protein TF-GR, consisting of a transcription factor (DNA binding domain and transcriptional activation domain) and the glucocorticoid binding domain, is expressed under the control of a strong promoter (e.g. CaMV 35S promoter). (2) In the absence of dexamethasone the activator is trapped by the formation of an inactive complex with HSP90. (3) The binding of dexamethasone mediates dissociation from HSP90 and allows binding of the activator to a target promoter that contains multiple TF-GR binding sites upstream of a short DNA fragment that encodes the TATA box. (4) Transcription from the target promoter is induced. TF can be any transcription factor that contains a DNA-binding domain and an activation domain. Aoyama and Chua have used the DNA-binding domain of yeast transcription factor GAL4 and the acid activation domain of Herpes simplexvirus protein 16 (Ref. 9).
1
2
3
4
CaMV35Spromoter tf-grtetR
CaMV 35S promoter
Transactivator Transcription initiation complex
TF-GR HSP90
+ dx No gene
expression
Gene X Target promoter
Gene X Target promoter
Product X Transcription initiation
complex
TF-GR/dx complex HSP90–TF-GR
complex
Fig. 5.The copper-inducible expression system. (1) Yeast trans-cription factor ACE1 is expressed under the control of a strong promoter (e.g. CaMV 35S promoter). (2) In the absence of copper, it cannot bind to its target promoter. (3) The binding of copper induces a conformational change, enabling the ACE1 to bind to specific cis sequences of the target promoter. (4) Transcription from the target promoter is induced. For the ethanol-inducible system, ACE1 is replaced by AlcR, and Cu is replaced by ethanol. However, it is not clear whether ethanol directly binds to AlcR.
1
2
3
4
CaMV35Spromoter ace 1tetR
CaMV 35S promoter
Transactivator Transcription initiation complex
ACE1
+ Cu No gene
expression
Gene X Target promoter
Gene X Target promoter
Product X Transcription initiation
complex
The ease with which the chemical is applied has to be addressed when discussing chemically inducible promoters. Tetracycline is most easily applied by infiltration into detached leaves14
. When plants are grown hydroponically, fresh tc has to be added every other day. Some browning of roots is observed under non-sterile hydroponic conditions. Soil drenching or application of tc to plants cultivated in rockwool has also been reported to be successful15,18
. Alternatively, tc can be applied by repeated leaf painting, a pro-cedure that is advantageous for the local induction of single organs (K. Krupinska, pers. commun.).
The tetracycline-inactivatable promoter The tc-inactivatable promoter8
is based on the same regulatory elements as the tc-inducible promoter (see Fig. 3)1
. It is especially useful for the study of protein or RNA stability19
: the expression of a transgene can be specifically turned off, after which the decay kinetics of the RNA or the protein can be analysed. In addition, it is a valuable system if the regeneration of transgenic plants is the only parameter affected by the transgene. In this case, regeneration can be performed in the presence of tc. Subsequent analysis can be performed without the chemical, so simplifying the experiments. The original target promoter Top10has been shown to be prone to silencing8
. Therefore reconstruction of the promoter is currently being carried out in an attempt to reduce its susceptibility to this epigenetic effect (C. Gatz and I. Lenk, unpublished).
Steroid-inducible promoters
Steroids such as the glucocorticoid dexamethasone (dx) are attrac-tive options as chemical inducers because they exhibit high speci-ficity for the transcriptional activator, the glucocorticoid receptor6
. Glucocorticoid-dependent transcription is based on the inhibitory interaction between the heat shock protein HSP90 and the ligand-binding domain of the receptor that occurs in the absence of the ligand20
(Fig. 4). Binding of the ligand leads to dissociation of the receptor from HSP90 and thus to release of a transcriptional acti-vator. Although the complete receptor protein does not work effi-ciently in transgenic plants21
, the ligand-binding domain has been shown to confer ligand-dependent activation of cis-located protein domains. The glucocorticoid receptor binding domain has been fused to different transcription factors: the maize transcription fac-tor R (Ref. 21), the Arabidopsis transcription factor Athb (Ref. 22), the flowering-time gene CONSTANS(Ref. 23) and the MADS box factor AP-3 (Ref. 24). In these examples, the protein product to be studied was directly controlled by the ligand-binding domain of the receptor. This post-transcriptional regulatory system is very power-ful. By expressing a steroid-regulated version of AP-3 in a suitable mutant background, the immediate effects of AP-3 on the floral mRNA population were analyzed by differential display while secondary effects were blocked by a protein synthesis inhibitor24
. This system has also been modified to establish a dx-inducible transcriptional control system9
that can be combined with any gene. Such a system was generated by fusing the glucocorticoid recep-tor ligand-binding domain to a fusion protein that consists of the DNA-binding domain of yeast GAL4 and the VP16 activation do-main. The target promoter consists of six GAL4 binding sites up-stream of the CaMV 35S promoter (Fig. 4). In transgenic tobacco plants, luciferase activity was induced 100-fold by dx. Because the background level in the absence of dx was arbitrarily set at 1, it is unclear whether there is any detectable background activity. Expression levels in the presence of inducers were not set in re-lation to the CaMV 35S promoter. Thus the data can be understood as ‘proof of principle’, but more data, especially on the expression levels, are required to obtain a good estimate of the efficiency of the system. Whether the post-transcriptional or the transcriptional
control system should be used depends on the experiment: the approach taken to clone target genes of AP-3 required the post-transcriptional control system because of the simultaneous use of a protein synthesis inhibitor24
.
By exploiting the same principle, but using domains from dif-ferent proteins, a steroid-inducible promoter has been established for transgenic maize. This system has been described in the con-text of an RNA extraction procedure by Pharmacia [Garnaat, C.W. and Roth, B. (1997) Science Tools from Pharmacia Biotech. 2, 10–11]. The DNA-binding domain and the ligand-binding domain were taken from the oestrogen receptor, whereas the activation do-main was taken from the maize transcription factor C1. The 17a -ethylenylestradiol-inducible target promoter consists of oestrogen response elements fused to a minimal plant promoter. By condition-ally expressing the male fertility gene MS45in a male-sterile back-ground, male fertility was chemically controlled. Male sterility is an important feature for the generation of hybrid seeds, and can be achieved in the absence of the chemical. However, when fertile lines are required then fertility can be restored by application of the inducer.
Different dx treatments have been described: the addition to medium in experiments using axenically grown plants; induction of plants in hydroponic culture9
; soil drenching23
; and the local treatment of leaves9
and flowers24 .
Copper-inducible gene expression
In contrast to tc or dx, copper plays an important role in plant metabolism: sufficient copper has to be present in the cell to drive essential biochemical processes, but accumulation to high levels leads to toxic effects. Nevertheless, a functional copper-inducible expression system has been established in plants10
. It is based on control elements that regulate the expression of copper detoxifi-cation genes in Saccharomyces cerevisiaein response to elevated copper concentrations. Regulation is mediated by the transcrip-tional activator ACE1, which binds to specific ciselements only when coordinated with copper25
. This simple mechanism was en-gineered into plants (Fig. 5). When the activator was driven by the CaMV 35S promoter, a 50-fold induction of the target promoter was observed with b-glucuronidase as a reporter enzyme. However, expression from this construct in roots was constitutive owing to plant activating sequence-1 left in the target promoter. As ex-pected, elimination of this element abolished the background activity26
. Unexpectedly, the promoter appeared to have lost its inducibility in leaves, although the data were not reported. This ‘root-specific’ copper-inducible system has been used to condition-ally express the iptgene in transgenic tobacco27
. As mentioned above, very low amounts of ipt lead to a visibly altered pheno-type. Transgenic tobacco was morphologically identical to con-trols under noninducing conditions, indicating that the promoter is not leaky in the absence of inducer. Following induction by addition of copper, typical cytokinin effects, such as decreased apical dominance and delayed senescence, were observed. These changes are considered to be minor in comparison with the aber-rant phenotype that elevated ipt levels normally elicit. These minor effects support the notion that the promoter is not highly induced in leaves. It is not clear whether the phenotypic effects resulted from residual copper inducible gene expression in the aerial parts of the plant or from the transport of cytokinins from induced roots to the shoot.
By placing ACE1 under the control of the nodule-specific nod 45promoter, the function of the system in an organ-specific man-ner was demonstrated26
in nodules and not in roots after the addition of copper ions to the nutrient solution. The nodule-specific system was used to express antisense constructs of aspartate aminotransferase-P2 in trans-genic L. corniculatusplants. When expression was induced, the aspartate aminotransferase-P2 activity declined dramatically, and a decrease in nodule asparagine concentration of up to 90% was observed. Whether the inducing amounts of copper are specific to the transgene only is not clear, and it is possible that detoxification mechanisms are also activated.
Ethanol-inducible gene expression
A promising alternative to the systems described above has re-cently been established12
. This novel promoter is based on the regu-latory elements of the Aspergillus nidulans alcApromoter, which is strongly inducible by ethanol. It is the most widely used pro-moter for overexpressing proteins in A. nidulans and other fila-mentous fungi, both for fundamental research and for applied biotechnology28
. The transcriptional activator AlcR, a DNA bind-ing protein belongbind-ing to the C6 zinc binuclear cluster family, binds to its target sequences within the alcApromoter when cells are grown in the presence of ethanol or other inducers such as ethyl methyl ketone29
. The system was adapted for plants by placing the
alcRcoding region under the control of the CaMV 35S promoter. The target promoter contains the TATA box as well as upstream sequences of the alcApromoter fused to position 223 of the CaMV 35S promoter12
. When stably transformed into tobacco these con-structs mediate ethanol-dependent expression of transgenes. It is not known if the DNA-binding activity is directly or indirectly affected by ethanol, but the principle of the regulation is similar to that of the copper inducible promoter (Fig. 5). The promoter activ-ity in the induced state is in the range of the CaMV 35S promoter. As this system has only recently been developed, some questions concerning the specificity of the inducer for the transgene, toxicity, background levels (especially under anaerobic conditions) and in-duction levels over a longer time course have yet to be addressed. As the system is put to greater use, the extent of its usefulness will emerge.
The system has already been used to analyse carbon metab-olism by conditionally expressing cytosolic invertase in transgenic plants12
. Constitutive expression of high levels of cytosolic inver-tase prevents plant maturation as chlorosis develops in the sink leaves; plants conditionally expressing invertase grew normally until induced with ethanol. Within four days of induction, the pheno-type of the youngest leaves was severely affected.
Although spraying seems to work12
, ethanol appears to be ef-fective if taken up through the roots, either in hydroponic culture using 0.1% ethanol, or by soil drenching with 1% ethanol. The local induction of single leaves is not yet possible because ethanol vapour induces the whole plant and its neighbours (U. Sonnewald, pers. commun.). Caddick et al. are optimistic that the system might function in field applications12
. It seems feasible that effi-cient ethanol formulations or non-volatile inducers will be devel-oped in the future. This would constitute a real breakthrough for agricultural biotechnology as chemically inducible promoters are still lacking. Neither the tc- nor the dx-inducible system are as broadly applicable.
Insecticide-inducible gene expression
An important step towards the development of a chemically in-ducible promoter for applications in the field utilizes a novel chemi-cally inducible expression system that responds to the already certified agrochemical RH5992 (Fig. 1) (I. Jepson, pers. commun.). The non-steroidal chemical RH5992 is effective as a lepidopteran-specific insecticide and acts by agonizing the effect of the insect
hormone ecdysone. The ecdysone receptor from Heliothis vir-escence was cloned by PCR, using degenerate oligonucleotides directed against the DNA-binding domain of the animal steroid/ thyroid superfamily. A chimeric transcriptional activator was assembled using the DNA-binding domain of the glucocorticoid receptor, the VP16 activation domain and the ligand-binding do-main of the H. virescenceprotein. Transcription from a suitable target promoter was RH5992-responsive. Because the H. virescence
protein has not been studied as thoroughly as the mammalian glucocorticoid receptor, any possible induction mechanism is still highly speculative. Overall, the approach is very attractive be-cause it uses a chemical that has already been tested for its en-vironmental compatibility, but its practical effectiveness has yet to be established.
Future prospects
It is feasible that chemically inducible promoters could be con-structed using novel regulatory elements from organisms such as
E. coli, mammals, fungi and insects, but various questions remain unanswered.
• How leaky are these promoters? Statistically relevant figures based on either b-glucuronidase or luciferase activity have to be presented; measurements close to background levels are often difficult to quantify. The iptgene, which gives rise to pheno-typical changes even at very low expression levels, provides a sensitive reporter system for these analyses.
• How do these promoters compare with the CaMV 35S promoter in the activated state?
• How homogenous is induction in different organs?
• To what extent do the induction kinetics depend on the size of the plant? Are lower leaves more rapidly induced than upper leaves, or vice versa?
• How fast does the response decline after omission of the in-ducer, and how does this depend on the duration of induction? • Is repeated application of the inducer necessary, even after long
exposure of the plants to the chemical?
• At what concentration is the inducer toxic? How does this com-pare with the dose–response curve of expression? Measure-ments made on photosystem II might be a good criterion for evaluating the vigour or fitness of the plants treated with the chemical.
• Does the chemical induce genes involved in the detoxification of xenobiotics?
• Does the transcriptional activator cause adverse effects in the plant? It has been reported that the VP16 domain might not be tolerated in higher amounts30
.
• How stable is gene expression, both somatically and meiotically? A comparative analysis of the different promoters under standard-ized conditions, will help in the evolution of transgene design.
Several additional refinements can be added to these systems. By combining activators with tissue-specific promoters it will be possible to induce gene expression in a given tissue. One possible development would be to generate a collection of activator plants (e.g. tobacco and Arabidopsis) that express the activator protein under the control of different tissue-specific or developmentally regulated promoters. After crossing ‘activator plants’ with plants encoding the gene of interest under the control of the target pro-moter (‘target plants’), gene expression will be inducible in the next generation. In contrast to the recently described activator LhG4, which consists of the Lac repressor and the GAL4 activation do-main31
Articles in Trends in Plant Science
To help our readers search through back issues for articles of interest, the Elsevier Science Tables of Contents (ESTOC) entries for Trends in Plant Science can be found at:
http://www.elsevier.nl:80/inca/publications/store/3/0/9/6/0/
The site also provides details of the editorial board; aims and scope of the journal; instructions to authors; and electronic access for sample copy requests, subscription information and orders.
References
1Gatz, C. (1997) Chemical control of gene expression, Annu. Rev. Plant Physiol. Plant Mol. Biol.48, 89–108
2Gorlach, J. et al.(1996) Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat, Plant Cell8, 629–643
3De Veylder, L., Van Montagu, M. and Inzé, D. (1997) Herbicide
safener-inducible gene expression in Arabidopsis thaliana, Plant Cell Physiol.38, 568–577
4Hillen, W. and Berens, C. (1994) Mechanisms underlying expression of Tn10
encoded tetracycline resistance, Annu. Rev. Microbiol. 48, 345–369
5Beckwith, J.R. and Zipser, D. (1970) The Lactose Operon, Cold Spring
Harbor Laboratory Press
6Evans, R.M. (1988) The steroid and thyroid hormone receptor superfamily,
Science240, 889–895
7Gatz, C., Frohberg, C. and Wendenburg, R. (1992) Stringent repression and
homogeneous de-repression by tetracycline of a modified CaMV 35S promoter in intact transgenic tobacco plants, Plant J.2, 397–404 8Weinmann, P. et al.(1994) A chimeric transactivator allows
tetracycline-responsive gene expression in whole plants, Plant J.5, 559–569
9Aoyama, T. and Chua, N-H. (1997) A glucocorticoid-mediated transcriptional
induction system in transgenic plants, Plant J.11, 605–612
10 Mett, V.L., Lochhead, L.B. and Reynolds, P.H.S. (1993) Copper controllable
gene expression system for whole plants, Proc. Natl. Acad. Sci. U. S. A.90, 4567–4571
11 Wilde, R.J. et al.(1992) Control of gene expression in tobacco cells using a
bacterial operator–repressor system, EMBO J.11, 1251–1259
12 Caddick, M.X. et al. (1998) An ethanol inducible gene switch for plants used
to manipulate carbon metabolism, Nat. Biotechnol.16, 177–180
13 Motyka, V. et al.(1996) Changes in cytokinin content and cytokinin oxidase activity in response to derepression of iptgene transcription in transgenic tobacco calli and plants, Plant Physiol.112, 1035–1043
14 Faiss, M. et al.(1997) Conditional transgenic expression of the iptgene indicates a function for cytokinins in paracrine signaling in whole tobacco plants, Plant J. 12, 401–415
15 Masgrau, C. et al.(1996) Inducible overexpression of oat arginine decarboxylase in transgenic tobacco plants, Plant J.11, 465–473 16 Kumar, A. et al.(1995) Potato plants expressing antisense and sense
S-adenosylmethionine decarboxylase (SAMDC) transgenes show altered
levels of polyamines and ethylene: antisense plants display abnormal phenotypes, Plant J.9, 147–158
17 Thompson A.J. and Myatt, S.C. (1997) Tetracycline-dependent activation of
an upstream promoter reveals transcriptional interference between tandem
genes within T-DNA in tomato, Plant Mol. Biol.34, 687–692
18Corlett, J.E., Myatt, S.C. and Thompson, A.J. (1996) Toxicity symptoms
caused by high expression of Tet repressor in tomato (Lycopersicon esculentum
Mill. L.) are alleviated by tetracycline, Plant Cell Environ.19, 447–454
19Gil, P. and Green, P.J. (1995) Multiple regions of the Arabidopsis SAUR-AC1
gene control transcript abundance: the 3′untranslated region functions as an
mRNA instability determinant, EMBO J.15, 1678–1686
20Picard, D. (1994) Regulation of protein function through expression of chimaeric proteins, Curr. Top. Biotechnol.5, 511–515
21Lloyd, A.M. et al.(1994) Epidermal cell fate determination in Arabidopsis: patterns defined by a steroid-inducible regulator, Science266, 436–439 22Aoyama, T. et al.(1995) Ectopic expression of the Arabidopsistranscriptional
activator Athb-1 alters leaf cell fate in tobacco, Plant Cell7, 1773–1785
23Simon, R., Igeno, M.I. and Coupland, G. (1996) Activation of floral meristem
identity genes in Arabidopsis, Nature384, 59–62
24Sablowski, R.W.M. and Meyerowitz, E.M. (1998) A homolog of NO APICAL
MERISTEMis an immediate target of the floral homeotic genes
APETALA3/PISTILLATA, Cell92, 93–103
25Dameron, C.T. et al. (1991) A copper-thiolate polynuclear cluster in the ACE1 transcription factor, Proc. Natl. Acad. Sci. U. S. A.88, 6127–6131
26Mett, V.L. et al.(1996) A system for tissue-specific copper-controllable gene expression in transgenic plants: nodule-specific antisense of aspartate
aminotransferase-P2, Transgenic Res.5, 105–113
27McKenzie, M.J. et al.(1998) Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter, Plant Physiol.116, 969–977 28Felenbok, M. (1991) The ethanol utilization regulon of Aspergillus nidulans:
the alcA–AlcRsystem as a tool for expression of recombinant proteins,
J. Biotechnol.17, 11–18
29Panozzo, C. et al.(1997) The zinc binuclear cluster activator AlcR is able to bind to single sites but requires multiple repeated sites for synergistic activation of the alcAgene in Aspergillus nidulans, J. Biol. Chem.272, 22859–22865
30Berger, S.L. et al. (1990) Selective inhibition of activated but not basal transcription by the acidic activation domain of VP16: evidence for transcriptional adaptors, Cell61, 1199–1208
31Moore, I. et al.(1998) A transcription activation system for regulated gene expression in transgenic plants, Proc. Natl. Acad. Sci. U. S. A.95, 376–381
Christiane Gatz*and Ingo Lenk are at the Albrecht von Haller Institute of Plant Sciences, University of Goettingen, Untere Karspuele 2, 37073 Goettingen, Germany.